Abstract
Background
Endocrine disruptors are one of the major threats to aquatic animals affecting their development and physiology. Amphibians are very sensitive to aquatic contaminants as their skin is semi-permeable. Several contaminants easily enter into their body, act as endocrine disruptors, and interfere in sexual development and metamorphosis. Endocrine disruptors have diverse effects in different species mainly due to the variations in developmental patterns. In the present study, I evaluated the endocrine disrupting potential of tamoxifen (anti-estrogen) in the tadpoles of Indian skipper frog Euphlyctis cyanophlyctis with undifferentiated type of gonad differentiation (testis differentiates through an ovarian phase).
Methods
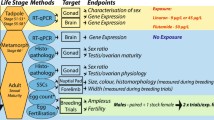
I exposed the tadpoles to four concentrations of tamoxifen (25, 50, 100, and 200 μg/l) during larval development (Gosner stages 25–42) and studied their metamorphosis, somatic and sexual development.
Results
Tamoxifen does not influence gonad differentiation and sex ratio (proportion of males and females). However, all the concentrations of tamoxifen stimulated gonad development resulted in testis maturation in males and increased oocytes size in females. Tamoxifen treatments delayed metamorphosis and stimulated somatic growth.
Conclusion
These results suggest that tamoxifen does not act as an anti-estrogen in E. cyanophlyctis while stimulate sexual development in both males and females through unknown mechanism. These results are useful to understand the mechanism of action of tamoxifen in lower vertebrates and develop E. cyanophlyctis as an indigenous amphibian model for endocrine disruption studies.
Similar content being viewed by others
Background
Use of pharmaceuticals has been increased drastically in past some decades, and many of these chemicals can be found in water-bodies as contaminants. Some of these chemical interfere in endocrine system (endocrine disrupters, ED) of aquatic animals and affect development, homeostasis leading to developmental and physiological abnormalities. However, many of the EDs can be useful to understand the mechanism and function of endocrine system (Orton & Tyler, 2015; Safholm, Ribbenstedt, Fick, & Berg, 2014).
Amphibians are one of the most sensitive groups to environmental contaminants as their skin is passively permeable for most of the chemicals (Quaranta, Bellantuono, Cassano, & Lippe, 2009). Several water contaminants act as ED and affect their sexual development and metamorphosis by interfering with hypothalamic–pituitary–gonadal axis and hypothalamic–pituitary–adrenal axis respectively (Kloas et al., 2009). Some of the EDs mimic steroid hormones and interact with their receptors (Kloas et al., 2009). EDs act as either agonists or antagonists and stimulate or inhibit gonadal differentiation and development, which could lead to abnormal sexual development and biased sex ratio (Flament, 2016). The effects of EDs can vary in different species and are dependent on the concentration used, duration of exposure, etc. (Flament, 2016; Hogan, Duarte, Wade, Lean, & Trudeau, 2008; Solomon et al., 2008). Moreover, differences in the gonad differentiation pattern and development rate are the possible intrinsic factors responsible for the variations in the effects of EDs on gonad development in amphibians (Mali & Gramapurohit, 2016; Phuge & Gramapurohit, 2015; Storrs & Semlitsch, 2008; Storrs-Mendez & Semlitsch, 2010). There are two patterns of gonadal differentiation in amphibians based on the direct (differentiated type, ovary and testis differentiate directly from indifferent gonads) or indirect (undifferentiated type, testis differentiate through an ovarian phase) differentiation of the testis (Mali & Gramapurohit, 2015). In addition, rate of the gonad development in amphibians can be categorized into different types (Haczkiewicz & Ogielska, 2013; Ogielska & Kotusz, 2004). For instance, ovary development is categorized as accelerated, basic, and retarded type based on the differentiation event before, during, and after metamorphosis, respectively (Ogielska & Kotusz, 2004). Moreover, studies regarding endocrine disruption in amphibians are mainly focused on the species with differentiated type of gonad differentiation, while species with undifferentiated type of gonad differentiation are neglected (Flament, 2016; Solomon et al., 2008).
