Abstract
Background
The Urgency Numeric Rating Scale (NRS) was developed as a content-valid single-item patient-reported outcome measure to assess severity of bowel urgency. Here, we evaluated the psychometric properties of the Urgency NRS.
Methods
Data were from a multicenter, randomized, placebo-controlled phase 3 trial in adults with moderately to severely active ulcerative colitis (NCT03518086). Patients completed the Urgency NRS using a daily electronic diary, from which weekly average Urgency NRS scores were calculated. Test–retest reliability, known-groups validity, construct validity, responsiveness, and score interpretation were assessed using the modified Mayo score, Inflammatory Bowel Disease Questionnaire (IBDQ), Patient Global Rating of Severity (PGRS), Patient Global Rating of Change (PGRC), and Geboes score.
Results
The study sample comprised 1,162 participants (40.2% female). Mean Urgency NRS score was higher (worse) at baseline than at week 12 (6.2 vs. 3.7). Test–retest reliability was strong, with intra-class correlation coefficients of 0.76–0.89. Baseline least-square mean Urgency NRS score was higher for participants with a PGRS score greater than the median (worse symptoms) than for those with a PGRS score less than or equal to the median (7.5 vs. 5.4; p < 0.0001), indicating good known-groups validity. Urgency NRS score was moderately correlated with IBDQ total and domain scores, PGRS, PGRC, and modified Mayo stool frequency, establishing its convergent validity. Correlations were weak for Geboes score and weak to moderate for modified Mayo endoscopic subscore and modified Mayo rectal bleeding, indicating that the Urgency NRS also had discriminant validity. Patients achieving clinical remission, clinical response, IBDQ remission, and PGRS score improvement showed significantly greater improvement on the Urgency NRS (p < 0.0001 for all), demonstrating responsiveness to change. A ≥ 3-point improvement in Urgency NRS score represented a meaningful improvement in bowel urgency and an Urgency NRS score of ≤ 1 point represented a bowel urgency remission threshold that was closely associated with clinical, endoscopic, and histologic remission.
Conclusions
The Urgency NRS is a valid and reliable patient-reported outcome measure that is suitable for evaluating treatment benefits in clinical trials in patients with moderately to severely active ulcerative colitis.
Similar content being viewed by others
Background
Ulcerative colitis (UC) is a chronic disease of unknown etiology that is characterized by inflammation of the colon and rectum. Common symptoms of UC include blood in the stool, diarrhea, and bowel urgency [1]. Bowel urgency is highly bothersome, and reducing its severity has been identified as a key factor influencing patients’ treatment decisions [2]. Clinical guidelines also identify bowel urgency as an important disease-related symptom signifying the severity of disease activity and recommend control of urgency as part of the management of UC [3]. However, bowel urgency has not been specifically assessed in clinical trials of UC treatments. Moreover, until recently, no validated patient-reported outcome (PRO) measures were available for specifically assessing changes in its severity resulting from treatment of UC.
To address this, we developed a new PRO measure, the Urgency Numeric Rating Scale (NRS), through a targeted literature review, concept elicitation interviews with UC patients, and expert input [4, 5]. Content validity of the Urgency NRS was previously established through cognitive interviews with adult UC patients [4, 5]. The aims of the present study were to evaluate the reliability, validity, and responsiveness of the instrument in a clinical trial setting; to identify a score change representing clinical meaningful improvement in bowel urgency; and to identify a bowel urgency severity threshold associated with inactive disease or remission.
Methods
The data used in this study were from LUCENT-1, a phase 3 randomized, double-blind, parallel-arm trial of the safety and efficacy of mirikizumab for UC induction treatment in adults with moderately to severely active UC (NCT03518086) [6]. Participants were randomly assigned 3:1 to receive an intravenous infusion of mirikizumab 300 mg or placebo at weeks 0, 4, and 8 during a 12-week treatment period. The primary outcome in LUCENT-1 was the percentage of participants in clinical remission of UC at week 12 based on a modified Mayo score (MMS) [7].
Participants
Participants were adults (aged 18–80 years) diagnosed with UC at least 3 months previously and with lack of response, loss of response, or intolerance to one of the following: corticosteroids, azathioprine, 6-mercaptopurine, infliximab, adalimumab, golimumab, vedolizumab, or tofacitinib. At baseline, their UC extended beyond the rectum and was moderately to severely active, as defined by an MMS total score of 4 to 9 and an endoscopic subscore ≥ 2. Patients with Crohn’s disease, unclassified inflammatory bowel disease, or UC not extending beyond the rectum, or who had undergone colectomy, were excluded. All participants provided written informed consent.
Patient-reported outcomes
Urgency NRS
The Urgency NRS [5] is a single-item measure of bowel urgency severity in the previous 24 h (Fig. 1). Bowel urgency is scored on an 11-point NRS ranging from 0 (no urgency) to 10 (worst possible urgency). Patients completed the Urgency NRS as part of a daily electronic diary (eDiary). Weekly average scores for the Urgency NRS were subsequently calculated (to the nearest whole number) for 7-day periods. A weekly score was considered missing if fewer than 4 days of scores were available in a given week.
