Abstract
Positron emission tomography (PET) using O-(2-[18F]fluoroethyl)-L-tyrosine (18F-FET) PET has been shown to be a useful tool for differentiating radiation therapy outcomes, such as brain metastasis recurrence or radiation necrosis. We present the case of a female patient with brain metastases from pulmonary mucinous adenocarcinoma with suspicion of tumor recurrence on follow-up magnetic resonance imaging (MRI) after radiosurgery. 18F-FET PET/computed tomography (CT) was indicative of radiation necrosis. Due to the patient's medical history and the discrepancy between the brain MRI and PET/CT results, surgical biopsies were decided, which were positive for brain metastasis recurrence. The diagnosis of metastasis recurrence may also be challenging on 18F-FET PET/CT. In case of discrepancies between MRI and PET/CT results, false-negative 18F-FET PET/CT remains a possibility and requires careful follow-up or biopsy.
Similar content being viewed by others
Introduction
O-(2-[18F]fluoroethyl)-L-tyrosine PET (18F-FET) is an artificial amino acid taken up by upregulated tumor cells but not incorporated into proteins. 18F-FET positron emission tomography/computed tomography (PET/CT) is increasingly used in daily practice, especially in the imaging of primary brain tumors and metastatic lesions. It demonstrated very good performance for the initial assessment of patients with new, isolated, untreated brain lesions (Dunet et al. 2012). It has also proven to be useful for the characterization of post-radiotherapy lesion changes in a few retrospective studies, mainly limited by the mixed histological population and absence of pre-therapy imaging. We present a case of false-negative 18F-FET PET/CT examination in a patient with treated brain metastases from pulmonary mucinous adenocarcinoma. Treated metastases had low to moderate tracer avidity, and quantitative metrics were consistent with radionecrosis. There were initial concerns regarding the discordance between PET/CT and magnetic resonance imaging (MRI), which showed signs of tumor recurrence. Surgical biopsies were decided after multidisciplinary discussions and showed the lesion corresponding to the tumoral tissue residue. This case illustrates that published cut-off values of 18F-FET parameters might not be appropriate for evaluating treated brain metastases from every cancer type in daily practice.
Case report
A 58-year-old woman with cerebral metastases from lung cancer of the right upper lobe presented with the progression of lesion size on follow-up MRI. She was initially diagnosed with mucinous adenocarcinoma 4 years previously, which was confirmed after a bronchoscopic biopsy and staged cT1a (0.6 cm) cN2 (station 4R) cM1c (five brain metastases), stage IVB (according to the TNM 8th edition). Histopathological analyses demonstrated no EGFR, ERBB2, or BRAF mutations, no ALK/ROS1 rearrangement, or PD-L1 expression, but KRAS (G12S, exon 2) and TP53 (C275S, exon 8) mutations. Initial cerebral MRI findings were suggestive of metastatic disease.
The primary lung tumor was treated with radio-chemotherapy, which included four cycles of cisplatin-pemetrexed from January 2018 to March 2018 and concomitant radiotherapy in the right upper lobe and mediastinum with a total dose of 66 Gy (i.e., 33 fractions of 2 Gy) from February 2018 to March 2018. The brain metastases were treated in October 2018 with stereotactic radiotherapy (SRT) at a dose of 20 Gy in a single fraction. Two new metastases in the left frontal lobe (December 2018) and right frontal lobe (March 2019) were subsequently treated with SRT with a single dose of 20 Gy.
The patient was followed up with MRI scans every 2–3 months. In November 2019, the follow-up MRI showed an increase in the size of two treated lesions, one in the right frontal lobe and the other in the right parietal lobe, suggestive of radionecrosis, and a 2-month control was recommended according to the multidisciplinary tumor board. The subsequent MRI showed an increase in the size of the right frontal lesion with an enhancing soft tissue component in its posterior part, whose perfusion (nrCBV = 2.3, nrCBF = 2.8) and spectroscopy (Cho/Cr ratio = 2.9) parameters suggested the persistence of tumor residue in this region (Fig. 1).
