Abstract
Background
Although reduced work ability is a substantial problem among people with inflammatory arthritis (IA), work ability is an underexposed area in clinical practice. Evidence on vocational interventions in IA is limited, but favourable results of delivery by a physiotherapist (PT) warrant the need for further research. Therefore, we aim to evaluate the (cost-)effectiveness of a multimodal, PT-led, vocational intervention in (self-)employed people with IA compared to usual care.
Methods
This randomized controlled trial will include 140 people with rheumatoid arthritis (RA) or axial spondyloarthritis (axSpA) who are (self-)employed and have reduced work ability (Work Ability Index – Single Item Scale (WAS) ≤ 7/10) and/or RA/axSpA related sick leave (≤ 6 months). Participants will be randomized 1:1 to the intervention or control condition (usual care). The intervention, delivered by primary care PTs, will be personalized to each patient, consisting of 10 to 21 sessions over 12 months. The intervention will be multimodal, comprising of 1) exercise therapy and a physical activity plan, 2) education/self-management support, 3) work-roadmap to guide participants in finding relevant other care, with optionally 4) online self-management course and 5) workplace examination. Assessments will be performed at baseline and after 3, 6, and 12 months. The primary outcome measure of effectiveness is work ability, as measured with the WAS at 12 months. For the cost-effectiveness analysis, the EuroQol (EQ-5D-5L), self-reported healthcare use, sick leave and productivity while at work will be used to estimate the trial based cost-utility from a societal perspective. A process evaluation, including assessments of adherence and treatment fidelity, will be undertaken using the registrations of the PTs and semi-structured interviews at 12 months follow-up in a random sample of the intervention group.
Discussion
The results of this study will provide insights in the (cost-)effectiveness of a multimodal, PT-led, vocational intervention in people with IA and a reduced work ability.
Trial registration
This study is registered in the International Clinical Trial Registry Platform (ICTRP) under number NL9343.
Similar content being viewed by others
Background
Rheumatoid Arthritis (RA) and axial SpondyloArthritis (axSpA) are chronic rheumatic diseases, characterized by inflammation of the joints, resulting in joint pain, stiffness, fatigue [1,2,3] and reduced health-related quality of life [3, 4]. These diseases usually begin in the fifth (RA) or third (axSpA) decade of life and thus affect people of working age [3, 5]. Despite breakthroughs in the pharmacological treatment, work ability of people with RA and axSpA is substantially reduced compared to the general population [6,7,8] and is characterized by substantial job loss [6], up to 38% of people with RA or axSpA lose their jobs already within the first few years of diagnosis [9], sick leave [4], and decreased productivity while at work (i.e., presenteeism) [10]. This causes considerable economic consequences for individuals as well as society [4, 11]. European yearly indirect costs in people with RA and axSpA were in 2015 estimated around €4.000 to €5.000 per person [12, 13].
Although reduced work ability is an important problem in RA and axSpA, the number of studies on vocational interventions for these patient groups is limited. Such interventions are referred to as job loss prevention, occupational rehabilitation or vocational rehabilitation and may be delivered by physiotherapists (PT), occupational therapists (OT), social workers, psychologists or other professionals, either monodisciplinary or multidisciplinary. Two recent systematic reviews [9, 14], including 6 randomized controlled trials (RCTs) [15,16,17,18,19,20] and 1 pilot-RCT [21], evaluated supervised vocational interventions on work-related outcomes in RA or axSpA. In these studies, the intervention was delivered by a multidisciplinary team [16, 18], or monodisciplinary by an OT [17, 20, 21], OT or PT [19] or rehabilitation counselor [15]. The RCTs showed conflicting evidence and overall a small effect on work-related outcomes. Interestingly, both studies [16, 18] evaluating a multidisciplinary vocational intervention on work-related outcomes found no effect, but all five studies involving monodisciplinary vocational interventions on work-related outcomes, reported an positive effect. Three studies found a medium effect [17, 20, 21] but from the results of two studies no effect sizes can be estimated [15, 19].
Although it is difficult to compare the magnitude of the treatment effects in the studies on PT or OT led vocational interventions due to variety in work-related outcome measures, the delivery of vocational interventions by a PT trained to treat people with inflammatory arthritis (IA) seems promising. Firstly from a patient-perspective but also from an economic perspective, as less expensive than a multidisciplinary intervention. In addition to the abovementioned positive results in IA, PT-led interventions have also been found to be effective on work-related outcomes in musculoskeletal pain in multiple studies [22,23,24]. Especially in patients with IA, delivery of vocational interventions by PTs may be an attractive option, as physiotherapy is relatively often used in this patient group (i.e., 25–50% of patients with IA visit a PT per year in the Netherlands [25]).