Inflammatory Bowel Disease Questionnaire
The Inflammatory Bowel Disease Questionnaire (IBDQ) [8, 9] is a 32-item PRO instrument comprising four domains: bowel symptoms, systemic symptoms, emotional functioning, and social functioning. Each item is scored on a 7-point Likert scale ranging from 1 (“a very severe problem”) to 7 (“not a problem at all”). The total score ranges from 32 to 224, with a higher score indicating better quality of life. The IBDQ was completed at screening, baseline, and week 12. IBDQ remission was defined as an IBDQ total score ≥ 170 [10].
Patient Global Rating of Severity
The Patient Global Rating of Severity (PGRS) is a single-item PRO measure for assessing overall disease symptom severity over the previous 24 h on a 6-point scale from 1 (“none”) to 6 (“very severe”). The PGRS was completed as part of the daily eDiary to assess UC severity. A weekly average score was calculated in the same way as for the Urgency NRS. At week 12, a ≥ 2-point improvement on the PGRS from baseline was prespecified as a large and meaningful improvement in symptom severity, and a PGRS score of 1 or 2 was considered indicative of UC symptom remission or minimal symptom severity [11,12,13].
Patient Global Rating of Change
The Patient Global Rating of Change (PGRC) is a single-item PRO measure of change in overall symptoms since starting a new medicine. Responses are graded on a 7-point scale from 1 (“very much better”) to 7 (“very much worse”). The PGRC was completed at weeks 4, 8, and 12 to assess change in UC severity. A PGRC score of 1 (“very much better”) or 2 (“much better”) at week 12 was prespecified as a large and meaningful improvement in symptom severity [11,12,13].
Disease activity measures
Modified Mayo score
The MMS comprises three subscores: stool frequency, rectal bleeding, and the endoscopic subscore. Each of the subscores is scored on a scale of 0 to 3. The MMS total score (range 0 to 9) is derived by summing the scores for the three subscales. In the present study, patients recorded stool frequency and rectal bleeding subscores daily as part of the eDiary. Weekly scores for the stool frequency and rectal bleeding subscales were calculated as the average of the three most recent non-missing daily scores in a 7-day scoring period. The weekly rectal bleeding and stool frequency subscores were considered missing if fewer than 3 days of diary data were available. The endoscopic subscore was scored based on analysis of biopsy samples collected at baseline and week 12 by both the site endoscopist and the blinded central reader using a predefined algorithm. Clinical remission based on Mayo subscores was defined as a stool frequency subscore of 0, or 1 with a ≥ 1-point decrease from baseline; a rectal bleeding subscore of 0; and an endoscopic subscore of 0 or 1 (excluding friability) [14]. Clinical response was defined as a decrease in MMS total score from baseline of ≥ 2 points and ≥ 30%; and a rectal bleeding subscore of 0 or 1, or that had decreased by ≥ 1 point from baseline [14]. An endoscopic subscore of 0 or 1 (excluding friability) indicated endoscopic remission [14].
Histology
Participants underwent lower endoscopy at baseline and week 12. Biopsy samples were collected during endoscopic procedures, provided it was safe to collect them, and were analyzed histopathologically by blinded central readers using the Geboes scoring system [15]. The Geboes scoring system assigns values to seven histologic features: 0 structural (architectural change), 1 chronic inflammatory infiltrate, 2a lamina propria eosinophils, 2b lamina propria neutrophils, 3 neutrophils in epithelium, 4 crypt destruction, and 5 erosion or ulceration. Erosion or ulceration is scored on 5 levels; other features are scored on 4 levels. Histologic remission was defined as a Geboes histologic score of 2b (absence of neutrophils in the epithelium and lamina propria; no crypt destruction, erosion, or ulceration) [16].
Statistical analysis
Psychometric analysis of the Urgency NRS
The psychometric properties of the Urgency NRS were analyzed using established methods [17] among patients with moderately to severely active UC using data from LUCENT-1. Trial participants from the modified intent-to-treat population, which included all randomized patients who received at least one dose of study drug, were pooled across treatment arms. The statistical analyses were conducted as specified in a psychometric validation analysis plan using SAS® version 9.4 or later (SAS Institute, Cary, NC). All analyses are as observed on the weekly average Urgency NRS score and other assessments unless otherwise specified.
Descriptive statistics were calculated for participant demographics and for average weekly Urgency NRS scores. In addition, the distributions of the daily Urgency NRS scores in the 1-week periods prior to the baseline and week 12 clinical visits were also evaluated. Possible floor and ceiling effects for Urgency NRS scores were evaluated to ensure that participants did not disproportionately report the lowest or highest possible score (0 or 10) at baseline or week 12.
Shrout and Fleiss intraclass correlation coefficients (ICC(2,1)) were estimated to evaluate the test–retest reliability among ‘stable’ patients [18, 19]. Two groups of stable participants were defined: those with no change in PGRS score between screening and baseline and those registering “no change” on the PGRC at week 4 compared to baseline. An ICC ≥ 0.70 was considered evidence of acceptable test–retest reliability [20]. ICC(2,1) was calculated using two-way random effects models with subject and time as random effects [21, 22]. Modified large sample confidence intervals were constructed for the ICC(2,1) according to Cappelleri and Ting (2003) [23].