The present study was undertaken to investigate the effect of tamoxifen (anti-estrogen; TM) on the gonad development and metamorphosis of Indian skipper frog Euphlyctis cyanophlyctis (Family, Dicroglossidae) with an undifferentiated type of gonad differentiation. Tamoxifen is an anti-estrogen used in breast cancer and infertility treatments (Chua et al., 2013; Jordan, 1994; Steiner, Terplan, & Paulson, 2005). TM is also reported to found in sewage and river water (Petrie, Barden, & Kasprzyk-Hordern, 2015; Zhang, Hibberd, & Zhou, 2008). Gonadal development of E. cyanophlyctis commences during early larval development where gonads in all the tadpoles differentiate into ovaries (around stage 27), and after stage 35, in some individuals, ovaries degenerate and transform into testis. At metamorphosis, males and females can be distinguished by studying gonad morphology. At metamorphosis, testes contain a mixture of germ and somatic cells while ovaries are composed of diplotene oocytes of different sizes (Phuge & Gramapurohit, 2013). Sexual development of this frog is sensitive to exogenous steroids, and changes in gonad development can be traced easily (Phuge, 2018; Phuge & Gramapurohit, 2015). Previously, I have studied the effects of sex steroids on gonad differentiation and development (Phuge & Gramapurohit, 2015). Along with the steroid treatments, effect of TM was studied. We treated the tadpoles of E. cyanophlyctis with four concentrations of TM and studied their gonadal and somatic development at metamorphosis.
Methods
Tadpoles were obtained and maintained in the laboratory following the same methodology described by (Phuge & Gramapurohit, 2015). Tadpoles of Gosner (1960) stage 25 were treated with four concentrations (25, 50, 100, and 200 μg/l) of TM up to stage 42 (forelimb emergence). For each treatment, 60 tadpoles in were reared in three aquaria (20 tadpoles per aquarium and five tadpoles per liter). TM was procured from Sigma-Aldrich, USA (catalog numbers TM─T5648; purity ≥ 98%). TM was dissolved in ethanol (100%) at concentration 4 mg/ml. The required amount of TM (with 50 μl/l ethanol) was directly added to the rearing water on every third day after complete water change. Control group was treated with 50 μl/l of ethanol.
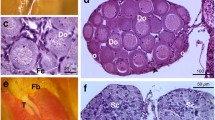
After stage 42 (forelimb emergence), tadpoles were maintained in small plastic containers (29 × 22 × 14 cm) with some water to facilitate metamorphosis. Larval period was calculated as days elapsed from stages 25–42. At the completion of metamorphosis, snout-vent length (SVL), body mass, and larval period were recorded for each froglet of each group. Snout-vent length was recorded to nearest 0.1 mm using a digital caliper (Mitutoyo) while body mass was recorded nearest to 1.0 mg using an electronic balance. Froglets were anaesthetized using 1% Tricaine methanesulfonate (MS 222); gonads were dissected out and fixed in Bouin’s fluid. Sexes of froglets and developmental stages of the testes were ascertained on the basis of gonad morphology following the criteria described previously (Phuge & Gramapurohit, 2015). To confirm the sex and developmental stage, gonads of ten males and ten females from each group were processed for histology. Bouin’s fixed gonads were dehydrated in ascending grades of ethanol, cleared in xylene, and embedded in paraffin wax (58–60 °C; MERCK). Serial transverse sections of tissue were cut at 5 μm using a rotary microtome (Leica RM 2125), stained with hematoxylin and eosin, observed and photographed under the microscope (Carl Zeiss, Axioscope A1). To determine the effect of TM on oocyte growth, diameter of five largest oocytes from the section passing through the center of the ovary was measured in micrometer. In the group treated with 100 μg/l of TM, only eight females survived. Therefore, ovaries from eight females of each group were considered for oocyte growth analysis.