In summary, studies on the effectiveness of vocational interventions delivered by a PT in people with RA or axSpA and reduced work ability are scarce. The aim of the study is therefore to evaluate the effectiveness and cost-effectiveness of a PT-led vocational intervention for people with RA or axSpA and reduced work ability as compared to usual care.
Methods
Study design
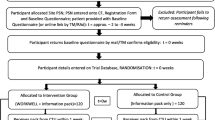
In a Dutch nationwide single-blind RCT, the effectiveness and cost-effectiveness of a multimodal, PT-led, vocational intervention compared to usual care in (self-)employed people with RA or axSpA and a reduced work ability will be evaluated. Measurements will take place at baseline, 3, 6 and 12 months (primary endpoint). An overview of the study is provided in the flowchart (Fig. 1).
Study flowchart [26]
Study setting
The intervention will be delivered by primary care PTs. People in the Netherlands have direct access to a PT. Since 2012, the costs of physiotherapy are not reimbursed by the basic health insurance. Full or partial reimbursement can be obtained by means of a complementary health insurance, otherwise patients must pay out-of-pocket. In the Netherlands, 76% of the people with RA or axSpA have such a complementary health insurance [25]. In this study, participants paid for the physiotherapy sessions using the same approach as described above.
Study context
In the Netherlands, employers are legally obliged to have a contract with an occupational healthcare service [27]. Employees with prolonged sick leave (i.e., ≥ 6 weeks) receive care from an occupational physician affiliated to such an occupational healthcare service [28, 29]. Employees can also approach an occupational physician in case of work-related problems as a preventive measure. Self-employed people in the Netherlands are expected to protect themselves against the risk of disability by taking out a disability insurance. In practice, however, only about a quarter of the self-employed people have a disability insurance [30]. People without a disability insurance are not entitled to counseling and benefits in the event of sick leave or job loss.
Participants
The study population will consist of (self-)employed people with RA or axSpA and reduced work ability.
Inclusion criteria
-
1.
Adult people (≥ 18 years) with a clinical diagnosis of RA or axSpA (confirmed by a rheumatologist),
-
2.
Being (self-)employed for a minimum of 12 h/week.
-
3.
Moderate to poor work ability (Work Ability Index-Single Item Scale (WAS) ≤ 7/10 [31] related to RA or axSpA and/or a self-reported history of sick leave in the last six months, related to RA or axSpA.
-
4.
Self-reported limitations in physical functioning related to RA/axSpA.
-
5.
Willingness to use physiotherapy (nearby participant).
-
6.
Willingness to pay for physiotherapy (through (partial) complementary health insurance or out-of-pocket).
-
7.
Sufficient command of Dutch language.
Exclusion criteria
-
1.
Pensionable age within two years, because this minimizes the perceived necessity of a vocational intervention.
-
2.
Persistent sick leave period of more than 6 months, because of presumed reduced potential of a vocational intervention with a prolonged sick leave period.
-
3.
Comorbid disease or situation other than RA or axSpA (including elective hospital admissions or major surgery in the coming 12 months) that significantly affects work ability, as this work ability is unlikely to be affected by an intervention targeting arthritis-related work disability.
-
4.
Pregnancy, because maternity leave during the intervention and follow-up period will hamper the execution of the intervention and interpretation of assessments.
-
5.
Being in a formal labour dispute, because this generally indicates non-health factors dominating perceived work ability which cannot be influenced by the intervention.
Study procedures
During the recruitment period, information about the study will be shared with all rheumatology departments and outpatient clinics in the Netherlands and through various public media. Potentially eligible participants interested in the study receive information about the study as much as necessary from a medical ethical perspective, with only minimal information about the content of the experimental intervention, namely that it is PT-delivered and includes exercise therapy and advices regarding work, and will be subsequently screened by the researcher (NB). If a potential participant meets the eligibility criteria and consents, the treating rheumatologist will be contacted to confirm the clinical diagnosis. Potential participants will be included after receiving a confirmation of the diagnosis and informed consent. Participants will formulate and rate one specific work-related limitation in physical functioning and two specific other limitations in physical limitations in daily life, using the Patient Specific Complaints Numeric Rating Scale (PSC NRS) [32] during a telephone conversation with the researcher (NB). Baseline data (T0) and follow-up assessments, administered at 3-months (T3), 6-months (T6) and 12-months (T12) after baseline, will be collected through online questionnaires.