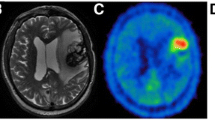
MRI and 18F-FET PET/CT findings. In our 58-year-old female patient, a follow-up brain MRI performed 16 months after radiosurgery showed an increase in the size of a treated right frontal lesion (white arrow) that appeared hyperintense on T2 weighted images (a), with central necrosis and peripheral thick contrast enhancement on T1 weighted images (b). The periphery of the lesion was bright on diffusion-weighted imaging (c) with moderately low ADC (d). On perfusion-weighted images, the nrCBV was 2.3 (e) and nrCBF was 2.8 (not shown) while MR spectroscopy (f) showed a high peak of choline with Cho/Cr ratio of 2.9, which overall indicated tumor residue. While 18F-FET PET/CT raw images showed moderate peripheral uptake (g, arrowhead), the maximum target-to-background ratio was 2.2 (i.e. lower than 2.55) and the time-activity-curve showed increasing uptake over time (h), which was interpreted as signs of radiation necrosis
18F-FET PET/CT was performed to further characterize these changes and showed a heterogeneous and low to moderate uptake of the radiotracer at the level of the right frontal lesion. The maximum tumor-to-brain ratio (TBRmax = lesion SUVmax divided by normal contralateral brain SUVmean) was 2.2 while the time-activity curve (TAC) displayed a cumulative pattern, a time-to-peak of > 40 min and a late phase slope of 0.178 SUV/h (estimated by linear regression on the 20–50 min post-injection frames) (Ceccon et al. 2017), which was overall in favor of a radionecrotic lesion (Fig. 1). No significant radiotracer uptake was observed in the other metastatic lesions treated with radiosurgery.
In this context, her case was re-discussed at the multidisciplinary brain metastases board, where surgical resection of the right frontal lesion was proposed.
Histopathological analyses of excisional biopsies of the lesion confirmed metastatic recurrence of her initial mucinous adenocarcinoma, while the dura mater biopsies showed no metastatic cells; however, revealed chronic calcified inflammatory reaction with foreign bodies.
Discussion
A current issue frequently encountered in the oncological management of brain metastases is differentiating tumor recurrence from treatment-related changes following stereotactic radiation therapy. Sometimes brain MRI does not always allow a clear differentiation between local brain tumor recurrence and progression from radiation-induced changes, including radiation necrosis. Radiation necrosis usually manifests within 6–12 months after radiotherapy treatment and occurs in approximately 5%–25% of all treated patients (Kumar et al. 2000; Shah et al. 2013). A similar rate is found in patients with brain metastases treated with radiosurgery (Minniti et al. 2011), taking into consideration that the rate could change according to the radiation total dose, field, number, and frequency of doses.
On MRI, a common characteristic of radiation necrosis on contrast-enhanced sequences is the geographic enhancement or Swiss cheese-like enhancement (Kumar et al. 2000). Nonetheless, conventional MRI is not always sufficient to differentiate tumor progression/recurrence from treatment-related effects (Mullins et al. 2005). Some sequences may have additive value in oncological imaging. Diffusion-weighted imaging, especially the apparent diffusion coefficient (ADC) sequences, can help differentiate tumor recurrence and radiation necrosis. Because of the low water mobility properties in high cellular lesions, the ADC will be low in the case of tumor recurrence. In contrast, an increased ADC is attributed to increased water mobility in cases of radiation necrosis (Asao et al. 2005). In addition, magnetic resonance spectroscopy (MRS) is an ongoing subject of research and may show that N-acetyl aspartate (NAA) and creatinine (Cr) decrease in cases of radiation necrosis, whereas high choline (Cho) levels are correlated with tumor recurrence (Rock et al. 2004; Sundgren et al. 2006). The Cho/Cr and Cho/NAA ratios have been described as good markers for differential diagnosis (Dowling et al. 2001; Plotkin et al. 2004). MR perfusion techniques using contrast enhancement can measure the relative cerebral blood volume (rCBV) and estimate vascularity and hemodynamics. Hyperperfusion is seen in tumor progression, and hypoperfusion is observed in radiation necrosis (Aronen and Perkio 2002; Ellika et al. 2007). It has been reported that rCBV values < 0.6 suggest radiation necrosis and values > 2.6 suggest tumor progression (Sugahara et al. 2000).