Sex ratio (number of males and females) in control group was checked for hypothetical 1:1 sex ratio using binomial test. Difference in the sex ratio in different groups was analyzed by chi-square test assuming the number of males and females in the control group as an expected values and number of males and females in TM-treated groups as obtained values. The difference in the mortality among control and TM-treated groups was analyzed using χ2 test. Size of the oocytes among control and TM-treated groups was compared using ANOVA followed by Tukey’s test for pair-wise comparisons. The data on larval life history traits (SVL, body mass and larval period) was log-transformed and confirmed for normal distribution and homogeneity using Shapiro-Wilk test and Levene’s test, respectively. Differences in the larval life history traits among control and TM-treated groups were analyzed using MANOVA, followed by Scheffe’s test for multiple pair-wise comparisons. All the tests were carried out in SPSS (version 19.0), and significance level was set at 0.05.
Results
Gonads of all the individuals were categorized into testis, ovaries, and intersexes following the criteria described previously (Phuge & Gramapurohit, 2015; Supplementary file). Sex ratio (number of males and females) in control group was not significantly different than the hypothetical 50:50 (Z = − 0.14, P = 0.44). There was no significant difference in sex ratio between control and all TM (25, 50, and 100 μg/l) treated groups (χ23 = 2, P > 0.057; Table 1). Testes of the males obtained in all the treatment groups were categorized into three groups based on the criteria described previously (Phuge & Gramapurohit, 2015; Supplementary file). In 80–90% of the males obtained in each TM-treated group, testis development was advanced up to seminiferous tubule formation, meiosis, and appearance of spermatozoa (Table 2). In the females obtained in all TM-treated groups, size of the oocytes was larger as compared to the control (F3,156 = 127.76, P < 0.0001; Fig. 1).
Overall, there was a significant difference in the tadpole mortality among control and TM-treatment groups (χ23 = 31.7, P < 0.0001). The mortality in 25 and 50 μg/l TM-treated groups was comparable to the control (χ21 ≤ 0.6241, P ≥ 0.43) while it was significantly higher in 100 μg/l TM-treated group (χ21 = 19.2, P = 0.0001; Table 1). A significant difference was observed in the larval life history traits of the control and TM-treated groups (Wilks’ λ F9,333 = 22.16, P < 0.0001). Further, univariate analysis showed that SVL (F3,139 = 67.51, P < 0.0001), body mass (F3,139 = 89.59, P < 0.0001), and larval period (F3,139 = 19.36, P < 0.0001) were significantly different among control and TM-treated groups (Fig. 2a–c). Froglets emerged from all TM-treated groups (25, 50, and 100 μl/l) were heavier and larger as compared to those from the control (P ≤ 0.009; Fig. 2 a and b). Larval period of the tadpoles treated with 25 μg/l TM was comparable to the control (P = 0.085) while tadpoles treated with 50 and 100 μg/l TM took significantly longer to metamorphose (P < 0.0001; Fig. 2c).
Effect of tamoxifen (TM) on life history traits of E. cyanophlyctis. a Effect of TM on snout-vent length (SVL). b Effect of TM on body mass. c Effect of tamoxifen on larval period. Asterisk (*) indicates significant difference in SVL, body mass, and larval period between control and particular group
Discussion
Amphibians provide a valuable model for endocrine disruption studies as their sexual development has no maternal influence, and metamorphosis is governed by thyroid hormones (Flament, 2016; Fort, Degitz, Tietge, & Touart, 2007; Scholz et al., 2013). The species with accelerated type of gonad development are good models to evaluate the potential of EDs that affect sexual development as the susceptibility of the gonad development can be assessed easily using histological methods (Phuge, 2018; Storrs & Semlitsch, 2008; Storrs-Mendez & Semlitsch, 2010). In endocrine disruption studies, histological endpoints are most reliable as they provide direct evidence (Olmstead et al., 2009; Phuge & Gramapurohit, 2015; Piprek, Pecio, Kubiak, & Szymura, 2012; Storrs & Semlitsch, 2008). Gonadal development of the frog E. cyanophlyctis is an accelerated type (Phuge & Gramapurohit, 2015), and therefore it is a good model to study endocrine disruption potential of variety of chemicals.
