Abstract
Background
Limited real-world data exists on clinical outcomes in systemic lupus erythematosus (SLE) patients by SLE Disease Activity Index 2000 (SLEDAI-2 K), hereafter, SLEDAI. We aimed to examine the association between SLEDAI score and clinical, patient-reported and economic outcomes in patients with SLE.
Methods
Rheumatologists from the United States of America and Europe provided real-world demographic, clinical, and healthcare resource utilization (HCRU) data for SLE patients. Patients provided self-reported outcome data, capturing their general health status using the EuroQol 5-dimension 3-level questionnaire (EQ-5D-3 L), health-related quality of life using the Functional Assessment of Chronic Illness Therapy (FACIT) and work productivity using the Work Productivity and Activity Impairment questionnaire (WPAI). Low disease activity was defined as SLEDAI score ≤ 4 and ≤ 7.5 mg/day glucocorticoids; patients not meeting these criteria were considered to have “higher” active disease. Data were compared between patients with low and higher disease activity. Logistic regression estimated a propensity score for SLE based on demographic and clinical characteristics. Propensity score matched analyses compared HCRU, patient-reported outcomes, income loss and treatment satisfaction in patients with low disease activity versus higher active disease.
Results
Data from 296 physicians reporting on 730 patients (46 low disease activity, 684 higher active disease), and from 377 patients’ self-reported questionnaires (24 low disease activity, 353 higher active disease) were analyzed. Flaring in the previous 12 months was 2.6-fold more common among patients with higher versus low active disease. Equation 5D-3 L utility index was 0.79 and 0.88 and FACIT-Fatigue scores were 34.78 and 39.79 in low versus higher active disease patients, respectively, indicating better health and less fatigue, among patients with low versus higher active disease. Absenteeism, presenteeism, overall work impairment, and total activity impairment were 47.0-, 2.0-, 2.6- and 1.5-fold greater in patients with higher versus low disease activity. In the previous 12 months there were 28% more healthcare consultations and 3.4-fold more patients hospitalized in patients with higher versus low disease activity.
Conclusion
Compared to SLE patients with higher active disease, patients with low disease activity experienced better health status, lower HCRU, less fatigue, and lower work productivity impairment, with work absenteeism being substantially lower in these patients.
Similar content being viewed by others
Introduction
Assessments of disease activity and response to therapy in systemic lupus erythematosus (SLE) have to take into account the complex, multi-organ manifestations of SLE [1], and the course of the disease over time, including the possibility of disease flares [2]. Several measures have been developed and validated to assess SLE disease activity in an appropriate and reproducible manner, including the British Isles Lupus Assessment Group (BILAG) index [3, 4], the Systemic Lupus Activity Measure (SLAM) [4, 5], and the Systemic Lupus Activity Questionnaire (SLAQ) [6]. The Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) assesses disease activity in the past 30 days using 24 weighted clinical and laboratory descriptors across nine organs/systems [7,8,9]. Modified versions of the SLEDAI include the SLEDAI-2000 (SLEDAI-2 K) [8, 9] and a version developed for use in the Safety of Estrogens in Lupus National Assessment study, the SELENA-SLEDAI [10]. We use the SLEDAI-2 K in this study, hereafter referred to as just SLEDAI.
Achieving good disease control, as evidenced via a disease activity measure, would be expected to improve a patient’s outcomes and result in lower healthcare resource utilization (HCRU) [11, 12]. European clinical guidelines recommend treatment goals in SLE of remission or low disease activity [13], and a definition of a Lupus Low Disease Activity State (LLDAS) that includes a SLEDAI score of ≤ 4 and prednisone ≤ 7.5 mg/d has been developed by an international panel of SLE experts [14,15,16]. As both the SLEDAI and the LLDAS have been employed primarily in clinical trials and longitudinal observational studies, [17, 18], exploring the relationship of SLEDAI scores and low disease activity with real-world patient outcomes is of interest.
The objective of the current study, therefore, was to quantify the effect of achieving low disease activity in a real-world patient cohort on the clinical, patient and economic impact of SLE. To achieve this, we compared flaring, patient-reported outcomes (PRO), HCRU, and income loss in SLE patients between those with low disease activity and higher active disease.
Methods
Study design and data collection
This was an analysis of data from the Adelphi Real World Lupus Disease Specific Programme™ (DSP), collected in the United States of America (USA) and Europe in 2010, 2013 and 2015, these data did not follow the same patients, but rather were comprised of three separate cohorts. Data from these three years were then aggregated for analyses in this study. The Lupus DSP is a real-world, non-interventional, point-in-time survey of rheumatologists and their patients with SLE; the full DSP methodology has been published previously [19].
Rheumatologists from a broad geographical area in the USA and five European countries (France, Germany, Spain, Italy and the United Kingdom [UK]) were identified from publicly available lists and invited to participate in the DSP if they were actively managing patients with SLE and saw ≥ 5 patients with SLE in a typical month. A total of 754 participating rheumatologists completed a patient record form for the next 5 consecutively consulting patients with SLE aged ≥ 18 years. Data recorded on the form included patient demographic and clinical characteristics, physician-defined current disease severity (mild, moderate or severe), most recent SLEDAI score, SLE management history, flaring, healthcare professional (HCP) consultations and hospitalizations during the 12 months prior to data collection.
Patients for whom a patient record form had been completed were invited to complete a patient self-completion form. The self-completion form included PRO questionnaires assessing health status, flare, fatigue and its impact, and productivity.
The definition of flare in SLE has not yet been universally decided or accepted [20], therefore, flare was defined in this study based on the clinical judgement of managing physicians. We collected data on the number of flares patients had experienced in the last 12 months as well as whether the patient was experiencing a flare at the time of consultation. As with flares, disease severity was based on the clinical opinion of the managing rheumatologist, thus a physician global assessment. Data on which classification criteria rheumatologists used to make their judgement of disease severity were not collected during this study.
Patient-reported outcome questionnaires
The EuroQoL 5D-3 L (EQ-5D-3 L), a widely used generic measure of health status [21, 22], consists of two parts: the descriptive system, which assesses health in five dimensions (Mobility, Self-care, Usual Activities, Pain / Discomfort, and Anxiety / Depression), each of which has three levels of response (no problems, some problems, extreme problems / unable to do), and the EQ-VAS, a 20 cm visual analog scale (VAS) on which the patient rates their perceived health from 0 (the worst imaginable health) to 100 (the best imaginable health). Application of country-specific scoring algorithms to the scores of the five domains provides a single health utility index, with a value of 1 indicating perfect health, a value of 0 indicating death, and a value of < 0 indicating a health state worse than death [23, 24].
The Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) scale assesses fatigue and its impact on daily activities and functioning in chronic disease [25, 26]. The questionnaire includes 13 items such as tiredness, weakness, listlessness, lack of energy, and the impact of these feelings on daily functioning (e.g., sleeping, and social activities). Each item is scored between 0 and 5, and all items contribute equally to the sum score, with 0 being the worst possible sum score and 52 being the best possible score, indicating no fatigue. The content validity and psychometric properties of the FACIT-Fatigue scale have been established in numerous chronic conditions, including SLE [27,28,29].
The Work Productivity and Activity Impairment-Lupus questionnaire (WPAI-Lupus) assesses the impact of SLE on work productivity and activity impairment over the past 7 days [30]. The questionnaire comprises 6 items, and results in the generation of scores for absenteeism, presenteeism, overall work impairment, and total activity impairment, each expressed as a percentage of work time missed or percentage impairment.
Statistical analyses
LLDAS is defined as a SLEDAI score ≤ 4 (with no activity in renal, central nervous, gastrointestinal, or cardiopulmonary systems, and no vasculitis, fever, or hemolytic anemia), a SELENA-SLEDAI physician global assessment ≤ 1, receiving ≤ 7.5 mg/day glucocorticoid, immunosuppressant or approved biologics only at well-tolerated standard maintenance doses, and demonstrating no new lupus disease activity compared with the previous assessment [14, 15]. In our analysis, a variation of the LLDAS, low disease activity, was defined as a SLEDAI score of ≤ 4 and a glucocorticoid dose of ≤ 7.5 mg/day. This definition of low disease activity was used to reflect the LLDAS as closely as possible, given that not all data included in the published definition were available. By contrast, higher active disease was defined as a SLEDAI score of > 4 or a glucocorticoid at a dose of > 7.5 mg/day. Patients were compared based on this objective assessment of disease activity, rather than subjective physician-reported disease severity.
Descriptive analyses of patient demographics and clinical characteristics were performed for the total study population and stratified by whether they had low disease activity or not. Means and standard deviations were calculated for continuous variables, and frequency counts and percentages for categorical variables. Results were compared using chi-squared tests for categorical variables, Fisher’s exact test for 2-by-2 categorical variables, and Mann-Whitney tests or t-tests for numeric variables.
To calculate the impact of disease activity on income, patient’s reported incomes were converted to US dollars for consistency, taking into account country, age and/or sex (where appropriate) [31,32,33,34,35,36]. Loss of income was calculated for each patient as the product of salary and WPAI absenteeism, presenteeism, and overall work impairment. Mean income loss was then compared in those with and without low disease activity.
-
Due to the observational nature of the data, any observed significant difference in an outcome using a bivariate test between two groups (e.g., a t-test) may be due to confounding factors. Propensity score matching is a statistical matching technique that attempts to balance pre-specified covariates between patient groups through the use of the propensity score (a measure of how likely a patient is to belong to either group, based on the covariates used) [37]. Therefore, analyses to compare HCRU, PRO scores and income loss in patients with low disease activity versus higher active disease were conducted utilizing propensity score matching. Low disease activity and higher active disease patient groups were matched on age, sex and ethnicity. Propensity scores were estimated using a logistic regression model. Patients in the low disease activity group were matched 1:1, with replacement and allowing for ties, to patients in the higher active disease group. A caliper was not applied. The balance in covariates between groups, following propensity score matching, was assessed by calculating standardized mean differences (SMDs), with an SMD between − 0.1 and 0.1 (not inclusive) taken to be indicative of adequate balance [38]. The treatment effect, or difference in outcomes between groups, was computed by taking the average of the difference between the outcomes in matched patients. The Abadie-Imbens standard error and the corresponding test statistic and associated p-value were also calculated [39]. Propensity score matching was repeated three times, for analyses of the following groups of variables: (1) physician-reported HCRU outcomes; (2) patient-reported EQ-5D-3 L utility index, FACIT-Fatigue scores and WPAI total activity impairment; (3) patient-reported (when employed) WPAI absenteeism, presenteeism, and overall work impairment (with their dollar adjusted equivalents).
Sensitivity analyses
To further investigate the definition of disease activity levels, patients were grouped according to the criteria for classifying them as having active disease. As glucocorticoids are used to manage short-term increases in disease activity (flares), patients receiving high dose glucocorticoids might not be considered to have higher active disease in general. The base case analyses were therefore re-run excluding patients classified as having active disease because they were receiving > 7.5 mg/day of glucocorticoid, rather than due to their SLEDAI score. All analyses were conducted in Stata v16.0 [40].
Results
Patients
Complete patient record forms were provided by 296 physicians for a total of 730 patients: 166 from the USA and 564 from Europe (France, n = 132; Germany, n = 96; Spain, n = 99; Italy, n = 113; UK, n = 124). Of these patients, 46 had low disease activity and 684 had higher active disease.
Patients with low disease activity and higher active disease were comparable for age, proportions of each sex, and time since diagnosis (Table 1). The proportion of non-White patients was numerically higher in the group with active disease than the group with low disease activity, although this difference was not statistically significant. Although disease severity as defined subjectively by physicians indicated greater severity in patients with higher active disease than those with low disease activity (p < 0.05), more than half of patients classified as having higher active disease were considered by their physicians to have mild SLE, and just over 20% of patients classified with low disease activity were assessed subjectively by their physicians to have moderate or severe SLE. In total, 94% of patients with higher active disease were receiving steroids at the time of data collection compared with 37% of patients with low disease activity, and more than two thirds of all patients had received steroids at some point in their treatment history. Overall, 4.1% of patients were receiving biologics, with a higher percentage of patients with low disease activity (6.5%) compared with higher active disease (3.9%) receiving these. Of these, 10 (1.4%) patients were receiving belimumab, with no significant difference in belimumab prescription between low and higher active disease groups (p = 0.4806).