Randomization and blinding
Randomization will be performed by the researcher (NB) using the software Castor (Castor. EDC©) at the participant level, in blocks of varying sizes (2–4-6 participants, with block size randomization) in a 1:1 ratio. Randomization will be stratified for disease (RA vs. axSpA), disease duration (< 5 vs. ≥ 5 years since diagnosis), and current sick leave (yes vs. no). We will stratify for disease duration because in the first few years after the diagnosis pharmacological treatment has started and symptoms could possibly fluctuate more than later in the disease course. The researcher will not be blinded for treatment allocation of the participants due to logistic reasons regarding contact with participants and treating PTs, but the researchers conducting the primary analyses will be blinded for the group allocation.
After completion of the baseline assessment, the participants will be informed by the researcher (NB) about their assigned condition (intervention/control). Given the nature of the intervention, participants and PTs involved in the treatment cannot be blinded to the treatment allocation.
Intervention
Recruitment and training of PTs
The intervention will be delivered by primary care PTs in the neighbourhood of the participants’ home. PTs will be primarily recruited from the ‘ReumanetNL’ network (www.reumanetnl.nl), a nationwide network of PTs with expertise in treating people with RA or axSpA. Participants in the experimental group who prefer to be treated by the PT that they are familiar with, are given this opportunity if this PT is willing to follow the study training and consent with the treatment protocol. To minimize contamination and because its (cost-)effectiveness is still unknown, PTs in the experimental arm are prohibited to provide the experimental intervention to participants outside the study and to participants in the control arm during their participation in the study.
Participating PTs are instructed to comply with the current Dutch physiotherapy guidelines for RA [33] and physiotherapeutic management recommendations for axSpA [34]. In addition they will have access to an online training environment and receive the treatment protocol on paper. The mandatory training for PTs will consist of multiple e-learnings on i) integration of work in the physiotherapy treatment, ii) Dutch occupational healthcare system and work-related laws/regulations, and iii) treatment protocol including a live question session. If deemed necessary, supplemental disease-specific trainings could be followed. The total duration of this mandatory training is approximately five hours. Participating PTs can contact a study team member with extensive expertise in physiotherapy for these populations at any time during the trial.
Intervention
The experimental group will receive a multimodal, PT-led, vocational intervention based on an integration of existing guidelines, programs, materials and clinical knowledge and experience [9, 33,34,35,36,37,38,39]. This intervention was developed according to the Medical Research Council framework for developing and evaluating complex interventions [40], in co-creation with people with RA or axSpA, PTs, (occupational) healthcare professionals (occupational physician, labour expert, OT, rheumatologist, nurse specialist), and researchers during six group meetings and was tested for feasibility in four patients. This intervention development process did result in several adaptations of the (draft) intervention, such as elaboration of the work-roadmap and the development of training courses for PTs in the trial [41].
The intervention consists of work-focused modalities embedded in the conventional physiotherapy treatment and comprises 10 to 21 PT sessions of 30 min (combination of face-to-face, online or telephone-based sessions) over a 12-month period, delivered in four steps (see Table 1). Due to the complexity of work ability, a multimodal approach with a long follow-up period is considered necessary to achieve sustainable changes in work ability. This approach should enable the PT to (i) gradually increase the intensity of the exercise therapy, (ii) monitor whether the participant succeeds in reaching sustainable lifestyle changes (e.g., being more physically active), (iii) adequately signpost the participant to other professionals over time, and (iv) monitor the impact of any work-related adaptations if applicable. The intervention will be multimodal, consisting of a combination of the following three mandatory and two optional treatment modalities:
Mandatory modalities
-
1.
Exercise therapy including personal physical activity plan;
-
2.
Education and self-management support;
-
3.
‘Work-roadmap’: adequately signposting participants to other professionals with regard to optimizing their work ability.
Optional modalities
-
4.
Online self-management course;
-
5.
Workplace examination.
These modalities will all be adjusted to the individuals’ needs based on the defined work treatment goals and aligned to the individuals’ specific (work-related) limitations in physical functioning. In Table 2 an extensive description of the content of the intervention modalities is given.
In addition to the intervention, participants are allowed to receive usual care.
Control
The control group will continue their usual care.
Outcome measures and data collection
The primary outcome is the participants’ reported work ability assessed by the Work Ability Index-Single Item Scale (WAS) [31]. The secondary outcomes are divided into four categories: 1) work-related outcomes; 2) clinical outcomes; 3) healthcare use and costs from the societal perspective and 4) expectancy of the treatment and global perceived effect. A detailed description of all outcome measures is shown in Table 3 and the timepoints on which they are assessed are displayed in Table 4.