Known-groups validity of the Urgency NRS was evaluated at baseline by comparing the distribution of the Urgency NRS between patients who had a PGRS \(\le\) median compared to those with a PGRS score > median at baseline. In addition, known groups validity of the Urgency NRS was evaluated at week 12 according to the following groups at week 12: PGRS score ≤ median or > median, clinical remission status, and clinical response status. Least-square (LS) mean scores on the Urgency NRS at baseline or week 12 were compared between known groups using analysis of variance models that included Urgency NRS score as the dependent variable and group as the independent variable. Cohen’s d was calculated as a standardized measure of mean difference between known groups at baseline and week 12. It was hypothesized that patients with more severe UC symptoms (higher PGRS scores at baseline and week 12, clinical non-responders at week 12, and clinical non-remitters at week 12) would have higher Urgency NRS scores.
Convergent validity was assessed by calculating Spearman correlation coefficients at baseline and week 12 between the Urgency NRS and IBDQ total and domain scores, PGRS, PGRC (week 12 only), Mayo rectal bleeding subscore, and Mayo stool frequency subscore. Discriminant validity was assessed by calculating Spearman correlations for the Urgency NRS with Geboes score and Mayo endoscopic subscore as objective measures at baseline and week 12. Cohen’s conventions were used to interpret the magnitude of the correlations: a correlation < 0.1 was considered negligible, between 0.1 and 0.3 was weak, between 0.3 to 0.5 was moderate, and > 0.5 was considered strong [24]. It was hypothesized that Urgency NRS scores would have moderate to strong correlations with IBDQ total score, PGRS, PGRC, Mayo stool frequency subscore, and Mayo rectal bleeding subscore and weak correlations with Geboes score and Mayo endoscopic subscore.
Responsiveness was evaluated by comparing mean changes in Urgency NRS scores from baseline to week 12 between groups of patients with and without meaningful improvements at week 12 according to clinical remission and clinical response (based on MMS total score and Mayo subscores), IBDQ remission, median PGRS score, uncollapsed PGRS score changes (4-point decrease through 1-point increase), and uncollapsed PGRC categories (“very much better” through “very much worse”). Effect sizes were calculated as a standardized measure of improvement on the Urgency NRS between groups at week 12 by dividing the difference in change from baseline between groups by the pooled standard deviation at baseline. One-way analysis of covariance models were used to compare the LS mean change from baseline between groups, with change in Urgency NRS score as the dependent variable, and baseline Urgency NRS score and the meaningful improvement group as independent variables. Scheffe’s correction was used for pairwise comparisons.
Urgency NRS score interpretation
Anchor-based analyses were conducted to identify a threshold for meaningful, within-patient improvement in Urgency NRS score, with PGRC, PGRS, and clinical remission serving as anchor variables [23, 25,26,27]. Spearman correlations were calculated between change from baseline to Week 12 on the Urgency NRS with change from baseline to Week 12 on the PGRS, MMS, and the Week 12 PGRC to assess the appropriateness of the anchor variables (correlation ≥ 0.3 was required). A large and meaningful improvement in symptom severity at week 12 was defined as a PGRS improvement of ≥ 2 points and a PGRC score of 1 (“very much better”) or 2 (“much better”). Sensitivity, specificity, positive predictive value, negative predictive value, and Youden’s index (YI) (sensitivity + specificity − 1) [28] were calculated for each possible Urgency NRS improvement threshold to correctly act as a surrogate for meaningful improvement compared to other levels of improvement, no change, or worsening according to the anchor variable. In addition, area under the receiver operating characteristic curve (AUROC) was calculated from a logistic regression with the anchor variable as the dependent variable and urgency improvement status as defined by the change from baseline threshold on the Urgency NRS as the independent variable [29]. The Urgency NRS score change that maximized YI and AUROC were considered candidate thresholds for meaningful within-patient change in Urgency NRS score.
Resolution or near resolution of symptoms is an important treatment goal in UC. Anchor-based analyses were performed to explore the levels of urgency severity that are most associated with patients being in remission or inactive disease and reflect bowel urgency remission at week 12. Clinical remission, endoscopic remission, histologic remission, and a PGRS score of 1 or 2 were used as binary remission anchor variables reflecting being or not being in a state of remission or inactive disease. Sensitivity, specificity, positive predictive value, negative predictive value, and YI were calculated for a sequence of thresholds on the Urgency NRS against the anchor variables as the ground truth. AUROC was calculated from a logistic regression with the anchor variable as the dependent variable and urgency remission status as defined by the Urgency NRS threshold at week 12 as the independent variable. Urgency NRS scores with the largest Youden’s index and AUROC values were considered as candidate thresholds, below which patients were considered to have bowel urgency remission.
Results
Participants
The modified intent-to-treat population comprised 1,162 participants, of whom 868 received mirikizumab 300 mg intravenously every 4 weeks and 294 received placebo. Median age was 41 years (range 18 to 79). Most participants were White (71.7%) or Asian (25.0%), and 40.2% were female (Table 1).
