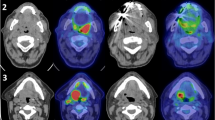
Abstract
Purpose
To determine the efficacy and safety of target volume determination by 18F-fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT) for intensity-modulated radiation therapy (IMRT) for locally advanced head and neck squamous cell carcinoma (HNSCC) extending into the oral cavity or oropharynx.
Methods
We prospectively treated 10 consecutive consenting patients with HNSCC using IMRT, with target volumes determined by PET-CT. Gross tumor volume (GTV) and clinical target volume (CTV) at the oral level were determined by two radiation oncologists for CT, magnetic resonance imaging (MRI), and PET-CT. Differences in target volume (GTVPET, GTVCT, GTVMRI, CTVPET, CTVCT, and CTVMRI) for each modality and the interobserver variability of the target volume were evaluated using the Dice similarity coefficient and Hausdorff distance. Clinical outcomes, including acute adverse events (AEs) and local control were evaluated.
Results
The mean GTV was smallest for GTVPET, followed by GTVCT and GTVMRI. There was a significant difference between GTVPET and GTVMRI, but not between the other two groups. The interobserver variability of target volume with PET-CT was significantly less than that with CT or MRI for GTV and tended to be less for CTV, but there was no significant difference in CTV between the modalities. Grade ≤ 3 acute dermatitis, mucositis, and dysphagia occurred in 55%, 88%, and 22% of patients, respectively, but no grade 4 AEs were observed. There was no local recurrence at the oral level after a median follow-up period of 37 months (range, 15–55 months).
Conclusions
The results suggest that the target volume determined by PET-CT could safely reduce GTV size and interobserver variability in patients with locally advanced HNSCC extending into the oral cavity or oropharynx undergoing IMRT.
Trial registration
UMIN, UMIN000033007. Registered 16 jun 2018, https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000037631
Similar content being viewed by others
Introduction
The advent of intensity-modulated radiation therapy (IMRT) for head and neck cancer has allowed a reduction in the radiation dose to risk organs, while maintaining the dose to the target volume (TV) (Pow et al. 2006; Kam et al. 2007). However, the dose distribution is steeper and more complex in IMRT than in conventional three-dimensional (3D) conformal radiation therapy, with the possibility of marginal recurrence (Schoenfeld et al. 2008; Eisbruch et al. 2004; Raktoe et al. 2013). Inter-institutional differences in treatment outcomes and adverse events (AEs) have also increased in the era of IMRT (Boero et al. 2016), suggesting that variables in target delineation could result in differences in clinical outcomes. More accurate and standardized TV determination and reduced interobserver variability are therefore needed for IMRT planning.
TV determination using 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) has been widely studied in patients with advanced head and neck squamous cell carcinoma (HNSCC). FDG-PET-based TVs are smaller than those obtained with computed tomography (CT) or magnetic resonance imaging (MRI), and have shown good agreement with local tumor extent determined by histopathology using surgically resected specimens (Lapa et al. 2021; Caldas-Magalhaes et al. 2012; Chatterjee et al. 2012; Daisne et al. 2004; Geets et al. 2006; Guido et al. 2009). Leclerc et al. reported that TV delineation based on FDG-PET could reduce the TV and radiation doses to the parotid gland and oral cavity, especially in patients with oropharyngeal and oral cancers (Leclerc et al. 2015). Other studies noted that TV determination by PET was particularly useful in cases with dental artifacts, suggesting that PET-based TV determination may be particularly useful in the oral cavity and oropharynx (Gardner et al. 2009; Anderson et al. 2014). Notably however, one study found no usefulness of TV determined by PET compared with MRI-based TV in patients with oropharyngeal cancer (Daisne et al. 2004). Furthermore, there is currently no uniform method for determining TV by PET. It has been suggested that TV delineation could be compromised by dental artifacts and may tend to be larger than it should be in tumors extending to the oral cavity or oropharynx, indicating the potential usefulness of PET-guided TV delineation for such tumors (Gardner et al. 2009). In the present study, we prospectively investigated the efficacy and safety of TV determination by FDG-PET using a multiple-threshold method in patients with HNSCC extending to the oral cavity or oropharynx (Okubo et al. 2010).