TM has been used as a reference anti-estrogen in several endocrine disruption studies. Previous studies in amphibians have shown that anti-estrogens TM and ICI 182780 (Faslodex) do not influence sex ratio (Bögi, Levy, Lutz, & Kloas, 2002; Mackenzie, Berrill, Metcalfe, & Pauli, 2003). However, TM restrains gonad development in X. laevis leading to non-functional state (Bögi et al., 2002). TM treatment also induces follicular atresia and reduces the number of oocytes in X. laevis (Cevasco et al., 2008). In Rana esculenta, TM acts as an anti-estrogen at lower concentration, while its action is estrogenic at higher concentration (Rastogi & Chieffi, 1975). These results suggest that TM affects gonad development depending on the species and the concentration used. TM is also reported to increase plasma estradiol, brain LHβ, and FSHβ mRNA levels in female frogs while in males frogs, it increases the number of germ cells in testis (Cevasco et al., 2008; Urbatzka, Bottero, Mandich, Lutz, & Kloas, 2007; Urbatzka, Lutz, Opitz, & Kloas, 2006). Interestingly, the effects of TM are contrasting in tadpoles and adult frogs. For instance, it acts as an anti-estrogen in adults of R. esculenta whereas in tadpoles, its action is estrogenic (Rastogi & Chieffi, 1975). Results of the present study showed that TM stimulates gonad development in both males and females. It needs to be clear that accelerated gonad development in TM-treated groups is not a result of prolonged larval duration. For instance, in the group treated with the lowest concentration (25 μg/l) of TM, mature testes were seen in 50% males, and larger oocytes were observed in females, where the larval period (119.3 ± 4.61) was not significantly different than control (105.76 ± 3.27; Fig. 2). In E. cyanophlyctis, testes mature after 2 months of metamorphosis (Phuge & Gramapurohit, 2013). The difference between larval period of control and tamoxifen-treated groups (TM 50 μg/l, 140.07 ± 7.57 and TM 100 μg/l, 155.96 ± 6.87) is less than 2 months.
Several studies reporting the effect of exogenous estrogen have shown its importance in ovary development of amphibians (Flament, 2016; Phuge & Gramapurohit, 2015). These estrogenic compounds produce female biased sex ratio, stimulate ovary development, and disturb testis development (Mali & Gramapurohit, 2016; Phuge & Gramapurohit, 2015; Storrs & Semlitsch, 2008; Storrs-Mendez & Semlitsch, 2010). But the role of estrogen in ovary differentiation of amphibians is not clear (Flament, 2016). In some studies, pharmaceutical anti-estrogens (including TM) have been used to understand the role of estrogen in ovary differentiation (Bögi et al., 2002; Mali & Gramapurohit, 2016; Ohtani, Miura, & Ichikawa, 2003; Rastogi & Chieffi, 1975). Anti-estrogenic chemicals inhibit/restrain ovary differentiation/development and produce male biased sex ratio (Bögi et al., 2002; Ohtani et al., 2003; Olmstead et al., 2009; Rastogi & Chieffi, 1975). TM was primarily designed as an estrogen receptor (ER) inhibitor and used for breast cancer treatment. However, its action as an ER agonist or antagonist depends on the tissue. For instance, it competitively binds to ER and downregulates their expression in mammary gland cells, while upregulates ER expression in ovary making it prone to cancer (Jordan, 1994; Osborne & Schiff, 2011). It seems that TM action is ambiguous in E. cyanophlyctis and stimulates gonad development through a mechanism currently unknown.