Distribution of Urgency NRS scores
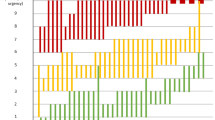
Collectively, participants registered the full range of weekly average Urgency NRS scores (0 to 10) at baseline and at week 12 (Fig. 2A). The mean (standard deviation) weekly Urgency NRS score was 6.2 (2.2) at baseline and 3.7 (2.6) at week 12. Median NRS score was also higher at baseline than at week 12 (6 vs. 3). The proportion of participants registering a score of 0 was 0.8% at baseline and 9.8% at week 12. A score of 10 was registered by 3.0% of participants at baseline and 1.6% at week 12. Figure 2B, C present the distributions of daily Urgency NRS scores in the 7 days prior to the baseline and week 12 visits, respectively. The distributions of daily scores were relatively uniform across days prior to both visits. There was therefore no evidence of any floor or ceiling effects among the weekly or daily values for the Urgency NRS at baseline or week 12. This suggests that weekly averages were appropriate to summarize daily Urgency NRS scores.
Test–retest reliability
The ICC(2,1) was estimated to be 0.89 (95% CI 0.87, 0.90) among stable participants with no change in PGRS score between screening and baseline and 0.76 (0.70, 0.82) among stable participants who registered “no change” on the PGRC at week 4 (Table 2). This indicated that the Urgency NRS had strong test–retest reliability.
Known-groups validity
When the participant sample was dichotomized based on the median baseline PGRS score of 4, the mean Urgency NRS score at baseline was higher for participants with a PGRS score above the median (7.5) than for those with a PGRS score less than or equal to the median (5.4; LS mean difference 2.1; p < 0.0001) (Table 3). Cohen’s d for the difference between PGRS groups was 1.07, indicating a large standardized mean difference in baseline Urgency NRS scores between PGRS groups at baseline. Thus, mean Urgency NRS scores at baseline were consistently higher (worse) for participants with more severe self-rated overall UC symptoms than for those with less severe self-rated UC.
Similarly, Urgency NRS scores at week 12 were significantly higher among patients with PGRS greater than the median score of 3 (5.4 vs. 2.5; LS mean difference = 2.7; p < 0.0001), patients without a clinical response (5.1 vs. 2.8; LS mean difference = 1.8; p < 0.0001), and patients not in clinical remission at week 12 (4.1 vs. 2.2; LS mean difference = 2.3; p < 0.0001). Cohen’s d for the mean Urgency NRS at week 12 between known groups was 1.39 by PGRS, 0.79 by clinical remission, and 1.00 by clinical response status. Known-groups validity was also demonstrated based on uncollapsed PGRS score changes (Additional file 1: Table S1). These results indicate that the Urgency NRS demonstrated good known-groups validity at baseline and week 12.
Convergent and discriminant validity
Correlations between Urgency NRS score and IBDQ total score and domain scores were moderate at baseline (− 0.31 to − 0.42) and moderate to large week 12 (− 0.46 to − 0.60) (Table 4). Large correlations were also observed with the PGRS at baseline (0.56) and week 12 (0.67) and with the PGRC at week 12 (0.52). Correlations with Mayo stool frequency were moderate at baseline (0.30) and moderate to large (0.49) at Week 12. Correlations with Mayo rectal bleeding were small to moderate at baseline (0.28) and moderate at week 12 (0.39). The Urgency NRS therefore demonstrated convergent validity. Conversely, correlations were very weak at baseline and weak to moderate at week 12 for the Urgency NRS with the objective Geboes score (0.02 at baseline and 0.28 at week 12) and Mayo endoscopic subscore (0.07 at baseline and 0.33 at week 12). The Urgency NRS therefore also demonstrated discriminant validity.
Responsiveness
Decreases (improvements) in Urgency NRS scores at week 12 were higher in participants who achieved clinical remission than in those with active disease (LS mean change from baseline − 3.8 vs. − 2.0; effect size (ES) = 0.80; p < 0.0001) (Table 5). Similarly, decreases in Urgency NRS scores were higher in clinical responders than in non-responders (LS mean change from baseline − 3.3 vs. − 1.0; ES = 1.07; p < 0.0001). Decreases in Urgency NRS scores were also higher in participants achieving IBDQ remission (LS mean change from baseline − 3.3 vs. − 1.3; ES = 0.70; p < 0.0001) and in participants with a week 12 PGRS score less than or equal to the median (LS mean change from baseline − 3.5 vs. − 0.9; ES = 1.05; p < 0.0001). Responsiveness was also demonstrated based on uncollapsed PGRS score changes (Additional file 1: Table S2) and uncollapsed PGRC categories (Additional file 1: Table S3). Collectively, these findings indicate that the Urgency NRS was able to detect changes in bowel urgency in patients whose UC severity and quality of life changed at week 12.
Meaningful within-patient improvement from baseline
The threshold with the maximum YI and AUROC for predicting a ≥ 2 point PGRS improvement was a 3-point Urgency NRS improvement (YI = 0.52, AUROC = 0.76) (Table 6, Fig. 3). A ≥ 3-point improvement on the Urgency NRS therefore yields the best balance between sensitivity and specificity of any Urgency NRS threshold at identifying large improvement in overall symptom severity based on the PGRS. A 3-point threshold for Urgency NRS improvement also maximized Youden’s index and AUROC for the PGRC (YI = 0.37, AUROC = 0.69). When clinical remission was used as the anchor, YI and AUROC were maximized (YI = 0.31, AUROC = 0.65) at an Urgency NRS improvement threshold of 3 points (Fig. 3 and Additional file 1: Table S4), indicating that a ≥ 3-point improvement on the Urgency NRS best corresponds to patients achieving clinical remission. Collectively, these analyses suggest that a ≥ 3-point improvement on the Urgency NRS represents a meaningful within-patient improvement in bowel urgency in moderate-to-severe UC patients.
