Abstract
Background
Dental age estimation of adults is a challenging procedure in forensic odontology. Radiographic techniques have enabled non-invasive age estimation of adults since the 90’s. Kvaal’s method based on the deposition of secondary dentin has demonstrated broad applicability in several populations. Population-specific studies with this method, however, are scarce in South America. This study tested the validity of Kvaal’s method in a sample of radiographs of Brazilian adults. The sample consisted of 192 periapical radiographs of 106 females (55.2%) and 86 males (44.8%) with ages between 20 and 80 years old. The permanent maxillary right (n = 84, 43.8%) and left (n = 108, 56.2%) central incisors were assessed based on Kvaal’s method.
Results
Lin’s coefficient of concordance between estimated (EA) and chronological (CA) ages was 0.800 (IC95% = 0.753; 0.847) (p < 0.001). The mean difference between EA and CA was − 2.84 years (p < 0.001). For females and males, separately, the mean differences between EA and CA were − 3.82 years (CI95% = − 5.38; − 2.25) (p < 0.001) and − 1.86 years (CI95% = − 3.56; − 0.17) (p = 0.031), respectively. Underestimations were predominant and increased with time.
Conclusion
After 28 years of the original publication, Kvaal’s method remains reliable for non-invasive dental age estimation of adults, especially for the analysis of the permanent maxillary central incisors of Brazilian individuals.
Similar content being viewed by others
Background
Dental age estimation has demonstrated great value in forensic science (Erbudak et al. 2012). Forensic applications of dental age estimation are useful in cases that involve the living and the deceased. In the living, it can be applied to assess the age of asylum seekers, alleged minor criminals, adopted children, and victims of human trafficking and exploitation (Pradella et al. 2017; Sobieska et al. 2018; Rocha et al. 2022). In the deceased, dental age estimation contributes to the process of human identification as part of the reconstructed biological profile of the victim (Goetten et al. 2021). The radiographic visualization of dental development is a reliable and common procedure used for dental age estimation of children and adolescents (Franco et al. 2013; Machado et al. 2022). However, after the apical closure of the third molars, in early adulthood, dental development stops (AlQahtani et al. 2010).
In adults, dental age estimation is based on regressive features of the human teeth, such as attrition, secondary dentin deposition, periodontosis, cementum apposition, root resorption, and root translucency (Gustafson 1950; Zdravkovic et al. 2022). Originally, the analysis of regressive dental features has been proposed based on invasive techniques that involve the ex vivo examination of gross dental anatomy, tooth sectioning, and slicing (Gustafson 1950). The analysis of secondary dentin deposition, however, is a feature that can be assessed radiographically—leading to a non-invasive approach (Kvaal et al. 1995). Dental age estimation techniques based on this parameter assess the secondary dentin deposition indirectly by quantifying the area of the pulp chamber and root canal (Kvaal et al. 1995). In other words, they consider the reduction of the pulpal space as a consequence of the deposition of secondary dentin (Kvaal et al. 1995).
State-of-the-art methods for adult dental age estimation based on secondary dentin deposition (and inherent reduction of the pulpal space) propose a volumetric analysis of cone beam computed tomography scans (Boedi et al. 2022a, b). These methods are advanced and require dedicated software and image devices. For this reason, they might not be promptly suitable for forensic applications in developing countries, in which forensic facilities are not equipped with computed tomography scans. In this context, forensic research has the duty to test the validity of existing methods that could be more feasible for population-specific applications.
The present study aimed to test the validity of the radiographic method proposed by Kvaal et al. (1995) in a sample of periapical radiographs of Brazilian adults.
Methods
Ethical aspects and study design
This study was performed with the approval of the Institutional Committee of Ethics in Human Research (protocol: 49916121.9.0000.5374). The study had an observational analytical cross-sectional model. For methodological purposes, the following heading-subheading structure was based on Equator guidelines, more specifically the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) checklist (Von Elm et al. 2014).