Several estrogenic and anti-estrogenic water-borne chemicals are known to influence larval life history traits of amphibians (Olmstead et al., 2009; Phuge & Gramapurohit, 2015; Storrs & Semlitsch, 2008). Some of these chemicals modulate thyroid hormone synthesis/function through hypothalamic–pituitary–thyroid axis and stimulate or delay metamorphosis (Carr & Patiño, 2011). TM may have direct or indirect effect on hypothalamic–pituitary–thyroid axis and probably disrupting thyroid function leading to the increased larval period. Long larval period could have resulted in increased body mass and size at metamorphosis in E. cyanophlyctis. Alternate explanation for increased size and body mass of the froglets in TM-treated groups could be that TM acts as ER agonist (by mimicking estrogens) and stimulates bone growth (Krum et al., 2008; Nakamura et al., 2007). Exogenous estrogen treatments during tadpole development have been reported to increase larval duration and body mass (Hogan, Lean, & Trudeau, 2006; Phuge & Gramapurohit, 2015). Moreover, 17β-estradiol is also known to accelerate skeletal growth in amphibians (Bauer-Dantoin & Meinhardt, 2010).
Water contaminations are a major threat to amphibians and contribute significantly in their decline (Hayes, Falso, Gallipeau, & Stice, 2010; Orton & Tyler, 2015). The frog E. cyanophlyctis is widely distributed and breeds in all kinds of water-bodies where its tadpoles may get exposed to variety of water contaminants. TM is frequently detected ED in sewage effluent and river waters (Petrie et al., 2015; Zhang et al., 2008). The concentrations of tamoxifen used in the present study were much higher than those found in contaminated water-bodies. However, tamoxifen is known to bioconcentrate in aquatic animals at much higher concentration than environmental relevant concentrations (Orias et al., 2015).
Conclusion
Present study clearly demonstrated that TM stimulates sexual development and somatic growth. Generally, endocrine disrupters have either stimulatory or inhibitory action on gonads (Brüggemann et al., 2018). In the present study, TM stimulated both testes and ovary development of E. cyanophlyctis. TM has diverse effects in amphibians depending on the tissue and the stage of the development. Results of the present study will be helpful to understand the mechanism of action of TM in amphibians and define additional criteria for endocrine disruptors. These results will also be useful in developing E. cyanophlyctis as an indigenous model to study endocrine disrupting potential of various pharmaceuticals.
Availability of data and materials
Data is available with corresponding author and will be made available on request.
References
Bauer-Dantoin, A. C., & Meinhardt, D. J. (2010). 17β-estradiol exposure accelerates skeletal development in Xenopus laevis tadpoles. Anatomical Record, 293(11), 1880–1886 https://doi.org/10.1002/ar.21226.
Bögi, C., Levy, G., Lutz, I., & Kloas, W. (2002). Functional genomics and sexual differentiation in amphibians. Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology, 133(4), 559–570.
Brüggemann, M., Licht, O., Fetter, É., Teigeler, M., Schäfers, C., & Eilebrecht, E. (2018). Knotting nets: Molecular junctions of interconnecting endocrine axes identified by application of the adverse outcome pathway concept: Molecular junctions of endocrine axes. Environmental Toxicology and Chemistry, 37(2), 318–328 https://doi.org/10.1002/etc.3995.
Carr, J. A., & Patiño, R. (2011). The hypothalamus-pituitary-thyroid axis in teleosts and amphibians: Endocrine disruption and its consequences to natural populations. General and Comparative Endocrinology, 170(2), 299–312 https://doi.org/10.1016/j.ygcen.2010.06.001.
Cevasco, A., Urbatzka, R., Bottero, S., Massari, A., Pedemonte, F., Kloas, W., & Mandich, A. (2008). Endocrine disrupting chemicals (EDC) with (anti)estrogenic and (anti)androgenic modes of action affecting reproductive biology of Xenopus laevis: II. Effects on gonad histomorphology. Comparative Biochemistry and Physiology. Toxicology & Pharmacology, 147(2), 241–251 https://doi.org/10.1016/j.cbpc.2007.10.001.