Youden’s Index from an anchor-based analysis of improvement in Urgency NRS from baseline to week 12. Clinical remission was defined as a Mayo stool frequency subscore of 0, or 1 with a ≥ 1-point decrease from baseline; a Mayo rectal bleeding subscore of 0; and a Mayo endoscopic subscore of 0 or 1 (excluding friability)
Threshold for bowel urgency remission
A threshold for bowel urgency remission—associated with clinical remission or inactive disease—was also explored by conducting anchor-based analyses with remission endpoints as anchor variables. An Urgency NRS threshold of 2 points or lower yielded the highest YI and AUROC with patients achieving UC symptom remission based on the PGRS (YI = 0.57, AUROC = 0.78), clinical remission (YI = 0.34, AUROC = 0.67), and histologic remission (YI = 0.25, AUROC = 0.62) (Table 7, Fig. 4, and Additional file 1: Table S5). For these three anchors, an Urgency NRS threshold of 1 point had lower YI and sensitivity than a threshold of 2 points, but higher specificity and higher or comparable positive predictive value. For endoscopic remission based on the Mayo endoscopic subscore, YI and AUROC were maximized at an Urgency NRS threshold of 3 points (YI = 0.27, AUROC = 0.63) and was also high with a threshold of 2 points (YI = 0.25, AUROC = 0.62) (Fig. 4 and Additional file 1: Table S5). Compared to a threshold of 2 points, a threshold of 1 point had lower values for YI and sensitivity but higher specificity and marginally higher positive predictive value. Collectively, these findings suggest that an Urgency NRS score of ≤ 2 points was best associated with patients achieving symptom, clinical, endoscopic, or histologic remission. An Urgency NRS score of ≤ 1 point would be a more conservative definition to identify patients with bowel urgency remission. Compared to a definition of ≤ 2 points, a definition of ≤ 1 point had lower YI but was superior in terms of specificity and generally superior in terms of positive predictive value.
Youden’s Index from an anchor-based analysis of remission on the Urgency NRS at week 12. Clinical remission was defined as a Mayo stool frequency subscore of 0, or 1 with a ≥ 1-point decrease from baseline; a Mayo rectal bleeding subscore of 0; and a Mayo endoscopic subscore of 0 or 1 (excluding friability). Histologic remission was defined as a Geboes histologic score of 2b. Endoscopic remission was defined as a Mayo endoscopic subscore of 0 or 1 (excluding friability)
Discussion
The present analysis used data from a phase 3 clinical trial to assess the measurement properties of the Urgency NRS, a content-valid PRO measure for capturing changes in the severity of bowel urgency in patients with UC [5]. The Urgency NRS showed good test–retest reliability based on data for stable participants without change on the PGRS and PGRC, and good known-groups validity based on PGRS score categories. It also showed convergent and discriminant validity. Correlations between the Urgency NRS and other assessments were stronger at week 12 than at baseline, presumably due to variability in patient outcomes resulting from different levels of response to active treatment versus placebo. Furthermore, the Urgency NRS was responsive to changes in UC severity.
For an NRS-based PRO measure to be useful in clinical trials, one must be able to interpret scores and score changes on the scale. This study found that an Urgency NRS score improvement of ≥ 3 points is clinically meaningful for patients with moderately to severely active UC and that an Urgency NRS score of ≤ 1 point represents bowel urgency remission. For three of the four anchors included in the bowel urgency remission analysis, both Youden’s index and area under the receiver operating characteristic curve were maximized at an Urgency NRS threshold of 2 points. Compared to a remission threshold of ≤ 2 points, an Urgency NRS threshold of ≤ 1 point represents a more conservative definition for bowel urgency remission and benefits from higher positive predictive value and higher specificity.
These results support the notion that patients in UC remission or with inactive disease may still register an Urgency NRS score greater than 0. In qualitative interviews with 19 patients with moderately to severely active UC, participants indicated that an Urgency NRS score of 1 to 3 reflected mild bowel urgency with minimal impact on daily life [30]. In addition, while a score of 0 on the Urgency NRS is defined as “no urgency,” a certain level of variability in bowel urgency should be expected, especially considering that bowel urgency can occur in healthy people without underlying inflammation [31] and that patients completed the Urgency NRS daily over a prolonged period of time. Therefore, achieving a mean score of 0 on this 11-point NRS scale may be an unrealistic treatment target. In the present analysis, we showed that an Urgency NRS score of up to 2 was most associated with achieving clinical remission, endoscopic remission, histologic remission, and resolution or very minimal overall symptom severity according to the PGRS. These results support the notion that patients in remission or inactive disease may still report minimal residual levels of bowel urgency on the Urgency NRS that they consider “normal.” This is in line with observations from the recent Study of a Prospective Adult Research Cohort with Inflammatory Bowel Disease (SPARC-IBD), where 39% of UC patients with mild urgency and 9% with moderate-to-severe urgency reported no abdominal pain, no bleeding, and normal bowel frequency [32].