Materials and methods
Study design and data collection
We prospectively analyzed data for 10 consecutive patients with locally advanced HNSCC, in whom the primary site involved the oral level and who were treated with IMRT at Juntendo University Hospital. Extension to the oral level was defined as extension of the gross tumor volume (GTV) to the oral cavity or oropharynx. The study protocol was approved by the Juntendo hospital ethics committee (Approval no.: 18-0030), and the study was conducted in accordance with the principles of the Declaration of Helsinki. The eligibility criteria were as follows: patients aged ≥ 20 years; ECOG performance status ≤ 2; patients with locally advanced HNSCC lesions extending to the oral level with dental artifacts, including patients with de novo or locally recurrent advanced HNSCC without prior radiation therapy; and patients scheduled for definitive or postoperative radiotherapy with a total dose > 50 Gy. The exclusion criteria were patients with uncontrolled diabetes or co-morbidities deemed difficult to treat, at the discretion of the attending radiation oncologist. Staging was performed in accordance with the Union for International Cancer Control (8th edition), based on physical examination, laryngoscopic examination, CT, PET-CT, and/or MRI. For patients with recurrent disease, the stage of disease at the time of recurrence was registered. The primary endpoints were size of the TV and interobserver variability, and the efficacy of TV determination by PET compared with CT and MRI. The secondary endpoints included AEs of IMRT determined by PET and their clinical outcomes. We considered a positive result if the size of the TV and the interobserver variability were smaller with PET than those contoured based on other modalities, while maintaining acceptable local control without severe AEs, compared with previous studies of IMRT for HNSCC.
Acquisition of images from planning CT, PET-CT, and MRI
A Type-S head and shoulder mask (CIVCO Medical Solutions, Iowa, USA) was used as the immobilization device. Planning CT (Aquilion LB, Canon Medical Systems, Tochigi, Japan) was performed with a 2-mm slice thickness. No iodine contrast agent was administered in any of the patients.
PET-CT image acquisitions were performed using a Canon Celesteion PCA-9000A (Canon Medical Systems). Patients were injected with 185 MBq (5 mCi) of FDG, left in a designated “quiet room” for an uptake period of 60 min, and then placed on a specialized flat table for radiotherapy planning. Patients, with thermoplastic masks, were placed in the supine position and the head and neck area was scanned, followed by a full-body PET-CT scan for staging purposes. About 30 min were required to complete the full-body PET-CT scan. The PET-CT images were reviewed by an experienced nuclear medicine radiologist (K.M.) and a radiation oncologist (Y.K.).
CT scans were acquired in the helical mode with a slice thickness of 2 mm and a pitch of 15 mm at 120 kV and tube current volume exposure control. All CT images were acquired using a matrix of 512 × 512 pixels. Voxel dimensions were 0.8 mm × 0.8 mm × 2.0 mm. PET images were acquired in the 3D mode using an axial field of view of 400 mm (two bed positions). The time for the one-bed position (196 mm) scan was 240 s. All PET images were acquired using a matrix of 208 × 208 pixels. The full width at half maximum at a distance of 10 cm from the center of the field of view reached 5.1 mm × 5.2 mm × 5.4 mm in the x, y, and z directions, respectively. The Fourier rebinning algorithm was combined with an ordered subsets expectation–maximization reconstruction (Daisne et al. 2004). Voxel dimensions were 2.0 mm × 2.0 mm × 2.0 mm.
Most patients underwent MRI with a 1.5-Tesla system (Avanto; Siemens, Munich, Germany) employing the 3D-gradient echo technique. The region from the skull base to the inferior margin of the sternal end of the clavicle was examined with a head and neck combined coil. After intravenous injection of gadolinium contrast agent (Prohance; Bracco Japan, Tokyo, Japan) at a dose of 0.2 mmol/kg body weight, T1-weighted fat-suppressed axial, coronal, and sagittal sequences were performed sequentially. The section thicknesses and intersection gaps were 2 mm and 0.9 mm for the axial plane, respectively.
Volume delineation
Following all scans, the PET, CT, and MRI datasets were converted to the digital imaging and communication in medicine (DICOM) format and transferred to a radiotherapy planning system (Eclipse instrument; Varian Medical Systems, Palo Alto, CA, USA). To compare the CT-based or MRI-based TVs with the PET-based TVs, the GTV and clinical target volume (CTV) of primary lesions were contoured by two observers (K.S. with > 35 years of experience and Y.K. with > 10 years of experience in HNSCC imaging and radiation therapy) with no knowledge of the other modality images, to produce GTVPET, GTVCT, GTVMRI, CTVPET, CTVCT, and CTVMRI. The nodal GTVs and nodal CTVs were delineated on the planning CTs using international guidelines, with no aid from FDG-PET scan information (Gregoire et al. 2013). The multiple-threshold method was adopted for contouring GTVPET in this study (Okubo et al. 2010). Briefly, a threshold value of 2.5 standard uptake value (SUV) was adopted for primary tumors of ≤ 2 cm, and threshold values of 35% and 20% of the maximum FDG activity of primary tumors were adopted for primary tumors of 2–5 cm and > 5 cm, respectively. Each observer decided whether to include or exclude borderline FDG uptake in close proximity to primary lesions that were suspected of accumulating FDG due to normal physiological uptake or inflammation. CTVs usually consisted of an arbitrary 10 mm margin around the GTV, with corrections made to exclude anatomical barriers such as bones, muscles, or the oropharyngeal cavity, with reference to physical examination and fiber laryngoscopy findings. Only primary lesion contours at the oral level were compared in this study. Co-registration of each image was performed carefully by one co-author (Y.K.) using fusion software on Eclipse.