Participants and settings
The sample consisted of 192 periapical radiographs collected from the Dental School’s image database (Table 1). The database was created with images taken between 2010 and 2022. Each radiograph belonged to patients (n = 192) of the dental clinic. Hence, the radiographs were obtained exclusively for clinical reasons, and the sample was collected retrospectively (there was no exposure of patients to ionizing radiation for research purposes). The inclusion criteria were periapical radiographs of female and male individuals with Brazilian nationality, radiographs showing at least one permanent maxillary central incisor (right: #11, or left: #21—Fédération Dentaire Internationale), and radiographs of individuals between 20 and 80 years old. The exclusion criteria consisted of radiographs showing decayed central incisors, radiographs that missed tooth parts during image acquisition, maxillary central incisors that were restored or that had root canal treatment, maxillary central incisors that had periapical lesions or root resorption, radiographs with poor image quality, and radiographs with missing information about patients’ sex, date of birth and date of image acquisition. Sample collection was performed between January 2021 and August/2022. The rationale behind the sample size and age range of the present study was based on Kvaal’s original study, in which a sample of 100 individuals between 20 and 87 years was investigated (Kvaal et al. 1995). The radiographs were imported to a personal computer equipped with a 15″ screen and image viewer for visualization, magnification, and eventual adjustments of brightness and contrast prior to the analyses.
Variable and measurements
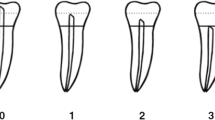
The first variable in this study was the chronological age of the individuals. This age was obtained by deducting the date of image acquisition minus the date of birth of each patient. For inferential statistics, the chronological age was converted from a continuous variable to a categorical variable. In other words, the following age categories were considered: 20–30; 31–40; 41–50; 51–60; 61–70; 71–80 years. The other variables consisted of the sex of the individuals and the estimated age. To estimate the dental age of adult individuals, Kvaal’s method was applied. The method is based on regressive equations for each tooth position. For single teeth, Kvaal et al. (1995) noticed that permanent maxillary central incisors had the highest coefficient of determination (r2 = 0.70) and the lowest standard error (9.5 years). For this reason, we selected the permanent maxillary central incisors in this study. Differently from Kvaal’s original study, we did not propose the combination of tooth positions because we did not have more tooth positions registered in our sample. The measurements included in Kvaal’s method were vertical: the maximum tooth length, root length on the mesial surface, maximum pulp length; and horizontal: width of the pulp and root at the cementum-enamel junction, pulp and root width midway between the cementum-enamel junction and the apex, and pulp and root width midway between the previous horizontal measurements (Fig. 1). Radiographic analysis was accomplished by the main observer (Forensic Odontologist with 5 years of experience in the field) in a dimmed room under standard viewing conditions (Vasconcelos et al. 2015). The number of daily radiographic analyses (full set of measurements) did not exceed 25 to avoid visual fatigue (Nascimento et al. 2018).
Illustrative representation of the measurements performed in this study showing the maximum tooth length (T), root length (R), maximum pulp length (P), root width at cementum-enamel junction (a), pulp width at cementum-enamel junction (a’), root (b) and pulp (b’) width midway between measurements a and c, and root (c) and pulp (c’) width midway between apex and cementum-enamel junction
Operator-dependent bias
To assess the intra-observer reproducibility, the main observer revisited 30% of the sample (n = 59 radiographs, randomly selected: www.random.org) and repeated the measurements 30 days from the main analysis. The measurements performed in the main analysis (T1) were compared with those repeated 30 days later (T2). To assess the inter-observer agreement, a second observer was recruited (an Oral Radiologist with 5 years of experience in the field). The second observer performed the measurements in 30% (n = 59) of the radiographs, and the measurements performed by the main and the second observers were compared. The comparisons were performed by means of intraclass correlation coefficient (ICC).
Quantitative variables and statistical methods
Descriptive and inferential statistics were performed. For the descriptive analysis, the absolute (n) and relative (%) frequencies of distribution were calculated within the categorical variables. For the continuous variables, the descriptive statistics consisted of mean, standard deviation, median, and quartiles. Kolmogorov–Smirnov test was used to assess the normality of distribution for the chronological and estimated ages. In this process, the metrics to assess data normality were the mean, median, asymmetry, and kurtosis. The concordance between chronological and estimated ages was assessed with Lin’s coefficient of concordance.
The difference between chronological and estimated ages was assessed with Student’s t test for paired samples. All the analyses based on concordance and differences were performed for the total sample and separately based on sex and age category. Finally, a sample-specific predictive linear regression was developed. Tooth measurements and sex were considered predictors of chronological age. The process to include the predictors followed a backward exclusion. In short, all the predictors were added at once and those with a p value > 0.05 were sequentially removed from the equation. Statistic tests were performed with Stata 17.0 (StataCorp LLC, College Station, TX, USA) considering the statistical significance of 5%.
Results
According to the ICC, the intra-observer reproducibility was between 0.89 and 1.00, while the inter-observer agreement was between 0.86 and 1.00.