At baseline, disease characteristics will be retrieved from the participants’ rheumatologist. Data from patient questionnaires will be collected and stored in the online database OnlinePROMS© (2020, Interactive Studios BV, Rosmalen, the Netherlands).
(Serious) Adverse events
All participants and PTs will be asked to immediately and proactively report serious adverse events (SAE) or adverse events (AE) to the researchers. SAEs and AEs, directly related to the intervention, will be recorded and followed until they have abated, or until a stable situation is reached. The researchers will report all intervention-related SAEs to the sponsor without undue delay after obtaining knowledge of the events.
Sample size calculation
The sample size was calculated with a conservatively estimated between-group effect size of 0.5 on the primary outcome measure WAS [31], based on an expected improvement in the experimental group from 6 (moderate work ability) to 8 (good work ability), an expected improvement in the control group from 6 to 7 (to take into account a potential ‘regression to the mean effect’) and a standard deviation of 2 [18, 46]. Based on previous studies [17, 21], this between-group effect size can be considered feasible. Based on two-sided testing, a significance level of 0.05, a power of 0.8, and this expected between-group effect size, 126 participants have to be recruited. Based on trials on comparable vocational interventions in IA [15, 17, 19], we took into account a drop-out rate of 10% (i.e., 126/90 * 100), which resulted in a sample size of 140 participants (70 in each arm). Considering a threefold higher prevalence of RA over axSpA in the Netherlands, we expect both groups to comprise more people with RA than axSpA.
Process evaluation
The process of the trial will be evaluated both quantitatively and qualitatively, comparable to previous and ongoing other trials from our group [63, 64]. For the quantitative evaluation, PTs will report process parameters after each treatment session (in OnlinePROMS®), including participant adherence, number of treatment sessions, the content of the applied treatment, and adverse events. Based on these parameters, PT treatment fidelity can be assessed. For the qualitative evaluation, a random sample of 10 participants and 10 PTs will be selected and invited for semi-structured interviews to discuss experiences, barriers and facilitators with regard to the intervention. The semi-structured interviews will be conducted after the selected participant has completed the 12-month assessment.
Statistical analysis
Primary analysis
A multilevel, regression analysis – with levels of patient and time point – will be performed using linear mixed modeling. Primary outcome measure WAS will be analyzed as a dependent variable, using the study group (intervention vs control), stratification variables (i.e., disease (RA vs. axSpA), disease duration (< 5 vs. > 5 years), current sick leave at randomization (yes or no)) and other potential confounders as independent variables. All analyses will be performed according to the intention-to-treat principle. Statistical significance will be accepted at p-values of less than 0.05 (two-sided testing).
Secondary analysis
Similar analyses will be performed for the secondary time points T3 and T6, as well as for the total follow-up period, and with all secondary outcome measures. Only Global Perceived Effect (GPE) will be analyzed as a dichotomous variable (‘completely recovered’ and ‘much recovered’ vs. all other responses) using logistic multilevel analysis. Furthermore, effect sizes from all clinical outcome measures will be calculated.
Economic evaluation
For the economic evaluation, a trial-based cost-utility analysis will be performed, relating costs to quality-adjusted life years (QALYs). Societal costs will include healthcare utilization, informal care and work-related costs, assessed using patient questionnaires at 3, 6 and 12 months. QALYs will be calculated using the Dutch tariff for the EQ-5D-5L [65], assessed at baseline, 3, 6, and 12 months. Analyses will be performed in accordance with the Dutch guidelines for economic evaluations [66], with extrapolation beyond the one-year trial period. The primary analysis will be from a societal perspective with friction cost method to value productivity, whereas secondary analyses follow the human-capital approach and a healthcare perspective [67]. Costs will be related to outcome using net-benefit analysis, with multiple imputation to account for missing data.
Data management
All the data of the participants will be pseudonymized with assignment of a study number to every participant. The key to the study numbers will be stored in a separate file on the server of the Leiden University Medical Center (LUMC). Only the research team, an auditor from the LUMC and national and international supervisory authorities can access the participants’ personal information. The collected data will be stored for 15 years on a local server at the LUMC and a backup of the data will be stored at the LUMC.