Flaring
The likelihood of having a flare and the mean number of flares in the 12 months prior to data collection were both 2.6-fold higher in patients with higher active disease than those with low disease activity (Fig. 1a and b; both p < 0.01).
Disease activity in patients that flared in the past 12 months. Association of disease activity with (a) Patients flaring in past 12 months (b) Number of flares in past 12 months. Patients were considered to have low disease activity if they had a SLEDAI score of ≤ 4 and were receiving a glucocorticoid dose of ≤ 7.5 mg/day; patients were considered to have active disease if they had a SLEDAI score of > 4 or were receiving a glucocorticoid at a dose of > 7.5 mg/day. CI, confidence interval; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index
Patient-reported outcomes and loss of income
Of those patients with data available to assess their disease activity, 377 (51.6% of total) completed a patient self-completion form, 88 from the USA and 289 from Europe (France, n = 78; Germany, n = 78; Spain, n = 48; Italy, n = 46; UK, n = 39). Of these 377 patients, 24 had low disease activity and 353 had higher active disease.
Propensity score matching for EQ-5D-3 L utility index, FACIT-Fatigue sum score and WPAI total activity impairment achieved balance for potential confounding variables (Additional file 2). Propensity score matching for WPAI absenteeism, presenteeism, overall work impairment, and lost income due to these achieved balance for age; however, propensity score matching was unable to balance groups for proportions of males and females and ethnicity, with SMDs of 18.1% and 14.8%, respectively, for these variables (Additional file 3). Post-matching compared with pre-matching, in the group with active disease, mean patient age was lower and there were higher proportions of male patients and White patients (Additional files 2 and 3).
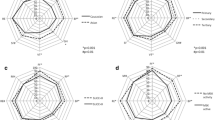
The mean EQ-5D-3 L utility index score in patients with low disease activity was 0.88 compared with 0.79 in patients with higher active disease, indicating better health status in patients with low disease activity compared to those with higher active disease (Fig. 2a; p < 0.05). Patients with low disease activity also reported lower levels of fatigue, with mean FACIT-Fatigue sum score of 39.79 compared with 34.78 in patients with higher active disease (Fig. 2b; p < 0.05). Patients with higher active disease also had 47-fold greater mean percentage absenteeism (Fig. 2c; p < 0.05), 2-fold greater mean percentage presenteeism (Fig. 2c; p < 0.001), 2.6-fold greater mean percentage overall work impairment (Fig. 2c; p < 0.001), and 1.5-fold greater mean percentage total activity impairment (Fig. 2c; p = 0.072) compared with patients with low disease activity.
Patient-reported outcomes by disease activity. Association of disease activity with PROs and income (a) EuroQoL-5D-3 L,a (b) Functional Assessment of Chronic Illness Therapy-Fatigue,b (c) Work Productivity and Activity Impairment-Lupus questionnaire, (d) Annual loss of income. a Higher scores on the EuroQoL-5D-3 L indicate better health status. b Higher scores on the Functional Assessment of Chronic Illness Therapy-Fatigue indicate lower levels of fatigue. *WPAI total activity impairment: Low disease activity (N = 24); Active disease (N = 24). Patients were considered to have low disease activity if they had a SLEDAI score of ≤ 4 and were receiving a glucocorticoid dose of ≤ 7.5 mg/day; patients were considered to have active disease if they had a SLEDAI score of > 4 or were receiving a glucocorticoid at a dose of > 7.5 mg/day. CI, confidence interval; EQ-5D-3 L, EuroQoL-5D-3 L; FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy-Fatigue; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; WPAI, Work Productivity and Activity Impairment questionnaire
Additionally, those patients with higher active disease had 37-fold, 1.9-fold and 2.1-fold higher annual loss of income associated with absenteeism (Fig. 2d; p < 0.05), presenteeism (Fig. 2d; p < 0.05) and overall work impairment (Fig. 2d; p < 0.01), compared to those with low disease activity.
Healthcare resource utilization
Propensity score matching for HCP consultations and hospitalizations achieved balance for all variables, with an SMD in the range of -10–10% (Additional file 1). In the previous 12 months, the numbers of consultations with HCPs was 28% lower in patients with low disease activity compared with patients with higher active disease (Fig. 3; p < 0.01) and the percentage of patients hospitalized at least once was 3.4-fold higher in patients with higher active disease compared with patients with low disease activity (Fig. 3; p < 0.01).
Healthcare resource utilization in past 12 months by disease activity. Patients were considered to have low disease activity if they had a SLEDAI score of ≤ 4 and were receiving a glucocorticoid dose of ≤ 7.5 mg/day; patients were considered to have active disease if they had a SLEDAI score of > 4 or were receiving a glucocorticoid at a dose of > 7.5 mg/day. CI, confidence interval; HCP, healthcare professional; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index
Sensitivity analyses
Excluding patients who were classified as having higher active disease solely due to receiving > 7.5 mg/day of glucocorticoid reduced the number of patients in the higher active disease group from 684 to 453. Results of the sensitivity analyses were broadly in line with those from the main analyses. The likelihood of having a flare and mean number of flares in the previous 12 months were 2.8-fold and 3.5-fold lower, respectively, in patients with low disease activity than those with higher active disease. In patients with low disease activity, the mean EQ-5D-3 L utility index score was 0.88 and the mean FACIT-Fatigue sum score 39.79 compared with 0.79 and 34.78, respectively, in patients with higher active disease, indicating better health status and lower fatigue levels in patients with low disease activity compared with patients with higher active disease. The mean percentages of absenteeism, presenteeism, overall work impairment, and total activity impairment were 25-fold, 2-fold, 2.2-fold and 1.6-fold lower, respectively, in patients with low disease activity versus higher active disease. A 27-fold, 1.7-fold and 1.8-fold lower annual loss of income was associated with absenteeism, presenteeism, and overall work impairment, respectively, in patients with low disease activity than those with higher active disease. The numbers of consultations with HCPs and the percentage of patients hospitalized in the previous 12 months were 1.4-fold and 5.7-fold lower, respectively, in patients with low disease activity compared with patients with higher active disease. All statistically significant differences between low disease activity and higher active disease groups found in the main analyses were reflected in the sensitivity analyses, except for absenteeism, for which there was no significant difference.