The asymmetry values were 0.0 and 0.1 for the chronological and estimated ages, with the Kolmogorov–Smirnov test showing p = 0.625 and 0.103, respectively (Table 2). These outcomes suggest normal distribution (Fig. 2).
The mean chronological age of the studied sample was 49.0 ± 14.8 years, while the mean estimated age was 46.0 ± 11.9 years. The mean values for each tooth measurement performed in this study were registered (Table 3).
The concordance between chronological and estimated ages was high, with Lin’s Coefficient of Concordance of 0.800 (CI95%: 0.753; 0.847). The mean difference between chronological and estimated ages was − 2.84 years (underestimation) for the total sample (p < 0.001). The upper bound of the confidence interval at 95% showed an underestimation of 4.04 years. Higher mean differences between chronological and estimated ages were observed in the older age categories (Figs. 3 and 4). When the analyses were performed separately based on sex, the mean difference between chronological and estimated ages was − 3.82 (CI95% − 5.38; − 2.25) years for females (p < 0.001), and − 1.86 (CI95% − 3.56; − 0.17) years for males (p = 0.31). Lin’s coefficient of concordance values were 0.78 and 0.82 for females and males, respectively (Table 4).
Analyses based on age showed that the worst predictions were in the older age groups, more specifically between 60 and 80 years, in which the underestimations were between − 8.95 and − 9.51 years (p < 0.001). In the other age groups, the mean difference between chronological and estimated ages was between − 5.1 and 4.56 years (Table 5).
The regressive equation generated via backward removal of statistically non-significant variables (p > 0.05) was
In the proposed equation, sex was significant for male individuals. Hence, “sex” should be converted to 1 for males and 0 for females (Table 6).
Discussion
Dental age estimation of adults usually results in higher differences between chronological and estimated ages compared to dental age estimation of children and adolescents. This phenomenon is related to the regressive dental parameters assessed to estimate age, which are less associated with chronological age compared to dental development. The difference between chronological and estimated ages is reflected by the mean error of the method. In 1995, Kvaal et al. proposed a non-invasive radiographic method based on the deposition of secondary dentin and the consequent reduction of the pulpal space. In their method, the authors established nine equations: three for the combination of different tooth positions and six for individual tooth positions. The specific equation for permanent maxillary central incisors was the best among the individual tooth equations, with a standard error of 9.5 years and a coefficient of determination (r2) of 0.70. Despite the promising outcomes, the authors stated that “the method ought to be tested on an independent sample”. The present study tested Kvaal’s original equation for central incisors in a sample of Brazilian adults.
Kvaal’s method already has been applied to a Brazilian sample. Recently, a study tested the performance of Kvaal’s equations for canines (Miranda et al. 2020). The mean error between chronological and estimated ages was about 6–7.5 years. In the present study, the mean difference between chronological and estimated ages was − 2.84 years. The lower error rates observed in the present study could be explained by the tooth position that was used. Kvaal et al. (1995) explain that while the best equations for adult age estimation with individual teeth was the one for permanent maxillary central incisors, the weakest predictions were obtained with mandibular canines (r2 = 0.56). The use of maxillary central incisors for adult age estimation with Kvaal’s method has been endorsed by studies with other country-specific samples, such as among the Serbian population (Zdravkovic et al. 2022). However, it must be noted that not only tooth positions should be selected based on the original method, but also the image acquisition technique.
Over the years, at least three studies have been published with Kvaal’s method in panoramic radiographs (Bosmans et al. 2005; Karkhanis et al. 2014; Li et al. 2019; Chandan et al. 2020). Originally, the method was designed in periapical radiographs, especially because linear measurements are necessary. Periapical radiographs show less distortion compared to panoramic ones. With periapical radiographs, Sharma and Srivastava (2010) found that mandibular first premolars were the best teeth for dental age estimation among Indian adults. Their outcomes, on the other hand, were influenced by important methodological limitations, namely the small sample size (n = 50) and the sampled age interval (15–60 years). In other words, the authors included individuals down to the age of 15 years, which are non-adults and are below the lower bound of the sample age interval established by Kvaal et al. in 1995.