Discussion
Work ability of people with RA or axSpA is considerably reduced compared to the general population [8]. Despite the observed need for vocational interventions, research on the (cost-)effectiveness of vocational interventions is limited. To our knowledge, this is the first study on the (cost-)effectiveness of a PT-led, vocational intervention in people with RA or axSpA. Based on existing evidence and clinical experience, we have integrated all potentially effective treatment modalities into a single intervention, delivered by PTs specifically trained for this purpose. We expect that the incorporation of these individually effective modalities, with an embedded focus on work, will lead to a moderate effect as well as substantial cost savings through reduced sick leave and improved work productivity. Therefore, we hypothesize that a multimodal, PT-led, vocational intervention in (self-)employed people with RA or axSpA and a reduced work ability is effective and cost-effective compared to usual care.
We would like to acknowledge two (potential) study limitations about the population. First, as we include two patient groups (i.e., RA and axSpA), our study sample could be considered relatively heterogenous. However, people with RA as well as axSpA experience comparable symptoms, including joint pain, stiffness, fatigue [1,2,3] and reduced health-related quality of life [3, 4]. Furthermore, we include a homogenous sample from a work perspective, namely people with reduced work ability but still at work or less than 6 months on sick leave, as this population is expected to profit most from our intervention. Therefore, we expect these two patient groups to respond similarly to our intervention. Second, since participants in this study cannot be blinded to their randomization, it cannot be ruled out that control participants will seek a similar (PT) intervention that may lead to contamination and thereby reduce the contrast between our two arms. However, we do not expect this to occur, as work is usually not addressed in current practice [68, 69]. Nevertheless, we will carefully record the use of any healthcare or other services during the 12-months of follow-up. This enables us to demonstrate the level of contamination.
Furthermore, we would like to mention three (potential) limitations regarding the design of the study. First, the number of sessions in our intervention (10 to 21 sessions during a 12-month follow-up period) appears to be relatively extensive compared to other vocational intervention studies in IA, in which the number of sessions was between one and 12 [15,16,17,18,19,20,21]. Due to the complexity of the concept of work ability, a multimodal approach with a long follow-up period is considered necessary to achieve sustainable changes in work ability. Second, although we tested a draft version of our intervention for feasibility in a small group of four patients, unexpected barriers in its execution may arise if applied on a larger scale which could impact the outcomes of the study. Third, for practical reasons, we only include people in whom the cost of the intervention is covered by their complementary health insurance (or if not, people are willing to pay it out-of-pocket). This may lead to a selection bias, although a majority of the people with IA (namely 76% [25]) in the Netherlands have this coverage. Because we carefully capture this eligibility criterium (inclusion criterium 6), we have insight into the extent of this barrier after the completion of the trial.
To conclude, as the study is still ongoing the results are not available yet, the scientific implications of this publication are limited. However, given the fact that limited research on the effectiveness of interventions to increase the work ability of people with RA or axSpA is available, for those interested in the evidence on this topic it is important to be aware of ongoing studies. The results of this study will provide insights in the (cost-)effectiveness of a multimodal, PT-led, vocational intervention in people with RA and axSpA and a reduced work ability.
Availability of data and materials
The data generated during this study will not be publicly available, but will be available upon reasonable request to the corresponding author.
Abbreviations
- IA:
-
Inflammatory arthritis
- RA:
-
Rheumatoid Arthritis
- axSpA:
-
Axial SpondyloArthritis
- RCT:
-
Randomized Controlled Trial
- PT:
-
Physiotherapist
- OT:
-
Occupational Therapist
- WAS:
-
Work Ability Index – Single Item Scale
- PSC NRS:
-
Patient Specific Complaints Numeric Rating Scale
- EQ-5D-5L:
-
EuroQol
- GPE:
-
Global Perceived Effect
- QALY:
-
Quality-adjusted Life Year
- ICTRP:
-
International Clinical Trial Registry Platform
- SAE :
-
Serious Adverse Event
- AE:
-
Adverse Event
- LUMC:
-
Leiden University Medical Center
References
Mielants H, Van den Bosch F. Extra-articular manifestations. Clin Exp Rheumatol. 2009;27(4 Suppl 55):S56-61.
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38.
Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390(10089):73–84.
Boonen A, Severens JL. The burden of illness of rheumatoid arthritis. Clin Rheumatol. 2011;30(Suppl 1):S3-8.
Myasoedova E, Davis J, Matteson EL, Crowson CS. Is the epidemiology of rheumatoid arthritis changing? Results from a population-based incidence study, 1985–2014. Ann Rheum Dis. 2020;79(4):440–4.
Berner C, Haider S, Grabovac I, Lamprecht T, Fenzl KH, Erlacher L, et al. Work ability and employment in rheumatoid arthritis: a cross-sectional study on the role of muscle strength and lower extremity function. Int J Rheumatol. 2018;2018:3756207.