Discussion
We analyzed real-word data from a large cohort of patients with SLE, collected from both the patients and their managing physicians, in the USA and Europe. Our findings demonstrated that patients achieving a low disease activity at a given point in time had lower previous flaring rates, fatigue, productivity impairment (in particular, lower levels of absenteeism), income loss and HCRU, and better health status, compared with patients who had higher active disease or had not achieved low disease activity during the same time.
Our finding that low disease activity was associated with lower levels of flaring over the last 12 months has been previously reported in a prospective cohort study across 13 centers in eight Asia Pacific countries, in which low disease activity was defined via the LLDAS [15].
We showed low disease activity to be associated with better health status, as assessed with the EQ-5D-3 L. The mean EQ-5D-3 L utility index of 0.79 that we observed among those with higher active disease was below population norms reported for females from Europe (0.89) and the USA (0.85), indicating that our analysis cohort (which consisted of almost 90% females) had poorer health status than the general population. The mean EQ-5D-3 L health utility index of 0.88 for patients with low disease activity suggests health status on a par with the general population [24]. Our finding that patients with low disease reported an average EQ-5D-3 L score 0.09 points higher than patients with higher disease suggests there is likely to be an observable difference in health-related quality of life (HRQoL) in these patients as this difference is higher than 0.07, the minimal clinically important difference (MCID) for this measure in SLE [41]. While few studies have examined EQ-5D-3 L scores in relation to disease activity in SLE, there are two studies that found an association of overall health status with disease activity. In the first study they reported modest correlations between SLEDAI score and EQ-5D-3 L VAS in Canada [42], and similar findings were reported in a second study in China between SLEDAI score and EQ-5D-3 L utility index [43]. A Swedish study also reported a significant association of EQ-5D-3 L utility index and disease activity, although in that study disease activity was measured using the patient-reported SLAQ and glucocorticoid dose [44].
Irreversible damage (as assessed by Systemic Lupus International Collaborating Clinics [SLICC] score/ American College of Rheumatology [ACR] damage index) is widely recognized to be associated with poorer quality of life, with the type of organ involvement potentially affecting disability and work ability differently [45, 46]. For example, a past study found that organ damage, specifically musculoskeletal damage, was negatively correlated with HRQoL, particularly the physical and social aspects [46]. A second study reported similar findings, with skin and joint involvement leading to a higher proportion of the patients experiencing activity impairment [45], indicating that patients with this type of organ damage should be examined and monitored closely in clinical practice [46].
Other PROs have been utilized in studies investigating the relationship of disease activity in SLE with HRQoL. A cross-sectional Brazilian study showed higher disease activity as assessed via the SLEDAI in women with SLE to be associated with poorer HRQoL assessed with the World Health Organization Quality of Life Group (WHOQOL-100) assessment instrument [47]. A Swiss cohort study reported similar findings, with increases in disease activity assessed by the SELENA-SLEDAI negatively correlated with the physical and mental component summaries of the SF-36, signifying reduced HRQoL [48].
We observed a mean FACIT-Fatigue sum score of 39.79 in patients with low disease activity and a mean sum score of 34.78 among those with higher active disease, these were both lower than the mean of 43.6 reported for > 1,000 and > 2,400 members of the general population in the US and Germany [25, 49]. This indicates that fatigue in both groups of SLE patients is worse than in the general population. Findings in the literature on the association between SLE disease activity and fatigue severity are mixed, with two published US studies reporting no significant association of disease activity with fatigue assessed [50, 51] while two studies observed similar findings as we reported here, with fatigue being worse in patients with more severe disease [52, 53]. One study exploring MCIDs in SLE reported that FACIT-F scores among patients experiencing “much more fatigue” differed from those feeling “somewhat more fatigued” by 15.0 points which is larger than the difference we found between patients with low and higher disease activity [54] suggesting that this difference in score may not have an impact on patient HRQoL.
We saw a much lower impact on productivity, and consequently on income, in patients with low disease activity. Absenteeism was only 0.17%, with a consequent income loss of $63/year, in patients with low disease activity, but in patients with higher active disease these were close to 8% and more than $2,300/year. When presenteeism was also considered, the difference between patients with low disease activity and higher active disease was even more marked: overall work impairment and annual income loss were 14% and $5,400 in patients with low disease activity but more than doubled to 37% and $11,400 in patients with higher active disease.
In an analysis of data from a large population-based US SLE cohort, overall work impairment assessed with the WPAI was correlated with disease activity assessed with the SLAQ [55]. Higher self-reported disease severity was also directly associated with higher levels of absenteeism and presenteeism in a survey of patients with SLE in the USA [56]. An analysis of a large cohort of US SLE patients reported that disease activity, assessed via the SLAQ, was a significant predictor of reduced work productivity [57]. Similarly, a study based in Germany found that high levels of disease activity as well as fatigue, pain and poor mental health and physical functioning were associated with impairment in work productivity and daily activities [58]. However, findings on the effect of disease activity on fatigue are mixed: a study of patients in Italy experiencing remission (no disease activity) for at least five years reported improvements in many of the physical components of HRQoL, including physical functioning and bodily pain, compared with those who had had shorter remission periods or were unremitted, but no differences in fatigue levels between these groups [59]. Published studies have also shown the indirect costs of SLE, including lost earnings, to be related to disease activity [57, 60]. Collectively, these data underscore the importance of disease activity in the burden of illness in SLE from the patient perspective.
In our study, patients with low disease activity had 28% fewer HCP consultations in the 12 months prior to data collection, compared with patients with higher active disease. In the 12 previous months, only 4% of patients with low disease activity were hospitalized, compared with 15% of those with higher active disease. The lower HCRU that we observed in patients with low disease activity is consistent with previous published studies. Reductions in disease activity assessed with the SLEDAI were associated with reductions in HCRU and healthcare costs in a retrospective longitudinal study of SLE patients in the USA [61]. Analysis of a cohort of SLE patients from various regions of Sweden reported indirect and direct healthcare costs (which relate to HCRU) around 50% higher in patients with a SLEDAI score ≥ 4 compared with patients with SLEDAI score < 4 [62]. In an SLE patient cohort from a single tertiary hospital in Australia, patients in LLDAS for ≥ 50% of the observation period incurred significantly lower (by 26%) annual direct healthcare costs than those in LLDAS for < 50% of the time [12]. A retrospective, observational cohort study of patients with SLE from the Japan Medical Data Center claims database identified increasing HCRU and cost with increasing disease severity, with disease severity defined based on an algorithm that included SLEDAI score with a number of other parameters [63].