Chua, M. E., Escusa, K. G., Luna, S., Tapia, L. C., Dofitas, B., & Morales, M. (2013). Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: A meta-analysis. Andrology, 1(5), 749–757 https://doi.org/10.1111/j.2047-2927.2013.00107.x.
Flament, S. (2016). Sex reversal in amphibians. Sexual Development, 10(5–6), 267–278 https://doi.org/10.1159/000448797.
Fort, D. J., Degitz, S., Tietge, J., & Touart, L. W. (2007). The hypothalamic-pituitary-thyroid (HPT) axis in frogs and its role in frog development and reproduction. Critical Reviews in Toxicology, 37(1–2), 117–161 https://doi.org/10.1080/10408440601123545.
Gosner, K. L. (1960). A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica, 16(3), 183–190.
Haczkiewicz, K., & Ogielska, M. (2013). Gonadal sex differentiation in frogs: How testes become shorter than ovaries. Zoological Science, 30(2), 125–134 https://doi.org/10.2108/zsj.30.125.
Hayes, T. B., Falso, P., Gallipeau, S., & Stice, M. (2010). The cause of global amphibian declines: A developmental endocrinologist’s perspective. Journal of Experimental Biology, 213(6), 921–933 https://doi.org/10.1242/jeb.040865.
Hogan, N. S., Duarte, P., Wade, M. G., Lean, D. R. S., & Trudeau, V. L. (2008). Estrogenic exposure affects metamorphosis and alters sex ratios in the northern leopard frog (Rana pipiens): Identifying critically vulnerable periods of development. General and Comparative Endocrinology, 156(3), 515–523 https://doi.org/10.1016/j.ygcen.2008.03.011.
Hogan, N. S., Lean, D. R. S., & Trudeau, V. L. (2006). Exposures to estradiol, ethinylestradiol and octylphenol affect survival and growth of Rana pipiens and Rana sylvatica tadpoles. Journal of Toxicology and Environmental Health, Part A, 69(16), 1555–1569 https://doi.org/10.1080/15287390500470759.
Jordan, V. C. (1994). Molecular mechanisms of antiestrogen action in breast cancer. Breast Cancer Research and Treatment, 31(1), 41–52 https://doi.org/10.1007/BF00689675.
Kloas, W., Urbatzka, R., Opitz, R., Würtz, S., Behrends, T., Hermelink, B., … Lutz, I. (2009). Endocrine disruption in aquatic vertebrates. Annals of the New York Academy of Sciences, 1163(1), 187–200 https://doi.org/10.1111/j.1749-6632.2009.04453.x.
Krum, S. A., Miranda-Carboni, G. A., Hauschka, P. V., Carroll, J. S., Lane, T. F., Freedman, L. P., & Brown, M. (2008). Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. The EMBO Journal, 27(3), 535–545 https://doi.org/10.1038/sj.emboj.7601984.
Mackenzie, C. A., Berrill, M., Metcalfe, C., & Pauli, B. D. (2003). Gonadal differentiation in frogs exposed to estrogenic and antiestrogenic compounds. Environmental Toxicology and Chemistry, 22(10), 2466 https://doi.org/10.1897/02-173.
Mali, P. V., & Gramapurohit, N. P. (2015). Pattern of gonadal differentiation and development up to sexual maturity in the frogs, Microhyla ornata and Hylarana malabarica: A comparative study. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology, 323(9), 666–678 https://doi.org/10.1002/jez.1958.
Mali, P. V., & Gramapurohit, N. P. (2016). Are sex steroids essential for gonadal differentiation of the ornate frog, Microhyla ornata? General and Comparative Endocrinology, 233, 63–72 https://doi.org/10.1016/j.ygcen.2016.05.019.
Nakamura, T., Imai, Y., Matsumoto, T., Sato, S., Takeuchi, K., Igarashi, K., … Kato, S. (2007). Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell, 130(5), 811–823 https://doi.org/10.1016/j.cell.2007.07.025.