Nikiphorou E, Ramiro S. Work disability in axial spondyloarthritis. Curr Rheumatol Rep. 2020;22(9):55.
Berkovic D, et al. Arthritis-related work outcomes experienced by younger to middle-aged adults: a systematic review. Occup Environ Med. 2021;78(4):225–36.
Madsen CMT, et al. A Systematic review of job loss prevention interventions for persons with inflammatory arthritis. J Occup Rehabil. 2021;31(4):866–85.
Verstappen SM. Rheumatoid arthritis and work: the impact of rheumatoid arthritis on absenteeism and presenteeism. Best Pract Res Clin Rheumatol. 2015;29(3):495–511.
Boonen A, van der Linden SM. The burden of ankylosing spondylitis. J Rheumatol Suppl. 2006;78:4–11.
Harvard S, Guh D, Bansback N, Richette P, Dougados M, Anis A, et al. Costs of early spondyloarthritis: estimates from the first 3 years of the DESIR cohort. RMD Open. 2016;2(1):e000230.
Raciborski F, Kłak A, Kwiatkowska B. Indirect costs of rheumatoid arthritis. Reumatologia. 2015;53(5):268–75.
Butink MHP, Webers C, Verstappen SMM, Falzon L, Betteridge N, Wiek D, et al. Non-pharmacological interventions to promote work participation in people with rheumatic and musculoskeletal diseases: a systematic review and meta-analysis from the EULAR taskforce on healthy and sustainable work participation. RMD Open. 2023;9(1):e002903.
Allaire SH, Li W, LaValley MP. Reduction of job loss in persons with rheumatic diseases receiving vocational rehabilitation: a randomized controlled trial. Arthritis Rheumatol. 2003;48(11):3212–8.
de Buck PD, le Cessie S, van den Hout WB, Peeters AJ, Ronday HK, Westedt ML, et al. Randomized comparison of a multidisciplinary job-retention vocational rehabilitation program with usual outpatient care in patients with chronic arthritis at risk for job loss. Arthritis Rheumatol. 2005;53(5):682–90.
Macedo AM, et al. Functional and work outcomes improve in patients with rheumatoid arthritis who receive targeted, comprehensive occupational therapy. Arthritis Rheumatol. 2009;61(11):1522–30.
van Vilsteren M, Boot CR, Twisk JW, van Schaardenburg D, Steenbeek R, Voskuyl AE, et al. Effectiveness of an integrated care intervention on supervisor support and work functioning of workers with rheumatoid arthritis. Disabil Rehabil. 2017;39(4):354–62.
Keysor JJ, LaValley MP, Brown C, Felson DT, AlHeresh RA, Vaughan MW, et al. Efficacy of a work disability prevention program for people with rheumatic and musculoskeletal conditions: a single-blind parallel-arm randomized controlled trial. Arthritis Care Res (Hoboken). 2018;70(7):1022–9.
Baldwin D, et al. Randomized prospective study of a work place ergonomic intervention for individuals with rheumatoid arthritis and osteoarthritis. Arthritis Care Res (Hoboken). 2012;64(10):1527–35.
Hammond A, O’Brien R, Woodbridge S, Bradshaw L, Prior Y, Radford K, et al. Job retention vocational rehabilitation for employed people with inflammatory arthritis (WORK-IA): a feasibility randomized controlled trial. BMC Musculoskelet Disord. 2017;18(1):315.
Eichler S, Salzwedel A, Rabe S, Mueller S, Mayer F, Wochatz M, et al. The effectiveness of telerehabilitation as a supplement to rehabilitation in patients after total knee or hip replacement: randomized controlled trial. JMIR Rehabil Assist Technol. 2019;6(2):e14236.
Sennehed CP, Holmberg S, Axén I, Stigmar K, Forsbrand M, Petersson IF, et al. Early workplace dialogue in physiotherapy practice improved work ability at 1-year follow-up—WorkUp, a randomised controlled trial in primary care. Pain. 2018;159:1456–64.
Wynne-Jones G, Artus M, Bishop A, Lawton SA, Lewis M, Jowett S, et al. Effectiveness and costs of a vocational advice service to improve work outcomes in patients with musculoskeletal pain in primary care: a cluster randomised trial (SWAP trial ISRCTN 52269669). Pain. 2018;159(1):128–38.
Sloot R, et al. Reumatische aandoeningen in Nederland: Ervaringen en kengetallen ('Rheumatic diseases in the Netherlands: Experiences and key figures'). 2016.