We observed discrepancies between subjective physician-reported disease severity and objectively defined disease activity, with 58% of patients classified as having higher active disease being considered by their physicians to have mild SLE. Excluding patients defined as having higher active disease on the basis of receiving glucocorticoid at a dose of > 7.5 mg/day reduced the patients classified as having higher active disease but considered by their physicians to have mild SLE to 48%, but this is still a marked misalignment of these means of assessing SLE. The lack of clear alignment of subjective physician-reported disease severity with the disease activity groups defined for the purposes of this analysis highlighted the challenges of assessing disease activity in SLE. One possible explanation for this misalignment, and a limitation of the SLEDAI, is that the presence of laboratory features alone, which could be chronic and not necessarily require treatment, may give patients a high disease activity score. The differences demonstrated in flaring, PROs and HCRU between patients with low disease activity and higher active disease even when including patients that physicians considered to have mild SLE in the active disease group indicated that the criteria used in defining disease activity, including SLEDAI score and receipt of high-dose glucocorticoid, are associated with the patient-reported and economic burden of SLE.
This study found an association between low disease activity and improved patient outcomes, functionality and HCRU. Although the clinical real-world effectiveness of biologic therapy fell outside the current scope, past studies have reported an association between advanced therapies, and improved patient outcomes. For example, treatment with belimumab has been shown to have a positive impact on HRQoL by improving physical functioning and fatigue [64]. In general, biologic agents have shown positive effects on HRQoL in randomized clinical trials [65]. However, of the total cohort, just 10 patients were receiving belimumab in our study.
There were potential limitations to our study. Despite the large number of study participants, missing data (particularly PRO data) resulted in small sample sizes being available for some analyses. Data were included for the next five consecutively consulting patients; the study sample was therefore pseudo-random, rather than truly random, and the study population could include a high proportion of patients who consult their physician more frequently than is typical in SLE, and who may be atypical in other ways. The point-in-time nature of the survey allowed assessment of the association between factors but precluded assessment of causality. Our methodology relied on accurate recall and reporting by physicians and patients, and missing data were expected but may have influenced results—always a challenge in this type of study. While the mean age and preponderance of females in our dataset reflected published demographics for SLE, our study population included a high proportion of White patients, although globally SLE has a higher prevalence in other ethnic groups. As non-White SLE patients have a higher likelihood of developing severe disease with poor outcomes [66, 67] caution should be exercised in extrapolation of these findings to a broader population.
Our data source did not include some of the data used to define low disease activity in the published definition of LLDAS [14, 15], and we were therefore not able to classify patients based exactly on that definition. However, patients that we defined as having higher active disease had a SLEDAI score ≥ 4 and/or were receiving ≥ 7.5 mg/day glucocorticoid, and therefore would be excluded from the LLDAS group based on the published definition. A total of 23 patients that we classified as having low disease activity were receiving an immunosuppressant or biologic, but insufficient data were available to ascertain whether all of these therapies were at well-tolerated standard maintenance doses, as required in the published definition of LLDAS.
As the definition of a flare in SLE has not yet been universally accepted, we defined flare in this study based on the clinical judgement of managing physicians, this may mean that different physicians classified the presence or absence of a flare using different criteria. Similarly, the classification criteria physicians used to make their judgement on whether a patient was experiencing a flare were not collected during this study. Because we collected data on both the number of flares patients had experienced in the last 12 months and whether the patient was experiencing a flare at the time of consultation, the outcome of a flare was, in part, retrospective, flares experienced in the last 12 months could have impacted the SLEDAI score at the time of evaluation. Similarly, disease severity was physician-defined based on their subjective assessment of the consulting patient and therefore there may be some variation in how physicians classify severity of SLE in this study.Finally, we recorded the most recent SLEDAI score recorded by the physician for each patient, this may have been collected up to six months prior to the day of the consultation.
Despite the limitations, real-world studies play an important role in exploring areas of concern that cannot be addressed in randomized clinical trials. Patients included in clinical trials represent only a small proportion of the consulting population as a result of the stringent eligibility criteria that patients are required to meet to be involved, for example, patients tend to be younger and without comorbidities [68]. Similarly, patients treated in the real-world setting may be less likely to be adherent to medication than those included in clinical trials and therefore better reflect outcomes of patients in the real world [69] As a result, data from real-world studies can complement clinical trials and provide insight into the effective of interventions in patients commonly seen in clinical practice.
Our analysis of a large real-world patient cohort from multiple countries and two continents provided insight into the association of clinical, patient-reported and economic outcomes with disease activity in SLE. Our findings indicated that patients with low disease activity experienced significantly better health status, less fatigue, and lower levels of productivity impairment, and were less burdensome to the healthcare system than those with higher active disease. We are hopeful that with an established definition of low SLE disease activity in place, there will be a useful guide for treatment goals and an aid to assess drugs in development and their potential to improve patient outcomes and reduce HCRU.
Data Availability
All data that support the findings of this study are the intellectual property of Adelphi Real World. All requests for access should be addressed directly to Ben Hoskin at ben.hoskin@adelphigroup.com.
References
Suarez-Avellaneda A, Quintana JH, Aragon CC, Gallego LM, Gallego CN, Bolanos JD, Ochoa MAG, Granados ME, Ruiz-Ordonez M. Systemic lupus erythematosus in the intensive care unit: a systematic review. Lupus. 2020;29(11):1364–76.
Tselios K, Gladman DD, Touma Z, Su J, Anderson N, Urowitz MB. Disease course patterns in systemic lupus erythematosus. Lupus. 2019;28(1):114–22.
Isenberg DA, Gordon C, Group BGBILA. From BILAG to BLIPS–disease activity assessment in lupus past, present and future. Lupus. 2000;9(9):651–4.
Mikdashi J, Nived O. Measuring disease activity in adults with systemic lupus erythematosus: the challenges of administrative burden and responsiveness to patient concerns in clinical research. Arthritis Res Ther. 2015;17:183.
Bae SC, Koh HK, Chang DK, Kim MH, Park JK, Kim SY. Reliability and validity of systemic lupus activity measure-revised (SLAM-R) for measuring clinical disease activity in systemic lupus erythematosus. Lupus. 2001;10(6):405–9.