For actual treatment, the CTV of the primary lesion (CTVPET) was divided into two categories: CTV1 was the GTV with a 5 mm margin to account for anatomic barriers, and CTV2 was the CTV1 extended by an additional 5 mm, based on international guidelines (Gregoire et al. 2018). The plan target volumes were created by adding a uniform margin of 5 mm around the CTVs. Organs at risk, such as the spinal cord, brainstem, parotid glands, oral cavity, and larynx, were contoured for all patients and planning organs at risk volumes (PRVs) were created for serial organs, such as the spinal cord or brainstem. In cases with no direct tumor invasion to the spinal cord or brainstem, the observation of dose constraints for these PRVs was prioritized over TV coverage.
Treatment planning
The sequential boost method was employed in cases with nasopharyngeal carcinoma extending to the oropharynx, with the CTV of the primary tumor and metastatic lymph nodes irradiated with 70 Gy in 35 fractions and the prophylactic lymph node area irradiated with 50 Gy in 25 fractions. Cases with non-nasopharyngeal HNSCC were treated with the simultaneous integrated boost method, with 70 Gy to CTV1 of the primary tumor and CTV of metastatic lymph nodes, 60 Gy to CTV2, and 54 Gy to prophylactic lymph node areas in 35 fractions. IMRT was delivered with 6-MV photons using a TomoTherapy HD unit (Accuray Inc., Sunnyvale, CA, USA). The dose constraints are listed in Appendix 1.
Analysis of recurrence
Tumor recurrences were determined by clinical examination and CT, MRI, or FDG-PET imaging. Local control (LC), progression-free survival (PFS), and overall survival (OS) were calculated from the date of study registration to the date of the event using the Kaplan–Meier method. Patients alive at the time of analysis were censored at their last follow-up visit. The location of local failure was compared with the PET-based dose distribution. The recurrent volume was defined in previous studies as: “in-field”, “extending outside the field” or “out-of-field” if it had received ≥ 95%, 20%–95%, or < 20% of the prescribed dose, respectively (Leclerc et al. 2015).
Delineation agreement analysis
The Dice similarity coefficient (DSC) was employed as a standard and intuitive metric for comparison in this study. Computation of the DSC involved doubling the overlap volume of the two given volumes (Voverlap) and subsequent division by the sum of the two volumes (V1, V2), as follows:
The DSC for the target volume was calculated by two observers (Y.K. and K.S.) for each modality, with an ideal value of 1. A value > 0.6 (or 0.8) was generally deemed to be very good (Bland 2015); however, clinical interpretation was challenging due to the greater tolerance of DSC for the same absolute error in larger volumes compared with smaller volumes. In addition to DSC, we therefore also determined the pairwise Hausdorff distance (HD) to compare agreement in absolute terms, independent of volume. HD is defined as the maximum distance from a point in one set to the nearest point in another set; a higher HD between two sets indicates the existence of a pocket of dissimilarity between the two sets, while a zero HD indicates that the sets are identical (Cignoni and Scopigno 1998).
Statistical analysis
The volumes determined for each modality and the interobserver variability (DSC, HD) were analyzed with Wilcoxon’s signed rank sum test. Factors associated with the DSC and HD of GTV, namely the primary site, stage, location of dental artifacts, distance between GTV and the dental artifacts, and size of GTV were analyzed. Fisher’s exact test and Spearman’s rank correlation coefficient test were used to analyze the correlations between the factors and the interobserver variability. AEs were assessed and documented according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. AEs occurring within 3 months after treatment were defined as acute AEs. All statistical analyses were assessed at a significance level of 0.05 using JMP 12 (SAS Institute; Minato-ku, Tokyo, Japan).