Other PRO instruments for capturing bowel urgency include the Patient Simple Clinical Colitis Activity Index (P-SCCAI) [33] and Ulcerative Colitis Patient-Reported Outcomes (UC-PRO) [34], validated multi-item instruments with individual items on bowel urgency. Also available are the Symptoms and Impacts Questionnaire for Ulcerative Colitis (SIQ-UC) [35] and Crohn’s and Ulcerative Colitis Questionnaire (CUCQ) [36, 37], which were developed to capture the symptoms and impacts of UC, including bowel urgency; and an unvalidated single-item measure used in SPARC-IBD [32]. However, the UC-PRO and CUCQ capture the frequency but not severity of bowel urgency. The CUCQ is further limited by its 2-week recall period, meaning that it is unable to capture daily fluctuations in symptoms. The P-SCCAI and SIQ-UC capture the severity of bowel urgency, but respectively use a binary response option and a 5-point response scale. By comparison, the Urgency NRS’s 11-point scale allows changes in bowel urgency to be better captured through a wider range of scores.
The validation work was conducted in accordance with current standards for evaluating the psychometric properties of PRO instruments [17, 29, 38,39,40] using a large patient sample. Another strength is that the analyses of thresholds for meaningful change and urgency remission included both PROs and objective clinical outcomes based on blinded assessments. Also, generalizability of the findings is enhanced by the inclusion of patients from a wide range of geographies, with similar demographics as those in other recent UC trials [41, 42]. However, there were very few Black participants in the trial so generalizability to this population remains to be tested.
One limitation of this validation study is that the psychometric evaluation only used weekly average Urgency NRS scores collected daily with 24-h recall periods. This reflected the intended use of the Urgency NRS in clinical trials. Psychometric properties for a one-time administration of the Urgency NRS with a longer recall period, which may be more applicable to clinical practice or real-world studies, was not examined. However, the psychometric properties of the Urgency NRS should, in theory, be very similar for a single assessment with a 7-day recall period as for a weekly average of daily scores. Minimal differences are seen in the distributions of Urgency NRS scores between daily and one-time assessments (as illustrated in Fig. 2), and the mean change from baseline should also be similar. As a result, Spearman correlations for convergent and discriminant validity and effect sizes for known-groups validity and responsiveness should be very similar. Assessments with longer recall periods have been shown to give higher estimates of ICC [43, 44]. Because high ICC values were seen among daily administration of the Urgency NRS, we should also expect high ICC values for one-time administration. Given the consistent results from the anchor-based analyses of meaningful within-patient improvement and bowel urgency remission, we believe the definitions for these endpoints would similarly hold for one-time administration of the Urgency NRS with a 1-week recall period, although this will require testing in future studies.
A limitation of using diagnostic test statistics (sensitivity, specificity, Youden’s Index, and AUROC) to define meaningful within-patient improvement and minimal to no bowel urgency is that they may only be applicable to the current sample; their generalizability to other samples or populations is unconfirmed. It would therefore be beneficial for future studies to reproduce or confirm our findings in other UC patient cohorts.
Conclusions
Improvement in the severity of bowel urgency is an important outcome to capture in UC clinical trials. We have developed and validated the Urgency NRS as a new PRO instrument for capturing changes in bowel urgency severity in patients with UC. The good psychometric properties of the Urgency NRS indicate that it can be used in clinical trials to evaluate treatment benefits in patients with moderately to severely active UC, and potentially in routine clinical practice.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- CUCQ:
-
Crohn’s and Ulcerative Colitis Questionnaire
- eDiary:
-
Electronic diary
- IBDQ:
-
Inflammatory Bowel Disease Questionnaire
- ICC:
-
Intraclass correlation coefficient
- LS:
-
Least-square
- MMS:
-
Modified Mayo score
- NRS:
-
Numeric rating scale
- PGRC:
-
Patient Global Rating of Change
- PGRS:
-
Patient Global Rating of Severity
- PRO:
-
Patient-reported outcome
- P-SCCAI:
-
Patient Simple Clinical Colitis Activity Index
- SD:
-
Standard deviation
- SE:
-
Standard error
- SIQ-UC:
-
Symptoms and Impacts Questionnaire for Ulcerative Colitis
- SPARC IBD:
-
Study of a Prospective Adult Research Cohort with Inflammatory Bowel Disease
- UC:
-
Ulcerative colitis
- UC-PRO:
-
Ulcerative Colitis Patient-Reported Outcomes
References
Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF (2017) Ulcerative colitis. Lancet 389:1756–1770. https://doi.org/10.1016/s0140-6736(16)32126-2
Louis E, Ramos-Goni JM, Cuervo J, Kopylov U, Barreiro-de Acosta M, McCartney S et al (2020) A qualitative research for defining meaningful attributes for the treatment of inflammatory bowel disease from the patient perspective. Patient 13:317–325. https://doi.org/10.1007/s40271-019-00407-5
Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD (2019) ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol 114:384–413. https://doi.org/10.14309/ajg.0000000000000152
Newton L, Randall JA, Hunter T, Keith S, Symonds T, Secrest RJ et al (2019) A qualitative study exploring the health-related quality of life and symptomatic experiences of adults and adolescents with ulcerative colitis. J Patient Rep Outcomes 3:66. https://doi.org/10.1186/s41687-019-0154-x
Dubinsky MC, Naegeli A, Dong Y, Lissoos T, Arora V, Irving P (2020) P126 The Urgency Numeric Rating Scale (NRS): a novel patient-reported outcome measure to assess bowel urgency in adult patients with ulcerative colitis [poster]. In: 15th Congress of ECCO (the European Crohn's and Colitis Organisation), February 12–15, 2020, Vienna, Austria.