Bakker NF, et al. Fysiotherapie WERKt: een gerandomiseerd, gecontroleerd onderzoek naar de (kosten) effectiviteit van een multimodale, gepersonaliseerde, werkgeoriënteerde interventie bij ontstekingsreuma ('Physiotherapy WORKs: a randomized controlled trial on the (cost-)effectiveness of a multi-modal, personalized, work-oriented intervention in inflammatory arthritis'). Nederlands Tijdschrift voor Reumatologie. 2021. 1. p. 45-46.
Ministry of Social Affairs and Employment. Arbowet ('Working Conditions Act’). 2017.
de Jong P, Everhardt T, Schrijvershof C. Toepassing van de wet Verbetering Poortwachter ('Application of The Dutch Eligibility for Permanent Incapacity Benefit (Restrictions) Act'). APE, 2011.
de Buck PD, et al. Communication between Dutch rheumatologists and occupational physicians in the occupational rehabilitation of patients with rheumatic diseases. Ann Rheum Dis. 2002;61(1):62-5.
Conen W, Debets M. Precariousness and social risks among solo self-employed in Germany and the Netherlands. 2019.
Ahlstrom L, et al. The work ability index and single-item question: associations with sick leave, symptoms, and health–a prospective study of women on long-term sick leave. Scand J Work Environ Health. 2010;36:404–12.
Beurskens AJ, et al. A patient-specific approach for measuring functional status in low back pain. J Manipulative Physiol Ther. 1999;22(3):144–8.
Hurkmans EJ, et al. KNGF guideline Rheumatoid arthritis. 2018. https://www.kngf.nl/binaries/content/assets/kennisplatform/onbeveiligd/guidelines/reumatoide-artritis-2020/kngf-rheumatoid-arthritis-ra-2018-practice-guideline.pdf.
van Weely SFE, et al. Aanbevelingen fysiotherapie bij mensen met axiale spondyloartritis ('Physiotherapy recommendations in people with axial spondyloarthritis'). 2019. https://reumanetnl.nl/wp‐content/uploads/2019/06/Aanbevelingen‐Fysiotherapie‐bij‐mensen‐met‐AxialeSpondyloartritus.pdf.
Boonen A, Lems WF. Arbeid als behandeldoel: Nieuwe richtlijn 'Reumatoïde artritis en participatie in arbeid' ('Work as a treatment goal: New guideline 'Rheumatoid arthritis and work participation''). Nederlands Tijdschrift voor Geneeskunde, 159. 2015. https://www.ntvg.nl/system/files/publications/a9593.pdf
Fit for Work, Fit for work? Spier- en gewrichtsaandoeningen en de Nederlandse arbeidsmarkt ('Fit for work? Muscle and joint disorders and the Dutch labour market'). 2013. https://fitforworknederland.nl/wp-content/uploads/2015/12/6_FitforWork_Quickscan_2013.pdf.
Gwinnutt JM, et al. 2021 EULAR recommendations regarding lifestyle behaviours and work participation to prevent progression of rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2022;82(1):48-56.
NVR-NVVG, Richtlijn Reumatoïde artritis en participatie in arbeid ('Guideline Rheumatoid Arthritis and Participation in Work'). 2015.
Ward MM, Deodhar A, Gensler LS, Dubreuil M, Yu D, Khan MA, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Care Res (Hoboken). 2019;71(10):1285–99.
Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ. 2021;374:n2061.
Bakker NF, Knoop J, Hutting N, Heerkens YF, Engels JA, Staal JB, et al. OPO201-pare development through co-creation of a personalized, multimodal, physiotherapist-led, work-oriented intervention to increase work ability in working people with rheumatoid arthritis or axial spondyloarthritis. Annals Rheum Dis. 2022;81(1):132-33.
de Buck PD, et al. A multidisciplinary job retention vocational rehabilitation programme for patients with chronic rheumatic diseases: patients' and occupational physicians' satisfaction. Ann Rheum Dis. 2004;63(5):562-8.
Stevens A, et al. Ready for goal setting? Process evaluation of a patient-specific goal-setting method in physiotherapy. BMC Health Serv Res. 2017;17(1):1-10.
Boot CR, et al. One-year predictors of presenteeism in workers with rheumatoid arthritis: disease-related factors and characteristics of general health and work. J Rheumatol. 2018;45(6):766-70.
Castillo-Ortiz J, et al. Work Outcome in Patients With Ankylosing Spondylitis: Results From a 12-Year Followup of an International Study. Arthritis Care Res (Hoboken). 2016;68(4):544-52.
Lambeek LC, et al. Randomised controlled trial of integrated care to reduce disability from chronic low back pain in working and private life. BMJ. 2010;340:c1035.