Results
Patient characteristics and treatment
Ten consecutive patients with HNSCC who received IMRT between March 2019 and July 2021 were included in this prospective study. Eight patients underwent MRI. One patient refused to continue treatment due to severe acute radiation-induced mucositis and their treatment was terminated at 46 Gy in 23 fractions. This patient was included in the analysis of TVs but excluded from the analysis of clinical outcomes. The patients’ characteristics are shown in Table 1. All nine patients who completed treatment received a radiation dose of 70 Gy in 35 fractions. Regarding the treatment modalities, two patients received radiation alone, four received concurrent chemoradiation with cisplatin, and four received intra-arterial chemoradiation for sinonasal cancer (Kosugi et al. 2021).
Clinical results
One patient died and eight were alive after a median follow-up period of 37 months (range, 15–55 months). Two patients were lost to follow-up at 10 and 13 months, respectively, both of whom were followed up with no recurrent or metastatic lesions but were subsequently lost to follow-up because of their advanced age and difficulty in attending the hospital. The 3-year LC, OS, and PFS rates were 88%, 88%, and 67%, respectively (Fig. 1). There was one local and two regional failures. Although the local recurrence occurred ‘‘in-field’’, it was not observed at the oral level, but the sinonasal cancer progressed at the skull base. The two regional recurrences were both outside the radiation field, including one patient who underwent salvage surgery with no subsequent disease progression, and one patient who underwent palliative irradiation and subsequently died of the disease.
AEs
The AEs associated with external radiotherapy with or without concurrent chemotherapy are summarized in Table 2. Grade ≤ 3 acute dermatitis, mucositis, and dysphagia were observed in 55%, 88%, and 22% of cases, respectively. No grade 4 AEs were observed.
Volume delineation
The GTV was compared in the 10 cases who underwent CT and PET-CT and in the eight who also underwent MRI. The GTV was smallest in GTVPET, followed by GTVCT and GTVMRI, with a significant difference between GTVPET and GTVMRI, but no significant difference between the other two groups. There was no significant difference between CTVPET, CTVCT, and CTVMRI (Fig. 2).
Box-and-whisker plots of GTV (a) and CTV (b) determined for each modality. The bottom and top of the boxes denote the 25th and 75th percentiles, respectively; line inside the box shows the median; crossmark shows the average; ends of the whiskers denote the maximum and minimum. Comparisons were made between each two groups with N = 10 and N = 8 comparable individuals. GTVmean (range) for each modality, GTVPET was 18.3 (0.8–90.3) cm3 for N = 10 and 18.4 (0.8–90.3) cm3 for N = 8, GTVCT was 23.3 (0.7–74.0) cm3 for N = 10 and 24.0 (1.9–74.0) cm3 for N = 8, GTVMRI was 33.9 (4.0–101.8) cm3 for N = 8. CTVmean (range) for each modality, CTVPET was 64.7 (13.0–216.6) cm3 for N = 10 and 62.5 (13.0–216.6) cm3 for N = 8, CTVCT was 64.7 (12.2–180.0) cm3 for N = 10 and 64.2 (12.2–180.0) cm3 for N = 8, CTVMRI was 86.0 (21.1–211.3) cm3 for N = 8
Delineation agreement
The DSC for GTVPET was 1, which was significantly higher than that for GTVCT or GTVMRI. Similarly, the DSC for CTVPET was significantly higher than that for CTVCT or CTVMRI (Fig. 3). HD was significantly smaller for GTVPET than for the other modalities, and also tended to be smaller for CTVPET (Fig. 4). DSC and GTVCT showed significant correlations (ρ = 0.63, P = 0.04), and HD and GTVMRI also showed significant correlations (ρ = 0.96, P = 0.0001). The associations of DSC and HD with other factors, such as primary site, de novo or recurrent disease, and distance between GTV and dental artifacts, were not clear (Tables 3 and 4).