Several PET/CT tracers have been investigated as imaging modalities to distinguish the treatment effect from tumors in clinical practice (Galldiks et al. 2019). Among them, 18F-FET PET showed a high diagnostic performance using tumor-to-brain ratios and dynamic parameters with a sensitivity of 95% and specificity of 91% (Galldiks et al. 2012). Presently, the differentiation of radiation injury from metastasis recurrence using amino acid PET has been the most thoroughly investigated indication (Galldiks et al. 2019), repeatedly demonstrating high diagnostic accuracy. Galldiks et al. reported that the combined evaluation of the TBRmean of 18F-FET uptake and the pattern of the TAC can differentiate local brain metastasis recurrence from radionecrosis with high accuracy (Galldiks et al. 2012). This study and others were confirmed by Ceccon et al., who reported that the optimal cut-off value was a TBRmax > 2.55 (sensitivity = 83%, specificity = 85%), TBRmean > 1.95 (sensitivity = 86%, specificity = 88%), time-to-peak < 32.5 min (sensitivity = 58%, specificity = 73%), and TAC slope < 0.125 SUV/h (sensitivity = 68%, specificity = 61%) for identification of recurrence (Ceccon et al. 2017). It is worth mentioning that these results were obtained mainly from retrospective analyses in small cohorts and that there was no histological confirmation of the diagnosis in many cases. In addition, it remains poorly known whether all metastases from different primary tumors behave the same regarding 18F-FET uptake at baseline or during follow-up, which could limit the reproducibility of these preliminary reports. Some authors have reported a wide range of lesion uptake in either primary solid tumors or related brain metastases (Galldiks et al. 2012; Unterrainer et al. 2017; Pauleit et al. 2005). In particular, as in our case, adenocarcinoma from various organs often demonstrates low 18F-FET uptake (Galldiks et al. 2012; Pauleit et al. 2005). Other potential confounding factors should also be considered when using quantitative metrics such as tumor genotype, intratumoral hemorrhage, corticoid intake (Stegmayr et al. 2019), or immunotherapy and targeted therapy (Galldiks et al. 2021), which could modify the lesion, microenvironment, and normal brain uptake. Finally, one point to be considered in the differential diagnosis is that very small lesions in which the SUV may not be sufficient to reach the threshold value of 2.55 due to the partial volume effect. Further histological correlation and large prospective studies are now needed, especially to optimize 18F-FET uptake and TAC slope cut-off values according to primary tumor types to detect progression and optimize patient management.
Conclusions
In conclusion, the causes of false-negative 18F-FET uptake have not been well investigated, and actual evidence of this in the literature is scarce. This case demonstrates important teaching points for the tumor board team involved in the management of patients with brain metastases. In the event of inconsistent findings between MRI and 18F-FET PET/CT, care must be taken before the final diagnosis, especially for pulmonary mucinous adenocarcinoma. Collaborative discussion between radio-oncologists, radiologists, and nuclear physicians can help favor one diagnosis over the other. Another point is that although 18F-FET PET/CT is sensitive and specific for detecting post-treatment changes in brain metastases, it is important to be careful about the pattern of lesion uptake, as in our case.