Calin A, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol. 1994;21(12):2281-5.
Wells G, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68(6):954-60.
Reilly MC, et al. Validity, reliability and responsiveness of the Work Productivity and Activity Impairment Questionnaire in ankylosing spondylitis. Rheumatology (Oxford). 2010;49(4):812-9.
Zhang W, et al. Validity of the work productivity and activity impairment questionnaire-general health version in patients with rheumatoid arthritis. Arthritis Res Ther. 2010;12(5):1-7.
Linton, S.J. and K. Halldén, Can we screen for problematic back pain? A screening questionnaire for predicting outcome in acute and subacute back pain. Clin J Pain. 1998;14(3):209-15.
Olafsen AH, Halvari H, Frølund C.W. The Basic Psychological Need Satisfaction and Need Frustration at Work Scale: A Validation Study. Front Psychol, 2021;12:697306.
Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152(10):2399-404.
Bartlett SJ, et al. Reliability and Validity of Selected PROMIS Measures in People with Rheumatoid Arthritis. PLoS One. 2015;10(9):e0138543.
Fries JF, et al. Progress in assessing physical function in arthritis: PROMIS short forms and computerized adaptive testing. J Rheumatol. 2009;36(9):2061-6.
Terwee CB, et al. Dutch-Flemish translation of 17 item banks from the patient-reported outcomes measurement information system (PROMIS). Qual Life Res. 2014;23(6):1733-41.
van Tubergen A, Black PM, Coteur G. Are patient-reported outcome instruments for ankylosing spondylitis fit for purpose for the axial spondyloarthritis patient? A qualitative and psychometric analysis. Rheumatology (Oxford). 2015;54(10):1842-51.
Maas F, Baron AJ, Wink FR, Bos R, Kamsma YPT, Bootsma H, Arends S, Spoorenberg J. Assessing physical activity in axial spondyloarthritis patients: modification of the SQUASH. Clin Exp Rheumatol. 2016;34(4):736.
Spinhoven P, et al. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27(2):363-70.
van den Hout WB, et al. Cost-utility and cost-effectiveness analyses of a long-term, high-intensity exercise program compared with conventional physical therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2005;53(1):39-47.
Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095-108.
EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208.
Knoop J, et al. Patients’ and clinicians’ experiences with stratified exercise therapy in knee osteoarthritis: a qualitative study. BMC musculoskelet disord. 2022;23(1):1-14.
van Wissen MA, et al. Effectiveness and cost‐effectiveness of longstanding exercise therapy versus usual care in patients with axial spondyloarthritis or rheumatoid arthritis and severe limitations: The protocols of two parallel randomized controlled trials. Physiother Res Int. 2022;27(1):e1933.
Versteegh M, et al. Dutch Tariff for the Five-Level Version of EQ-5D. Value Health. 2016;19(4):343-52.
Kanters TA, et al. Update of the Dutch manual for costing studies in health care. PLoS One. 2017;12(11):e0187477.
van den Hout WB. The value of productivity: human-capital versus friction-cost method. Ann Rheum Dis. 2010;69(Suppl 1):i89-91.
Hutting N, et al. Physical therapists and importance of work participation in patients with musculoskeletal disorders: a focus group study. BMC Musculoskelet Disord. 2017;18(1):196.
Oswald W, et al. Work participation of patients with musculoskeletal disorders: is this addressed in physical therapy practice? J Occup Med Toxicol. 2017;12:27.
Acknowledgements
Not applicable.
Funding
This study is funded by the Dutch Arthritis Society (ReumaNederland) and the Scientific College of Physical Therapy (Wetenschappelijk College Fysiotherapie; WCF) of the Royal Dutch Society for Physical Therapy (Koninklijk Nederlands Genootschap voor Fysiotherapie; KNGF). The funders had no role in the design, organization and execution of the study.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the conception and design of the study and writing of this manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study is approved by the Medical Ethical Committee of Leiden-Den Haag-Delft (NL75919.058.20) and will be conducted in agreement with the declaration of Helsinki (2013) and in compliance with the General Data Protection Regulations and the Dutch Medical Research Involving Human Subjects Act. Written informed consent will be obtained from all eligible participants in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bakker, N.F., van Weely, S.F.E., Hutting, N. et al. Effectiveness and cost-effectiveness of a multimodal, physiotherapist-led, vocational intervention in people with inflammatory arthritis: study protocol of the Physiotherapy WORKs trial. BMC Rheumatol 7, 31 (2023). https://doi.org/10.1186/s41927-023-00357-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41927-023-00357-4