Romero-Diaz J, Isenberg D, Ramsey-Goldman R. Measures of adult systemic lupus erythematosus: updated version of british Isles Lupus Assessment Group (BILAG 2004), european Consensus Lupus Activity measurements (ECLAM), systemic lupus activity measure, revised (SLAM-R), systemic lupus activity questionnaire for Population Studies (SLAQ), systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), and systemic Lupus International collaborating Clinics/American college of rheumatology damage index (SDI). Arthritis Care Res (Hoboken). 2011;63(Suppl 11):37–46.
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630–40.
Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288–91.
Touma Z, Urowitz MB, Ibanez D, Gladman DD. SLEDAI-2K 10 days versus SLEDAI-2K 30 days in a longitudinal evaluation. Lupus. 2011;20(1):67–70.
Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, Lockshin M, Merrill JT, Belmont HM, Askanase AD, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353(24):2550–8.
Hammond ER, Desta B, Near AM, Wang X, Jiang M. Frequency, severity and costs of flares increase with disease severity in newly diagnosed systemic lupus erythematosus: a real-world cohort study, United States, 2004–2015. Lupus Sci Med 2021, 8(1).
Yeo AL, Koelmeyer R, Kandane-Rathnayake R, Golder V, Hoi A, Huq M, Hammond E, Nab H, Nikpour M, Morand EF. Lupus Low Disease Activity State and reduced Direct Health Care costs in patients with systemic Lupus Erythematosus. Arthritis Care Res (Hoboken). 2020;72(9):1289–95.
Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, Cervera R, Doria A, Gordon C, Govoni M, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78(6):736–45.
Franklyn K, Lau CS, Navarra SV, Louthrenoo W, Lateef A, Hamijoyo L, Wahono CS, Chen SL, Jin O, Morton S, et al. Definition and initial validation of a Lupus Low Disease Activity State (LLDAS). Ann Rheum Dis. 2016;75(9):1615–21.
Golder V, Kandane-Rathnayake R, Huq M, Nim HT, Louthrenoo W, Luo SF, Wu Y-JJ, Lateef A, Sockalingam S, Navarra SV, Zamora L, Hamijoyo L, Katsumata Y, Harigai M, Chan M, O’Neill S, Goldblatt F, Lau CS, Li ZG, Hoi A, Nikpour M, Moran EF. For Asia-Pacific Lupus collaboration: Lupus low disease activity state as a treatment endpoint for systemic lupus erythematosus: a prospective validation study. Lancet Rheumatol. 2019;1:e95–102.
van Vollenhoven RF, Bertsias G, Doria A, Isenberg D, Morand E, Petri MA, Pons-Estel BA, Rahman A, Ugarte-Gil MF, Voskuyl A et al. 2021 DORIS definition of remission in SLE: final recommendations from an international task force. Lupus Sci Med 2021, 8(1).
Morand EF, Trasieva T, Berglind A, Illei GG, Tummala R. Lupus Low Disease Activity State (LLDAS) attainment discriminates responders in a systemic lupus erythematosus trial: post-hoc analysis of the phase IIb MUSE trial of anifrolumab. Ann Rheum Dis. 2018;77(5):706–13.
Schwarting A, Schroeder JO, Alexander T, Schmalzing M, Fiehn C, Specker C, Perna A, Cholmakow-Bodechtel C, Koscielny VB, Carnarius H. First Real-World Insights into Belimumab Use and Outcomes in Routine Clinical Care of systemic Lupus Erythematosus in Germany: results from the OBSErve Germany Study. Rheumatol Ther. 2016;3(2):271–90.
Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: Disease-Specific Programmes - a means to understand. Curr Med Res Opin. 2008;24(11):3063–72.
Petri M, Buyon J, Kim M. Classification and definition of major flares in SLE clinical trials. Lupus. 1999;8(8):685–91.
EuroQol Group. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208.
Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–43.
Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D). Br J Rheumatol. 1997;36(5):551–9.
Szende A, Oppe M, Devlin N. EQ-5D value sets: Inventory, comparative review and user guide. Netherlands: Springer; 2007.
Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528–38.
Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63–74.
Kosinski M, Gajria K, Fernandes AW, Cella D. Qualitative validation of the FACIT-fatigue scale in systemic lupus erythematosus. Lupus. 2013;22(5):422–30.
Lai JS, Beaumont JL, Ogale S, Brunetta P, Cella D. Validation of the functional assessment of chronic illness therapy-fatigue scale in patients with moderately to severely active systemic lupus erythematosus, participating in a clinical trial. J Rheumatol. 2011;38(4):672–9.
Strand V, Simon LS, Meara AS, Touma Z. Measurement properties of selected patient-reported outcome measures for use in randomised controlled trials in patients with systemic lupus erythematosus: a systematic review. Lupus Sci Med 2020, 7(1).
Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4(5):353–65.
Salary and Cost of Living Comparison [Internet]. Available from: http://www.salaryexplorer.com.
OECD. OECD Statistics [Internet]. Available from: https://stats.oecd.org.
Statista. The statistics portal for market data, market research and market studies [Internet]. Available from: https://www.statista.com.
NBC. International: Top News And Analysis [Internet]. CNBC. Available from: https://www.cnbc.com.
Deutsche Welle. News and current affairs from Germany and around the world | DW [Internet]. Available from: https://www.dw.com.
The Local - Germany’s News in English [Internet]. Available from: https://www.thelocal.de//.
Rosenbaum P, Rubin DB. The Central Role of the Propensity score in Observational Studies for Causal Effects. Biometrika. 1983;70:41–55.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107.
Abadie A, Imbens G. Matching on the estimated propensity score. Econometrica. 2016;84:781–807.
StataCorp. Stata Statistical Software: release 16. In. College Station. TX: StataCorp LLC; 2019.
Mikdashi J. Measuring and monitoring health-related quality of life responsiveness in systemic lupus erythematosus patients: current perspectives. Patient Relat Outcome Meas. 2018;9:339–43.
Wang C, Mayo NE, Fortin PR. The relationship between health related quality of life and disease activity and damage in systemic lupus erythematosus. J Rhuematol. 2001;28(3):525–32.
Wang SL, Wu B, Zhu LA, Leng L, Bucala R, Lu LJ. Construct and criterion validity of the Euro Qol-5D in patients with systemic lupus erythematosus. PLoS ONE. 2014;9(6):e98883.