Box-and-whisker plots of DSC of GTV (a) and CTV (b) determined for each modality. Comparisons were made between each two groups with N = 10 and N = 8 comparable individuals. DSCmean (range) of GTV for each modality, DSC of GTVPET was 1.0 (1.0–1.0) for N = 10 and N = 8, DSC of GTVCT was 0.45 (0–0.77) for N = 10 and 0.49 (0–0.77) for N = 8, DSC of GTVMRI was 0.52 (0.16–0.76) for N = 8. DSCmean (range) of CTV for each modality, DSV of CTVPET was 0.92 (0.86–0.99) for N = 10 and 0.92 (0.86–0.99) for N = 8, DSC of CTVCT was 0.63 (0–0.86) for N = 10 and 0.66 (0–0.86) for N = 8, DSC of CTVMRI was 0.69 (0.58–0.85) for N = 8. DSC = Dice similarity coefficient
Box-and-whisker plots of HD of GTV (a) and CTV (b) determined for each modality. Comparisons were made between each two groups with N = 9, N = 8, and N = 7 comparable individuals. HDmean (range) of GTV for each modality, HD of GTVPET was 0 (0–0) for N = 8 and N = 7, HD of GTVCT was 1.24 (0.64–2.10) cm for N = 8 and 1.25 (0.64–2.10) cm for N = 7, HD of GTVMRI was 1.25 (0.57–2.90) cm for N = 7. HDmean (range) of CTV for each modality, HD of CTVPET was 0.97 (0.58–1.40) cm for N = 9 and 0.91 (0.58–1.40) cm for N = 7, HD of CTVCT was 1.24 (1.00–1.57) cm for N = 9 and 1.22 (1.00–1.49) cm for N = 7, DSC of CTVMRI was 1.60 (0.71–3.05) cm for N = 7. HD = Hausdorff distance
Discussion
Accurate TV determination and reduced interobserver variability are important factors in IMRT radiotherapy planning. For head and neck IMRT, primary site contouring guidelines have been published to equalize TV among observers (Gregoire et al. 2018); however, the availability and implementation of guidelines alone are not sufficient to ensure uniform delineation (Veen et al. 2019). The guidelines also state that FDG-PET may be useful for delineating TV in patients with locally advanced HNSCC; unfortunately however, TV determination using FDG-PET has not become common practice. We suggest two main reasons for this: first, there is currently no uniform method for determining TV using FDG-PET (Okubo et al. 2010), and second, there are few reports on the long-term safety of TV determined by FDG-PET, especially for IMRT (Leclerc et al. 2015; Matsuura et al. 2017; Wang et al. 2006; Vernon et al. 2008). Previous guidelines also stated that “PET volumes should preferably be delineated using user-independent segmentation algorithms”, but the SUV threshold for automatic TV delineation has not been standardized and various values have been proposed. Furthermore, the use of a single threshold for lesions of various sizes may increase the risk of overestimating or underestimating lesions. Hosono et al. proposed a multiple-threshold method based on lesion size to resolve the risk of a single threshold, and also reported the long-term clinical results of radiotherapy for TV determined by this method (Okubo et al. 2010; Matsuura et al. 2017). In the current study, we investigated the usefulness and safety of IMRT for TV by PET using a multiple-threshold method for lesions at the oral level, where the usefulness of TV determination by PET is controversial (Daisne et al. 2004; Leclerc et al. 2015; Okubo et al. 2010).
We performed IMRT for TV by PET in 10 patients and treatment was completed in nine patients. Regarding safety, the 3-year LC, OS, and PFS of patients who completed treatment were 88%, 88%, and 67%, respectively, which were comparable to those in previous studies of oropharyngeal cancer and maxillary sinus cancer (Eisbruch et al. 2010; Gillison et al. 2019; Homma et al. 2023). Local recurrence occurred in one patient “in-field”, but this was sinonasal carcinoma that progressed at the skull base following skull base extension before treatment, with no recurrent lesions at the oral level, and the TV determination was facilitated by PET. In addition to de novo lesions, patients with recurrent lesions were also included in this study and were treated with IMRT for TV determined by PET, with no local recurrence. This represents an important result, because there have been no previous reports on the safety of TV by PET for recurrent lesions. All patients in the present study, except for one case of nasopharyngeal carcinoma, were irradiated with high-doses of CTV1, a 5 mm extension of GTV, according to international guidelines (Gregoire et al. 2018). For recurrent cases however, the guidelines recommend extensions > 5 mm from the GTV for CTV, which is in the high-dose area due to anatomical structural disruption. TV determination by PET using a multistep method may safely reduce the high-dose irradiation area, even in recurrent cases. In the current study, the high-dose (70 Gy) irradiated area at the oral cavity level was reduced by an average of 11.7 (range: 0.3–31.2) cm3 in PET compared with MRI, indicating that the high-dose irradiated area was reduced by 5.9% (range; 0.1%–13.2%) of the oral cavity volume (data not shown). AEs ≤ grade 3 were less common than in previous studies of chemoradiotherapy for head and neck cancer (Gillison et al. 2019; Homma et al. 