D’Haens G, Kobayashi T, Morris N, Lissoos T, Hoover A, Li X et al (2022) OP26 efficacy and safety of mirikizumab as induction therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 LUCENT-1 study. J Crohns Colitis 16:i028-i29
Schroeder KW, Tremaine WJ, Ilstrup DM (1987) Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 317:1625–1629. https://doi.org/10.1056/NEJM198712243172603
Guyatt G, Mitchell A, Irvine EJ, Singer J, Williams N, Goodacre R et al (1989) A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 96:804–810
Irvine EJ (1999) Development and subsequent refinement of the inflammatory bowel disease questionnaire: a quality-of-life instrument for adult patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 28:S23–S27. https://doi.org/10.1097/00005176-199904001-00003
CADTH. Appendix 5 Validity of Outcome Measures. In: Golimumab (Simponi) (Subcutaneous Injection): Adult Patients with Moderately to Severely Active Ulcerative Colitis Who Have Had an Inadequate Response to, or Have Medical Contraindications for, Conventional Therapies. Ottawa, ON, Canada: Canadian Agency for Drugs and Technologies in Health; 2014.
Butler J, Spertus JA, Bamber L, Khan MS, Roessig L, Vlajnic V et al (2022) Defining changes in physical limitation from the patient perspective: insights from the VITALITY-HFpEF randomized trial. Eur J Heart Fail 24:843–850. https://doi.org/10.1002/ejhf.2481
Butler J, Shahzeb Khan M, Lindenfeld J, Abraham WT, Savarese G, Salsali A et al (2022) Minimally clinically important difference in health status scores in patients with HFrEF vs. HFpEF. JACC Heart Fail 10:651–661. https://doi.org/10.1016/j.jchf.2022.03.003
Reaney M, Addepalli P, Allen V, Spertus JA, Dolan C, Sehnert AJ et al (2022) Longitudinal psychometric analysis of the Hypertrophic Cardiomyopathy Symptom Questionnaire (HCMSQ) using outcomes from the phase III EXPLORER-HCM trial. Pharmacoecon Open 6:575–586. https://doi.org/10.1007/s41669-022-00340-8
FDA (2016) Ulcerative colitis: clinical trial endpoints. Guidance for Industry. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). https://www.fda.gov/files/drugs/published/Ulcerative-Colitis--Clinical-Trial-Endpoints-Guidance-for-Industry.pdf.
Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Lofberg R (2000) A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 47:404–409. https://doi.org/10.1136/gut.47.3.404
Magro F, Doherty G, Peyrin-Biroulet L, Svrcek M, Borralho P, Walsh A et al (2020) ECCO Position paper: harmonization of the approach to ulcerative colitis histopathology. J Crohns Colitis 14:1503–1511. https://doi.org/10.1093/ecco-jcc/jjaa110
Cappelleri JC, Zou KH, Bushmakin AG, Alvir JMJ, Alemayehu D, Symonds T (2013) Patient-reported outcomes: measurement, implementation and interpretation. Chapman & Hall/CRC Press, Boca Raton
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86:420–428. https://doi.org/10.1037//0033-2909.86.2.420
Vaz S, Falkmer T, Passmore AE, Parsons R, Andreou P (2013) The case for using the repeatability coefficient when calculating test-retest reliability. PLoS ONE 8:e73990. https://doi.org/10.1371/journal.pone.0073990
Litwin MS (1995) How to measure survey reliability and validity. SAGE Publications, Thousand Oaks
Schuck P (2004) Assessing reproducibility for interval data in health-related quality of life questionnaires: which coefficient should be used? Qual Life Res 13:571–586. https://doi.org/10.1023/B:QURE.0000021318.92272.2a
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163. https://doi.org/10.1016/j.jcm.2016.02.012
Cappelleri JC, Ting N (2003) A modified large-sample approach to approximate interval estimation for a particular intraclass correlation coefficient. Stat Med 22:1861–1877. https://doi.org/10.1002/sim.1402
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates, Hillsdale
Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR, Clinical Significance Consensus Meeting Group (2002) Methods to explain the clinical significance of health status measures. Mayo Clin Proc 77:371–383. https://doi.org/10.4065/77.4.371
Revicki DA, Cella D, Hays RD, Sloan JA, Lenderking WR, Aaronson NK (2006) Responsiveness and minimal important differences for patient reported outcomes. Health Qual Life Outcomes 4:70. https://doi.org/10.1186/1477-7525-4-70
Revicki D, Hays RD, Cella D, Sloan J (2008) Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 61:102–109. https://doi.org/10.1016/j.jclinepi.2007.03.012
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32–35. https://doi.org/10.1002/1097-0142(1950)3:1%3c32::aid-cncr2820030106%3e3.0.co;2-3
de Vet HCW, Terwee CB, Mookink LB, Knol DL (2011) Measurement in medicine: a practical guide. Cambridge University Press, New York