When it comes to the individual assessments of error rates investigated per age category, the best performances of the method were detected for the age categories in the interval between 20 and 60 years. In all these categories, the mean difference between chronological and estimated ages was below 5.1 years. The age category of 31–40 years had the best performance of the method, showing a mean difference of 1.32 years and a lack of statistically significant difference between chronological and estimates ages. Miranda et al. (2020) also applied a stratified assessment of age categories to understand the performance of the method. The authors observed a progressive increase in the mean error of the method with time. This is to say that the older age categories had the higher error rates. Their mean error rates varied between 4.63 and 10.74 years—using canines as the teeth for age estimation. Similarly, our outcomes showed a progressive increase in the mean difference between chronological and estimated ages in the age interval between 51 and 80 years. In these categories (51–60, 61–70, and 71–80 years), only underestimations were detected (up to − 9.51 years). In the present and previous (Miranda et al. 2020) studies, the outcomes of Kvaal’s method fit within the error rates usually observed for adult dental age estimation. The studies among Brazilian individuals agree that better performances of the method could be achieved among young adults—preferably below the age of 50 years, according to the present study.
Future studies in the field are encouraged to test the performance of the sample-specific equation proposed in the present study. The rationale behind the equation was to reduce even more the difference between chronological and estimated ages among Brazilian adults. Hence, external validation is necessary endorsed. Because the proposed equation was able to exclude three original measurements proposed by Kvaal et al. (1995), it is estimated that less labor time would be needed to estimate age with our equation. Additionally, our equation detected sex as an important component of age estimation, leading to an equation with weighted sex (1 for males and 0 for females). Specifically, when females and males were assessed separately in the present study, a statistically significant difference was observed between chronological and estimated ages. In which the mean difference in females was almost twice higher than in males. This is evidence that methods for adults might need equations that weight sex for age estimation.
Conclusions
The present test of the validity of Kvaal’s method for adult dental age estimation confirmed the reliability of the method among Brazilians. Permanent maxillary central incisors led to a mean difference between chronological and estimated ages that was about five years or less for adults between twenty and fifty years old. The mean error rate among females was almost twice higher than in males. External validation of the proposed sample-specific dental age estimation equation is encouraged.
Availability of data and materials
Available upon reasonable request to the authors.
Abbreviations
- CA:
-
Chronological age
- EA:
-
Estimated age
- STROBE:
-
Strengthening the reporting of observational studies in epidemiology
- ICC:
-
Intraclass correlation coefficient
References
AlQahtani SJ, Hector MP, Liversidge HM (2010) Brief communication: The London atlas of human tooth development and eruption. Am J Phys Anthropol 142(3):481–90. https://doi.org/10.1002/ajpa.21258
Boedi RM, Shepherd S, Mânica S, Franco A (2022a) CBCT in dental age estimation: a systematic review and meta-analysis. Dentomaxillofac Radiol 51(4):20210335. https://doi.org/10.1259/dmfr.20210335
Boedi RM, Shepherd S, Oscandar F, Mânica S, Franco A (2022b) Regressive changes of crown-root morphology and their volumetric segmentation for adult dental age estimation. J Forensic Sci 67(5):1890–1898. https://doi.org/10.1111/1556-4029.15094
Bosmans N, Ann P, Aly M, Willems G (2005) The application of Kvaal’s dental age calculation technique on panoramic dental radiographs. Forensic Sci Int 153(2–3):208–212. https://doi.org/10.1016/j.forsciint.2004.08.017
Chandan PK, Arora KS, Das M, Kaur P, Mohaptra S, Pareek S (2020) Assessment of validity and reliability of Kvaal’s method for age estimation among a population sample - a retrospective study. Indian J Dent Res 31(2):186–190. https://doi.org/10.4103/ijdr.IJDR_209_18
Erbudak HO, Ozbek M, Uysal S, Karabulut E (2012) Application of Kvaal et al.’s age estimation method to panoramic radiographs from Turkish individuals. Forensic Sci Int 219(1–3):141–146. https://doi.org/10.1016/j.forsciint.2011.12.012
Franco A, Thevissen P, Fieuws S, Souza PHC, Willems G (2013) Applicability of Willems model for dental age estimations in Brazilian children. Forensic Sci Int 231(1–3):401.e1–4. https://doi.org/10.1016/j.forsciint.2013.05.030