2023). Regarding TVs, GTVPET was significantly smaller than GTVMRI, in contrast with previous findings (Daisne et al. 2004). This apparent discrepancy may be due to differences in the GTVPET imaging algorithm and fusion accuracy between MRI and PET-CT. GTVPET was smaller than GTVCT, but the difference was not significant. This may be because all patients enrolled in this study had dental artifacts, and CT images were more difficult to visualize in this group. In fact, compared with the other modalities, the interobserver variability for GTVCT was larger, the DSC was smaller, and HD was larger. Given that MRI has been reported to be more useful than CT for TV determination in patients with metallic dental implants, we believe that our results provide positive information regarding TV determination by PET (Gardner et al. 2009). The results for CTV were not significantly different among the different modalities, although CTVPET had the smallest volume. Previous studies of HNSCC reported that local recurrence usually occurred in the high-dose area, highlighting the need for accurate delineation of the GTV (Leclerc et al. 2015). DSC and HD, as measures of interobserver variability, showed less difference in PET than in the other modalities for both GTV and CTV. Interestingly, the DSC of GTVPET was 1, indicating precise matching between the two observers. At the oral level, physiologic or inflammatory accumulation of FDG in close proximity to the GTV, specifically physiologic accumulation in the tonsils or inflammatory accumulation due to dental caries, could result in interobserver variability in GTVPET (Haerle et al. 2013); however, the current results showed perfect agreement in GTVPET by combining the multiple-threshold method, physical examination, and endoscopic examination. These results suggest that TV determination by PET could safely reduce the high-dose irradiation area and also reduce interobserver variability. Regarding the factors associated with DSC and HD in GTVCT and GTVMRI, GTV showed a significant correlation, in accordance with previous studies (Veen et al. 2019). In contrast, there was no correlation with clinical stage, site of primary lesion, location of dental artifacts, or distance between the GTV and dental artifacts. This might be because the usefulness of TV determination by PET at the oral level was compounded not only by visibility due to dental artifacts, but also by the fusion accuracy between modalities due to mandibular mobility or neck curvature and anatomic complexity, which may not have been a significant factor in this limited number of cases (Gardner et al. 2009; Anderson et al. 2014). Identifying the factors responsible for the high interobserver variability of TV in CT and MRI, which may in turn make PET-CT more effective in determining TV, could help to resolve the problems of accessibility for PET-CT and limited medical resources. A larger prospective study with a unified TV delineation method by PET is needed to clarify this.
The study had several limitations. First, the number of patients was very small, and the primary sites were limited to the oropharynx, nasopharynx, and paranasal sinus among the HNSCC. Therefore, caution should be exercised when interpreting the results of this study. Second, although the single energy metal artifact reduction algorithm has demonstrated usefulness for CT metallic artifact reduction and PET-MRI with regard to cases with dental artifacts, we were unable to use it in this study (Funama et al. 2015; Huellner 2021); however, the usefulness of TV determination by PET at the oral level may be influenced by factors other than dental artifacts. Third, FDG-PET is not recommended for superficial lesions. The lack of spatial resolution of the PET camera with the partial volume effect does not allow a sufficiently accurate delineation of TV. It is therefore essential to set the TV not only by PET-CT, but also by physical examination and fiber findings. Fourth, this study did not use an iodine contrast agent in CT scans, although the international contouring guidelines recommend the use of a contrast agent (Gregoire et al. 2018); however, the greater usefulness of MRI compared with CT for TV determination has been reported in cases with dental artifacts, and we do not believe that this will affect the current results in terms of the usefulness of PET (Gardner et al. 2009; Anderson et al. 2014). Fifth, no post-treatment quality of life studies were conducted, so the impact of IMRT of the TV determined by FDG-PET using a multiple-threshold method on it is unknown. Further study is needed regarding FDG-PET-based TV determination and long-term clinical outcomes, including patients’ quality of life such as salivary gland function or swallowing function.
Conclusions
Carrying out IMRT of the TV determined by FDG-PET using a multiple-threshold method could safely reduce the GTV and interobserver variability in patients with HNSCC lesions extending to the oral level.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author.