Availability of data and material
All data generated or analysed during this study are included in this published article.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- Cho:
-
Choline
- Cr:
-
Creatinine
- 18F-FET:
-
O-(2-[18F]fluoroethyl)-L-tyrosine
- MRS:
-
Magnetic resonance spectroscopy
- NAA:
-
N-Acetyl aspartate
- PET/CT:
-
Positron emission tomography/ computed tomography
- rCBV:
-
Relative cerebral blood volume
- TAC:
-
Time activity curve
- TBR:
-
Tumor-to-brain ratio
References
Aronen HJ, Perkio J (2002) Dynamic susceptibility contrast MRI of gliomas. Neuroimaging Clin N Am 12(4):501–523
Asao C, Korogi Y, Kitajima M, Hirai T, Baba Y, Makino K et al (2005) Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am J Neuroradiol 26(6):1455–1460
Ceccon G, Lohmann P, Stoffels G, Judov N, Filss CP, Rapp M et al (2017) Dynamic O-(2–18F-fluoroethyl)-L-tyrosine positron emission tomography differentiates brain metastasis recurrence from radiation injury after radiotherapy. Neuro Oncol 19(2):281–288
Dowling C, Bollen AW, Noworolski SM, McDermott MW, Barbaro NM, Day MR et al (2001) Preoperative proton MR spectroscopic imaging of brain tumors: correlation with histopathologic analysis of resection specimens. AJNR Am J Neuroradiol 22(4):604–612
Dunet V, Rossier C, Buck A, Stupp R, Prior JO (2012) Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: a systematic review and metaanalysis. J Nucl Med 53(2):207–214
Ellika SK, Jain R, Patel SC, Scarpace L, Schultz LR, Rock JP et al (2007) Role of perfusion CT in glioma grading and comparison with conventional MR imaging features. AJNR Am J Neuroradiol 28(10):1981–1987
Galldiks N, Stoffels G, Filss CP, Piroth MD, Sabel M, Ruge MI et al (2012) Role of O-(2-(18)F-fluoroethyl)-L-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J Nucl Med 53(9):1367–1374
Galldiks N, Langen KJ, Albert NL, Chamberlain M, Soffietti R, Kim MM et al (2019) PET imaging in patients with brain metastasis-report of the RANO/PET group. Neuro Oncol 21(5):585–595
Galldiks N, Abdulla DSY, Scheffler M, Wolpert F, Werner JM, Hullner M et al (2021) Treatment monitoring of immunotherapy and targeted therapy using (18)F-FET PET in patients with melanoma and lung cancer brain metastases: initial experiences. J Nucl Med 62(4):464–470
Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE et al (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217(2):377–384
Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A et al (2011) Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 6:48
Mullins ME, Barest GD, Schaefer PW, Hochberg FH, Gonzalez RG, Lev MH (2005) Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol 26(8):1967–1972
Pauleit D, Stoffels G, Schaden W, Hamacher K, Bauer D, Tellmann L et al (2005) PET with O-(2–18F-Fluoroethyl)-L-Tyrosine in peripheral tumors: first clinical results. J Nucl Med 46(3):411–416
Plotkin M, Eisenacher J, Bruhn H, Wurm R, Michel R, Stockhammer F et al (2004) 123I-IMT SPECT and 1H MR-spectroscopy at 3.0 T in the differential diagnosis of recurrent or residual gliomas: a comparative study. J Neurooncol 70(1):49–58
Rock JP, Scarpace L, Hearshen D, Gutierrez J, Fisher JL, Rosenblum M et al (2004) Associations among magnetic resonance spectroscopy, apparent diffusion coefficients, and image-guided histopathology with special attention to radiation necrosis. Neurosurgery 54(5):1111–1117 (discussion 7–9)
Shah AH, Snelling B, Bregy A, Patel PR, Tememe D, Bhatia R et al (2013) Discriminating radiation necrosis from tumor progression in gliomas: a systematic review what is the best imaging modality? J Neurooncol 112(2):141–152
Stegmayr C, Stoffels G, Kops ER, Lohmann P, Galldiks N, Shah NJ et al (2019) Influence of dexamethasone on O-(2-[(18)F]-Fluoroethyl)-L-tyrosine uptake in the human brain and quantification of tumor uptake. Mol Imaging Biol 21(1):168–174
Sugahara T, Korogi Y, Tomiguchi S, Shigematsu Y, Ikushima I, Kira T et al (2000) Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol 21(5):901–909
Sundgren PC, Fan X, Weybright P, Welsh RC, Carlos RC, Petrou M et al (2006) Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imaging 24(9):1131–1142
Unterrainer M, Galldiks N, Suchorska B, Kowalew LC, Wenter V, Schmid-Tannwald C et al (2017) (18)F-FET PET uptake characteristics in patients with newly diagnosed and untreated brain metastasis. J Nucl Med 58(4):584–589
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
JP analyzed and interpreted the patient PET CT images and participate in writing the manuscript. MM and VD analyzed and interpreted the patients MRI examinations and were contributors in writing the manuscript. LS and SA were on charge of treating the patient by radiation, following her during the entire time. SA is the main author and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Ready if needed.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alshehri, S., Prior, J., Moshebah, M. et al. Negative 18F-FET PET/CT in brain metastasis recurrence: a teaching case report. European J Hybrid Imaging 5, 21 (2021). https://doi.org/10.1186/s41824-021-00115-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41824-021-00115-0