Ogielska, M., & Kotusz, A. (2004). Pattern and rate of ovary differentiation with reference to somatic development in anuran amphibians. Journal of Morphology, 259(1), 41–54 https://doi.org/10.1002/jmor.10162.
Ohtani, H., Miura, I., & Ichikawa, Y. (2003). Role of aromatase and androgen receptor expression in gonadal sex differentiation of ZW/ZZ-type frogs, Rana rugosa. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 134(2), 215–225 https://doi.org/10.1016/S1532-0456(02)00252-1.
Olmstead, A. W., Kosian, P. A., Korte, J. J., Holcombe, G. W., Woodis, K. K., & Degitz, S. J. (2009). Sex reversal of the amphibian, Xenopus tropicalis, following larval exposure to an aromatase inhibitor. Aquatic Toxicology, 91(2), 143–150 https://doi.org/10.1016/j.aquatox.2008.07.018.
Orias, F., Simon, L., Mialdea, G., Clair, A., Brosselin, V., & Perrodin, Y. (2015). Bioconcentration of 15N-tamoxifen at environmental concentration in liver, gonad and muscle of Danio rerio. Ecotoxicology and Environmental Safety, 120, 457–462 https://doi.org/10.1016/j.ecoenv.2015.06.033.
Orton, F., & Tyler, C. R. (2015). Do hormone-modulating chemicals impact on reproduction and development of wild amphibians?: Endocrine disruption in amphibians. Biological Reviews, 90(4), 1100–1117 https://doi.org/10.1111/brv.12147.
Osborne, C. K., & Schiff, R. (2011). Mechanisms of endocrine resistance in breast cancer. Annual Review of Medicine, 62(1), 233–247 https://doi.org/10.1146/annurev-med-070909-182917.
Petrie, B., Barden, R., & Kasprzyk-Hordern, B. (2015). A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Research, 72, 3–27 https://doi.org/10.1016/j.watres.2014.08.053.
Phuge, S. K. (2018). Effect of fromestane on gonadal sex differentiation and sex ratio in the frog, Euphlyctis cyanophlyctis , with undifferentiated type of gonadal differentiation. Journal of Herpetology, 52(2), 171–175 https://doi.org/10.1670/17-019.
Phuge, S. K., & Gramapurohit, N. P. (2013). Gonadal sex differentiation, development up to sexual maturity and steroidogenesis in the skipper frog, Euphlyctis cyanophlyctis. General and Comparative Endocrinology, 181, 65–71 https://doi.org/10.1016/j.ygcen.2012.08.017.
Phuge, S. K., & Gramapurohit, N. P. (2015). Sex hormones alter sex ratios in the Indian skipper frog, Euphlyctis cyanophlyctis: Determining sensitive stages for gonadal sex reversal. General and Comparative Endocrinology, 220, 70–77 https://doi.org/10.1016/j.ygcen.2014.04.030.
Piprek, R. P., Pecio, A., Kubiak, J. Z., & Szymura, J. M. (2012). Differential effects of testosterone and 17 -estradiol on gonadal development in five anuran species. Reproduction, 144(2), 257–267 https://doi.org/10.1530/REP-12-0048.
Quaranta, A., Bellantuono, V., Cassano, G., & Lippe, C. (2009). Why amphibians are more sensitive than mammals to xenobiotics. PLoS One, 4(11), e7699 https://doi.org/10.1371/journal.pone.0007699.
Rastogi, R. K., & Chieffi, G. (1975). The effects of antiandrogens and antiestrogens in nonmammalian vertebrates. General and Comparative Endocrinology, 26(1), 79–91 https://doi.org/10.1016/0016-6480(75)90217-8.
Safholm, M., Ribbenstedt, A., Fick, J., & Berg, C. (2014). Risks of hormonally active pharmaceuticals to amphibians: A growing concern regarding progestagens. Philosophical Transactions of the Royal Society, B: Biological Sciences, 369(1656), 20130577–20130577 https://doi.org/10.1098/rstb.2013.0577.