Abbreviations
- IMRT:
-
Intensity-modulated radiation therapy
- TV:
-
Target volume
- 3D:
-
Three-dimensional
- AEs:
-
Adverse events
- FDG-PET:
-
Fluorodeoxyglucose positron emission tomography
- HNSCC:
-
Head and neck squamous cell carcinoma
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- GTV:
-
Gross tumor volume
- ECOG:
-
Eastern cooperative oncology group (with reference to patient performance status)
- DICOM:
-
Digital imaging and communication in medicine
- CTV:
-
Clinical target volume
- SUV:
-
Standard uptake value
- PRVs:
-
Planning organs at risk volumes
- LC:
-
Local control
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- DSC:
-
Dice similarity coefficient
- HD:
-
Hausdorff distance
References
Anderson CM, Sun W, Buatti JM, Maley JE, Policeni B, Mott SL et al (2014) Interobserver and intermodality variability in GTV delineation on simulation CT, FDG-PET, and MR images of head and neck cancer. Jacobs J Radiat Oncol 1(1):006
Bland M (2015) An introduction to medical statistics
Boero IJ, Paravati AJ, Xu B, Cohen EE, Mell LK, Le QT et al (2016) Importance of radiation oncologist experience among patients with head-and-neck cancer treated with intensity-modulated radiation therapy. J Clin Oncol 34(7):684–690
Caldas-Magalhaes J, Kasperts N, Kooij N, van den Berg CA, Terhaard CH, Raaijmakers CP et al (2012) Validation of imaging with pathology in laryngeal cancer: accuracy of the registration methodology. Int J Radiat Oncol Biol Phys 82(2):e289–e298
Chatterjee S, Frew J, Mott J, McCallum H, Stevenson P, Maxwell R et al (2012) Variation in radiotherapy target volume definition, dose to organs at risk and clinical target volumes using anatomic (computed tomography) versus combined anatomic and molecular imaging (positron emission tomography/computed tomography): intensity-modulated radiotherapy delivered using a tomotherapy Hi Art machine: final results of the VortigERN study. Clin Oncol 24(10):e173–e179
Cignoni PRC, Scopigno R (1998) Metro: measuring error on simplified surfaces. Comput Graph Forum 17:167–174
Daisne JF, Duprez T, Weynand B, Lonneux M, Hamoir M, Reychler H et al (2004) Tumor volume in pharyngolaryngeal squamous cell carcinoma: comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology 233(1):93–100
Eisbruch A, Marsh LH, Dawson LA, Bradford CR, Teknos TN, Chepeha DB et al (2004) Recurrences near base of skull after IMRT for head-and-neck cancer: implications for target delineation in high neck and for parotid gland sparing. Int J Radiat Oncol Biol Phys 59(1):28–42
Eisbruch A, Harris J, Garden AS, Chao CK, Straube W, Harari PM et al (2010) Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00–22). Int J Radiat Oncol Biol Phys 76(5):1333–1338
Funama Y, Taguchi K, Utsunomiya D, Oda S, Hirata K, Yuki H et al (2015) A newly-developed metal artifact reduction algorithm improves the visibility of oral cavity lesions on 320-MDCT volume scans. Phys Med 31(1):66–71
Gardner M, Halimi P, Valinta D, Plantet MM, Alberini JL, Wartski M et al (2009) Use of single MRI and 18F-FDG PET-CT scans in both diagnosis and radiotherapy treatment planning in patients with head and neck cancer: advantage on target volume and critical organ delineation. Head Neck 31(4):461–467
Geets X, Daisne JF, Tomsej M, Duprez T, Lonneux M, Gregoire V (2006) Impact of the type of imaging modality on target volumes delineation and dose distribution in pharyngo-laryngeal squamous cell carcinoma: comparison between pre- and per-treatment studies. Radiother Oncol 78(3):291–297
Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ et al (2019) Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 393(10166):40–50
Gregoire V, Ang K, Budach W, Grau C, Hamoir M, Langendijk JA et al (2014) Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol 110(1):172–81
Gregoire V, Evans M, Le QT, Bourhis J, Budach V, Chen A et al (2018) Delineation of the primary tumour Clinical Target Volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: AIRO, CACA, DAHANCA, EORTC, GEORCC, GORTEC, HKNPCSG, HNCIG, IAG-KHT, LPRHHT, NCIC CTG, NCRI, NRG Oncology, PHNS, SBRT, SOMERA, SRO, SSHNO. TROG Consensus Guidelines Radiother Oncol 126(1):3–24
Guido A, Fuccio L, Rombi B, Castellucci P, Cecconi A, Bunkheila F et al (2009) Combined 18F-FDG-PET/CT imaging in radiotherapy target delineation for head-and-neck cancer. Int J Radiat Oncol Biol Phys 73(3):759–763