Bexelius C, Wachtmeister K, Skare P, Jonsson L, Vollenhoven R. Drivers of cost and health-related quality of life in patients with systemic lupus erythematosus (SLE): a swedish nationwide study based on patient reports. Lupus. 2013;22(8):793–801.
Bjork M, Dahlstrom O, Wettero J, Sjowall C. Quality of life and acquired organ damage are intimately related to activity limitations in patients with systemic lupus erythematosus. BMC Musculoskelet Disord. 2015;16:188.
Takase YIT, Doi H, Tsuji H, Hashimoto M, Ueno K, Inaba R, Kozuki T, Taniguchi M, Tabuchi Y, Watanabe R, Kitagori K, Akizuki S, Murakami K, Nakashima R, Yoshifuji H, Itaya T, Yamamoto W, Uozumi R, Tanaka M, Ohmura K, Morinobu A. Correlation between irreversible organ damage and the quality of life of patients with systemic lupus erythematosus: the Kyoto Lupus Cohort survey. Lupus. 2021;30(10):1577–85.
dos Reis MG, da Costa IP. Health-related quality of life in patients with systemic lupus erythematosus in Midwest Brazil. Rev Bras Reumatol. 2010;50(4):408–22.
Chaigne B, Chizzolini C, Perneger T, Trendelenburg M, Huynh-Do U, Dayer E, Stoll T, von Kempis J, Ribi C, Swiss Systemic Lupus Erythematosus Cohort Study G. Impact of disease activity on health-related quality of life in systemic lupus erythematosus - a cross-sectional analysis of the swiss systemic Lupus Erythematosus Cohort Study (SSCS). BMC Immunol. 2017;18(1):17.
Montan I, Lowe B, Cella D, Mehnert A, Hinz A. General Population norms for the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scale. Value Health. 2018;21(11):1313–21.
Azizoddin DR, Gandhi N, Weinberg S, Sengupta M, Nicassio PM, Jolly M. Fatigue in systemic lupus: the role of disease activity and its correlates. Lupus. 2019;28(2):163–73.
Jump RL, Robinson ME, Armstrong AE, Barnes EV, Kilbourn KM, Richards HB. Fatigue in systemic lupus erythematosus: contributions of disease activity, pain, depression, and perceived social support. J Rheumatol. 2005;32(9):1699–705.
Rendas-Baum R, Baranwal N, Joshi AV, Park J, Kosinski M. Psychometric properties of FACIT-Fatigue in systemic lupus erythematosus: a pooled analysis of three phase 3 randomised, double-blind, parallel-group controlled studies (BLISS-SC, BLISS-52, BLISS-76). J Patient Rep Outcomes. 2021;5(1):33.
Arnaud L, Gavand PE, Voll R, Schwarting A, Maurier F, Blaison G, Magy-Bertrand N, Pennaforte JL, Peter HH, Kieffer P, et al. Predictors of fatigue and severe fatigue in a large international cohort of patients with systemic lupus erythematosus and a systematic review of the literature. Rheumatology (Oxford). 2019;58(6):987–96.
Goligher EC, Pouchot J, Brant R, Kherani RB, Avina-Zubieta JA, Lacaille D, Lehman AJ, Ensworth S, Kopec J, Esdaile JM, et al. Minimal clinically important difference for 7 measures of fatigue in patients with systemic lupus erythematosus. J Rheumatol. 2008;35(4):635–42.
Drenkard C, Bao G, Dennis G, Kan HJ, Jhingran PM, Molta CT, Lim SS. Burden of systemic lupus erythematosus on employment and work productivity: data from a large cohort in the southeastern United States. Arthritis Care Res (Hoboken). 2014;66(6):878–87.
Garris C, Oglesby A, Sulcs E, Lee M. Impact of systemic lupus erythematosus on burden of illness and work productivity in the United States. Lupus. 2013;22(10):1077–86.
Panopalis P, Yazdany J, Gillis JZ, Julian L, Trupin L, Hersh AO, Criswell LA, Katz P, Yelin E. Health care costs and costs associated with changes in work productivity among persons with systemic lupus erythematosus. Arthritis Rheum. 2008;59(12):1788–95.
Kernder A, Dusing C, Richter J, Brinks R, Fischer-Betz R, Winkler-Rohlfing B, Aringer M, Schneider M, Chehab G. Factors detrimental to work productivity and daily activities in systemic lupus erythematosus patients - analysis of the german LuLa study. Lupus. 2021;30(12):1931–7.
Margiotta DPE, Fasano S, Basta F, Pierro L, Riccardi A, Navarini L, Valentini G, Afeltra A. The association between duration of remission, fatigue, depression and health-related quality of life in italian patients with systemic lupus erythematosus. Lupus. 2019;28(14):1705–11.
Meacock R, Dale N, Harrison MJ. The humanistic and economic burden of systemic lupus erythematosus: a systematic review. PharmacoEconomics. 2013;31(1):49–61.
Kan H, Guerin A, Kaminsky MS, Yu AP, Wu EQ, Denio A, Priti J, Narayanan S, Molta C. A longitudinal analysis of costs associated with change in disease activity in systemic lupus erythematosus. J Med Econ. 2013;16(6):793–800.
Jonsen A, Hjalte F, Willim M, Carlsson KS, Sjowall C, Svenungsson E, Leonard D, Bengtsson C, Rantapaa-Dahlqvist S, Pettersson S, et al. Direct and indirect costs for systemic lupus erythematosus in Sweden. A nationwide health economic study based on five defined cohorts. Semin Arthritis Rheum. 2016;45(6):684–90.
Tanaka Y, Mizukami A, Kobayashi A, Ito C, Matsuki T. Disease severity and economic burden in japanese patients with systemic lupus erythematosus: a retrospective, observational study. Int J Rheum Dis. 2018;21(8):1609–18.
Gomez A, Qiu V, Cederlund A, Borg A, Lindblom J, Emamikia S, Enman Y, Lampa J, Parodis I. Adverse health-related quality of life outcome despite adequate clinical response to treatment in systemic Lupus Erythematosus. Front Med (Lausanne). 2021;8:651249.
Gomez A, Parodis I. Do biological agents improve health-related quality of life in patients with systemic lupus erythematosus? Results from a systematic search of the literature. Autoimmun Rev. 2022;21(11):103188.