Scholz, S., Renner, P., Belanger, S. E., Busquet, F., Davi, R., Demeneix, B. A., … Embry, M. R. (2013). Alternatives to in vivo tests to detect endocrine disrupting chemicals (EDCs) in fish and amphibians – screening for estrogen, androgen and thyroid hormone disruption. Critical Reviews in Toxicology, 43(1), 45–72 https://doi.org/10.3109/10408444.2012.737762.
Solomon, K. R., Carr, J. A., Du Preez, L. H., Giesy, J. P., Kendall, R. J., Smith, E. E., & Van Der Kraak, G. J. (2008). Effects of atrazine on fish, amphibians, and aquatic reptiles: A critical review. Critical Reviews in Toxicology, 38(9), 721–772 https://doi.org/10.1080/10408440802116496.
Steiner, A. Z., Terplan, M., & Paulson, R. J. (2005). Comparison of tamoxifen and clomiphene citrate for ovulation induction: A meta-analysis. Human Reproduction, 20(6), 1511–1515 https://doi.org/10.1093/humrep/deh840.
Storrs, S. I., & Semlitsch, R. D. (2008). Variation in somatic and ovarian development: Predicting susceptibility of amphibians to estrogenic contaminants. General and Comparative Endocrinology, 156(3), 524–530 https://doi.org/10.1016/j.ygcen.2008.03.001.
Storrs-Mendez, S. I., & Semlitsch, R. D. (2010). Intersex gonads in frogs: Understanding the time course of natural development and role of endocrine disruptors. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 314B(1), 57–66 https://doi.org/10.1002/jez.b.21310.
Urbatzka, R., Lutz, I., Opitz, R., & Kloas, W. (2006). Luteinizing hormone, follicle stimulating hormone, and gonadotropin releasing hormone mRNA expression of Xenopus laevis in response to endocrine disrupting compounds affecting reproductive biology. General and Comparative Endocrinology, 146(2), 119–125 https://doi.org/10.1016/j.ygcen.2005.10.005.
Urbatzka, R., Bottero, S., Mandich, A., Lutz, I., & Kloas, W. (2007). Endocrine disrupters with (anti)estrogenic and (anti)androgenic modes of action affecting reproductive biology of Xenopus laevis: I. Effects on sex steroid levels and biomarker expression. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 144(4), 310–318 https://doi.org/10.1016/j.cbpc.2006.10.008.
Zhang, Z., Hibberd, A., & Zhou, J. L. (2008). Analysis of emerging contaminants in sewage effluent and river water: Comparison between spot and passive sampling. Analytica Chimica Acta, 607(1), 37–44 https://doi.org/10.1016/j.aca.2007.11.024.
Acknowledgements
Author is grateful to DST and Savitribai Phule Pune University (SPPU) for research fellowship. The research work is a part of authors Ph.D. thesis submitted to SPPU, and Dr. Narahari P. Gramapurohit was a guide for the thesis.
Funding
The research was supported by grants from the Department of Science and Technology (SR/SO/AS-63-2009).
Author information
Authors and Affiliations
Contributions
Study designed, executed, and presented by SKP. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In India, Indian researchers do not require permission to collect animals unless the locality of collection is in wildlife protected area (The Gazette of India, REGD. NO. D. L.–33004/99, section 17). The experiment was carried out following the guidelines of institutional committee for animal ethics (No. 538/CPCSEA).
Consent for publication
No human subjects are included. No individual person’s data are included.
Competing interests
Author declares no competing interest regarding the publication of this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Phuge, S. Tamoxifen stimulates gonad development and somatic growth in the tadpoles of Indian skipper frog, Euphlyctis cyanophlyctis. JoBAZ 81, 19 (2020). https://doi.org/10.1186/s41936-020-00161-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-020-00161-3