Haerle SK, Hany TF, Ahmad N, Burger I, Huber GF, Schmid DT (2013) Physiologic [18F]fluorodeoxyglucose uptake of floor of mouth muscles in PET/CT imaging: a problem of body position during FDG uptake? Cancer Imaging 13(1):1–7
Homma A, Mikami M, Matsuura K, Onimaru R, Yoshida D, Shinomiya H et al (2023) Dose-finding and efficacy confirmation trial of the superselective intra-arterial infusion of cisplatin and concomitant radiotherapy for locally advanced maxillary sinus cancer (JCOG1212): results of the efficacy confirmation phase in patients with T4aN0M0: RADPLAT for T4aN0M0 Maxillary Sinus Cancer. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2023.11.031
Huellner MW (2021) PET/MR in head and neck cancer—an update. Semin Nucl Med 51(1):26–38
Kam MK, Leung SF, Zee B, Chau RM, Suen JJ, Mo F et al (2007) Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol 25(31):4873–4879
Kosugi Y, Kawamoto T, Oshima M, Fujimaki M, Ohba S, Matsumoto F et al (2021) Invasion of the pterygoid plates: an indicator for regional lymph node failure in maxillary sinus cancer. Radiat Oncol 16(1):2
Lapa C, Nestle U, Albert NL, Baues C, Beer A, Buck A et al (2021) Value of PET imaging for radiation therapy. Strahlenther Onkol 197(9):1–23
Leclerc M, Lartigau E, Lacornerie T, Daisne JF, Kramar A, Gregoire V (2015) Primary tumor delineation based on (18)FDG PET for locally advanced head and neck cancer treated by chemo-radiotherapy. Radiother Oncol 116(1):87–93
Matsuura T, Nishimura Y, Nakamatsu K, Kanamori S, Ishikawa K, Tachibana I et al (2017) Clinical outcomes of IMRT planned with or without PET/CT simulation for patients with pharyngeal cancers. Int J Clin Oncol 22(1):52–58
Okubo M, Nishimura Y, Nakamatsu K, Okumura M, Shibata T, Kanamori S et al (2010) Radiation treatment planning using positron emission and computed tomography for lung and pharyngeal cancers: a multiple-threshold method for [(18)F]fluoro-2-deoxyglucose activity. Int J Radiat Oncol Biol Phys 77(2):350–356
Pow EH, Kwong DL, McMillan AS, Wong MC, Sham JS, Leung LH et al (2006) Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 66(4):981–91
Raktoe SA, Dehnad H, Raaijmakers CP, Braunius W, Terhaard CH (2013) Origin of tumor recurrence after intensity modulated radiation therapy for oropharyngeal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 85(1):136–141
Schoenfeld GO, Amdur RJ, Morris CG, Li JG, Hinerman RW, Mendenhall WM (2008) Patterns of failure and toxicity after intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys 71(2):377–385
van der Veen J, Gulyban A, Nuyts S (2019) Interobserver variability in delineation of target volumes in head and neck cancer. Radiother Oncol 137:9–15
Vernon MR, Maheshwari M, Schultz CJ, Michel MA, Wong SJ, Campbell BH et al (2008) Clinical outcomes of patients receiving integrated PET/CT-guided radiotherapy for head and neck carcinoma. Int J Radiat Oncol Biol Phys 70(3):678–684
Wang D, Schultz CJ, Jursinic PA, Bialkowski M, Zhu XR, Brown WD et al (2006) Initial experience of FDG-PET/CT guided IMRT of head-and-neck carcinoma. Int J Radiat Oncol Biol Phys 65(1):143–151
Acknowledgements
We thank Susan Furness, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This study was supported by a Grant-in-Aid for Scientific Research (22K07779) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Contributions
YK and NM prepared the manuscript, conducted the literature search, and reviewed and edited the manuscript. YK, KS, NM, TK, YM, TK, MO, NO, JT, KI, SK, AI, NH, MF, SO, FM, KM, and NS reviewed the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This prospective analysis was approved by the ethics committee of Juntendo University (Approval no.: 18-0030). Informed consent was obtained from all individual participants included in the study.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Competing interests
KS received a research grant from Canon Medical Systems Corporation for the submitted paper. NM and NS received research funding from Chiyoda Technol, Boston Scientific, and Elekta outside of the submitted article. NO received research funding from Elekta outside of the submitted article. YK, TK, YM, TK, MO, JT, KI, SK, AI, NH, MF, SO, FM, and KM declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kosugi, Y., Sasai, K., Murakami, N. et al. Efficacy and safety of FDG-PET for determining target volume during intensity-modulated radiotherapy for head and neck cancer involving the oral level. EJNMMI Rep. 8, 6 (2024). https://doi.org/10.1186/s41824-024-00197-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41824-024-00197-6