Lewis MJ, Jawad AS. The effect of ethnicity and genetic ancestry on the epidemiology, clinical features and outcome of systemic lupus erythematosus. Rheumatology (Oxford). 2017;56(suppl1):i67–i77.
Maningding E, Dall’Era M, Trupin L, Murphy LB, Yazdany J. Racial and ethnic differences in the prevalence and time to Onset of Manifestations of systemic Lupus Erythematosus: the California Lupus Surveillance Project. Arthritis Care Res (Hoboken). 2020;72(5):622–9.
Saunders C, Byrne CD, Guthrie B, Lindsay RS, McKnight JA, Philip S, Sattar N, Walker JJ, Wild SH, Scottish Diabetes Research Network Epidemiology G. External validity of randomized controlled trials of glycaemic control and vascular disease: how representative are participants? Diabet Med. 2013;30(3):300–8.
Hubbard TE, Paradis R. Real-World Evidence: A New Era for Health Care Innovation In.: The Network for Excellence in Health Innovation; 2015.
European Pharmaceutical Market Research Association (EphMRA). : Code of Conduct September 2019, 2020(29 May).
US Department of Health and Human Services: Summary of the HIPAA Privacy Rule. 2003, 2020(29 May).
Health Information Technology (HITECH). : Health Inform Technol Act. 2009, 2020(29 May).
DeTora LM, Toroser D, Sykes A, Vanderlinden C, Plunkett FJ, Lane T, Hanekamp E, Dormer L, DiBiasi F, Bridges D, et al. Good publication practice (GPP) Guidelines for Company-Sponsored Biomedical Research: 2022 update. Ann Intern Med. 2022;175(9):1298–304.
Acknowledgements
Medical writing support under the guidance of the authors was provided by Carole Evans, PhD on behalf of Adelphi Real World, and was funded by Johnson and Johnson, in accordance with Good Publication Practice (GPP) guidelines [73]. The FACIT and all related works are owned and copyrighted by, and the intellectual property of David Cella, Ph.D. Permission for use of the FACIT questionnaires is obtained by contacting Dr. Cella at information@facit.org.
Funding
Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Adelphi Lupus DSP. The analysis described here used data from the Adelphi Lupus DSP and was funded by Janssen. Janssen is one of multiple subscribers to the DSP. Janssen did not influence the original survey through either contribution to the design of questionnaires or data collection.
Author information
Authors and Affiliations
Contributions
BH, CA, DB and JP participated in the conception and design of the study; in the acquisition of data; analyzed and interpreted the data. BH, CA, DB, JP, ZT, KC, PB and CSK participated in manuscript writing and revision of drafts. All authors have reviewed the manuscript; given final approval of the version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Data collection was undertaken in line with European Pharmaceutical Marketing Research Association [70] guidelines and as such it does not require ethics committee approval. Each survey was performed in full accordance with relevant legislation at the time of data collection, including the US Health Insurance Portability and Accountability Act 1996 [71], and Health Information Technology for Economic and Clinical Health Act legislation. [72]. All patients gave written informed consent before any study-related procedures were performed.
Consent for publication
Not applicable.
Competing interests
Zahi Touma collaborated with Janssen employees on this study, has acted as a consultant for AstraZeneca, Biogen IDEC, EMD Serono, Pfizer Inc., GlaxoSmithKline, Janssen Inc., UCB, Eli Lilly and Sarkana Pharma. Karen Costenbader collaborated with Janssen employees on this study, has acted as a consultant for AstraZeneca and Neutrolis, and has research collaborations with Glaxo Smith Kline, Lilly, Exagen Diagnostics, Gilead, Amgen and Merck. Ben Hoskin, Christian Atkinson, David Bell, and James Pike are employed by Adelphi Real World. Chetan Karyekar is an employee and shareholder of Janssen pharmaceuticals. Pamela Berry is an employee and shareholder of Janssen pharmaceuticals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
41927_2023_355_MOESM1_ESM.docx
Additional file 1. Patient characteristics before and after propensity score matching for physician-reported outcomes. Patients were considered to have low disease activity if they had a SLEDAI score of ≤ 4 and were receiving a glucocorticoid dose of ≤ 7.5 mg/day; patients were considered to have active disease if they had a SLEDAI score of > 4 or were receiving a glucocorticoid at a dose of > 7.5 mg/day. SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; SMD, standardized mean difference.
41927_2023_355_MOESM2_ESM.docx
Additional file 2. Patient characteristics before and after propensity score matching for general health status and fatigue. Domains included: EQ-5D-3 L utility index, FACIT-fatigue sum score and WPAI total activity impairment. Patients were considered to have low disease activity if they had a SLEDAI score of ≤ 4 and were receiving a glucocorticoid dose of ≤ 7.5 mg/day; patients were considered to have active disease if they had a SLEDAI score of > 4 or were receiving a glucocorticoid at a dose of > 7.5 mg/day. EQ-5D-3 L, EuroQoL-5D-3 L; FACIT-fatigue, Functional Assessment of Chronic Illness Therapy-fatigue; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; SMD, standardized mean difference; WPAI, Work Productivity and Activity Impairment questionnaire
41927_2023_355_MOESM3_ESM.docx
Additional file 3. Patient characteristics before and after propensity score matching for work productivity and activity. Domains include: WPAI absenteeism, WPAI presenteeism, WPAI overall work impairment, lost income due to absenteeism, presenteeism, and overall work impairment. Patients were considered to have low disease activity if they had a SLEDAI score of ≤ 4 and were receiving a glucocorticoid dose of ≤ 7.5 mg/day; patients were considered to have active disease if they had a SLEDAI score of > 4 or were receiving a glucocorticoid at a dose of > 7.5 mg/day. EQ-5D-3 L, EuroQoL-5D-3 L; FACIT-fatigue, Functional Assessment of Chronic Illness Therapy-fatigue; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; SMD, standardized mean difference; WPAI, Work Productivity and Activity Impairment questionnaire.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article?s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article?s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Touma, Z., Costenbader, K.H., Hoskin, B. et al. Patient-reported outcomes and healthcare resource utilization in systemic lupus erythematosus: impact of disease activity. BMC Rheumatol 8, 22 (2024). https://doi.org/10.1186/s41927-023-00355-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41927-023-00355-6