Goetten IFS, Silva RF, Franco A (2021) Skeletal and dental age estimation of the living in a criminal scenario–case report. Rom J Legal Med 29(1):105–108. https://doi.org/10.4323/rjlm.2021.105
Gustafson G (1950) Age determinations on teeth. J Am Dent Assoc 41:45–54. https://doi.org/10.14219/jada.archive.1950.0132
Karkhanis S, Mack P, Franklin D (2014) Age estimation standards for a Western Australian population using the dental age estimation technique developed by Kvaal et al. Forensic Sci Int 235:104.e1–6. https://doi.org/10.1016/j.forsciint.2013.12.008
Kvaal SI, Kolltveit KM, Thomsen IO, Solheim T (1995) Age estimation of adults from dental radiographs. Forensic Sci Int 74:175–185. https://doi.org/10.1016/0379-0738(95)01760-G
Li MJ, Chu G, Han MQ, Chen T, Zhou H, Guo YC (2019) Application of the Kvaal method for age estimation using digital panoramic radiography of Chinese individuals. Forensic Sci Int 301:76–81. https://doi.org/10.1016/j.forsciint.2019.05.015
Machado MVF, Soares MQS, Baz AMSA, Junqueira JLC, Franco A (2022) A large sample-sized study on dental development of children treated at the Central Dental Clinic (OCEx) of the Brazilian army. Clin Oral Invest 26(8):5439–5447. https://doi.org/10.1007/s00784-022-04511-z
Miranda JC, Azevedo ACS, Rocha M, Michel-Corsato E, Biazevic MGH (2020) Age estimation in Brazilian adults by Kvaal’s and Cameriere’s methods. Braz Oral Res 34:e051. https://doi.org/10.1590/1807-3107bor-2020.vol34.0051
Nascimento EHL, Gaêta-Araujo H, Vasconcelos KF, Freire BB, Oliveira-Santos C, Haiter-Neto F, Freitas DQ (2018) Influence of brightness and contrast adjustments on the diagnosis of proximal caries lesions. Dentomaxillofac Radiol 47:20180100. https://doi.org/10.1259/dmfr.20180100
Pradella F, Pinchi V, Focardi M, Grifoni R, Palandri M, Norelli GA (2017) The age estimation practice related to illegal unaccompanied minors immigration in Italy. J Forensic Odontostomatol 35:141–148
Rocha LT, Ingold MS, Panzarella FK, Santiago BM, Oliveira RN, Bernardino IM, Makeeva I, Junqueira JL, Mânica S, Franco A (2022) Applicability of Willems method for age estimation in Brazilian children: performance of multiple linear regression and artificial neural network. Egypt J Forensic Sci 12:9. https://doi.org/10.1186/s41935-022-00271-9
Sharma M, Srivastava A (2010) Radiographic evaluation of dental age of adults using Kvaal’s method. J Forensic Dent Sci 2(1):22–26. https://doi.org/10.4103/0974-2948.71053
Sobieska E, Fester A, Nieborak M, Zadurska M (2018) Assessment of the dental age of children in the Polish population with comparison of the Demirjian and the Willems methods. Med Sci Monit 24:8315–8321. https://doi.org/10.12659/MSM.910657
Vasconcelos KF, Nicolielo LFP, Nascimento MC, Haiter-Neto F, Bóscolo FN, Van Dessel J, EzEldeen M, Lambrichts I, Jacobs R (2015) Artefact expression associated with several cone-beam computed tomographic machines when imaging root filled teeth. Int Endod J 48:994–1000. https://doi.org/10.1111/iej.12395
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative (2014) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 12(12):1495–1499. https://doi.org/10.1016/j.ijsu.2014.07.013
Zdravkovic D, Jovanovic M, Papic M, Ristic V, Milojevic Samanovic A, Kocovic A, Sovrlic M, Vuletic M, Misic A, Mladenovic R, Milosavljevic M, Todic J (2022) Application of the Kvaal method in age estimation of the Serbian population based on dental radiographs. Diagnostics (basel) 12(4):911. https://doi.org/10.3390/diagnostics12040911
Acknowledgements
The authors would like to express their gratitude to the staff of the Institutional Oral Radiology clinics.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SM participated in the acquisition, analysis, and interpretation of data and contributed to the writing of the manuscript. AP also participated in the acquisition, analysis, and interpretation of data and contributed to the writing of the manuscript. MS was responsible for the conception and design of the study, supervision, and revision of the final manuscript. MA also was responsible for the conception and design of the study, supervision, and revision of the final manuscript. AF participated in the conception and design of the study, guidance, and review of the final manuscript, in addition to being responsible for the administration of the project. All authors mentioned above have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed with the approval of the Institutional Committee of Ethics in Human Research (protocol: 49916121.9.0000.5374).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
STROBE Statement—Checklist of items that should be included in reports of cross-sectional studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mantovani, S., Petta, A., Arakelyan, M. et al. Kvaal’s dental age estimation method applied to Brazilian adults—a cross-sectional test of validity. Egypt J Forensic Sci 14, 1 (2024). https://doi.org/10.1186/s41935-023-00381-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41935-023-00381-y