Newton L, Guobyte A, McFadden S, Symonds T, Delbecque L, Donaldson J et al (2021) P253 A qualitative study exploring meaningful improvement in bowel urgency among adults with moderate to severe ulcerative colitis [poster]. In: 16th Congress of ECCO (the European Crohn's and Colitis Organisation), July 2–3 and 8–10, 2021 (virtual)
Rangan V, Mitsuhashi S, Singh P, Ballou S, Hirsch W, Sommers T et al (2018) Risk factors for fecal urgency among individuals with and without diarrhea, based on data from the national health and nutrition examination survey. Clin Gastroenterol Hepatol 16:1450–8 e2. https://doi.org/10.1016/j.cgh.2018.02.020
Dawwas GK, Jajeh H, Shan M, Naegeli AN, Hunter T, Lewis JD (2021) Prevalence and factors associated with fecal urgency among patients with ulcerative colitis and Crohn’s disease in the Study of a Prospective Adult Research Cohort with Inflammatory Bowel Disease. Crohns Colitis 360(3):otab046
Bennebroek Evertsz F, Nieuwkerk PT, Stokkers PC, Ponsioen CY, Bockting CL, Sanderman R et al (2013) The patient simple clinical colitis activity index (P-SCCAI) can detect ulcerative colitis (UC) disease activity in remission: a comparison of the P-SCCAI with clinician-based SCCAI and biological markers. J Crohns Colitis 7:890–900. https://doi.org/10.1016/j.crohns.2012.11.007
Higgins PDR, Harding G, Revicki DA, Globe G, Patrick DL, Fitzgerald K et al (2017) Development and validation of the Ulcerative Colitis Patient-Reported Outcomes signs and symptoms (UC-pro/SS) diary. J Patient Rep Outcomes 2:26. https://doi.org/10.1186/s41687-018-0049-2
Dulai PS, Jairath V, Khanna R, Ma C, McCarrier KP, Martin ML et al (2020) Development of the symptoms and impacts questionnaire for Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther 51:1047–1066. https://doi.org/10.1111/apt.15726
Alrubaiy L, Cheung WY, Dodds P, Hutchings HA, Russell IT, Watkins A et al (2015) Development of a short questionnaire to assess the quality of life in Crohn’s disease and ulcerative colitis. J Crohns Colitis 9:66–76. https://doi.org/10.1093/ecco-jcc/jju005
Hutchings HA, Alrubiay L, Watkins A, Cheung WY, Seagrove AC, Williams JG (2017) Validation of the Crohn’s and Ulcerative Colitis questionnaire in patients with acute severe ulcerative colitis. United Eur Gastroenterol J 5:571–578. https://doi.org/10.1177/2050640616671627
Nunnally JC, Bernstein IH (1994) Psychometric theory. McGraw-Hill, New York, NY
Hays RD, Revicki D (2005) Reliability and validity (including responsiveness). In: Fayers P, Hays RD (eds) Assessing quality of life in clinical trials: methods and practice, 2nd edn. Oxford University Press, New York, pp 25–39
FDA. Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims U.S. Food and Drug Administration 2009. https://www.fda.gov/media/77832/download. Accessed 11 June 2020.
Danese S, D'Haens G, Rubin D, Panaccione R, Zhou W, Ilo D et al. OP021 Patients with ulcerative colitis report improvements in abdominal pain, bowel urgency, and fatigue with 8-week upadacitinib treatment in two phase 3 trials: U-ACHIEVE AND U-ACCOMPLISH [oral presentation]. UEG Week, October 3–5, 2021 (virtual).
Ghosh S, Sanchez Gonzalez Y, Zhou W, Clark R, Xie W, Louis E et al (2021) Upadacitinib treatment improves symptoms of bowel urgency and abdominal pain, and correlates with quality of life improvements in patients with moderate to severe ulcerative colitis. J Crohns Colitis. https://doi.org/10.1093/ecco-jcc/jjab099
Stull DE, Leidy NK, Parasuraman B, Chassany O (2009) Optimal recall periods for patient-reported outcomes: challenges and potential solutions. Curr Med Res Opin 25:929–942. https://doi.org/10.1185/03007990902774765
Topp J, Andrees V, Heesen C, Augustin M, Blome C (2019) Recall of health-related quality of life: how does memory affect the SF-6D in patients with psoriasis or multiple sclerosis? A prospective observational study in Germany. BMJ Open 9:e032859. https://doi.org/10.1136/bmjopen-2019-032859
Acknowledgements
The authors thank Stephen Gilliver of Evidera for providing medical writing support, which was funded by Eli Lilly and Company in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Funding
This study was funded by Eli Lilly and Company.
Author information
Authors and Affiliations
Contributions
MS, LD, TH, TL, GH, and LS were involved in the design and conceptualization of the study and analysis and interpretation of results. MCD, DA, and JDL were involved in the analysis and interpretation of results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was compliant with the International Council for Harmonisation (ICH) guidelines on good clinical practice. All participants provided written informed consent. All informed consent forms and protocols were approved by the appropriate ethical review boards prior to initiation of the study.
Consent for publication
Not applicable.
Competing interests
MCD has received consulting fees from AbbVie, Arena, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Genentech, Gilead, Janssen, Pfizer, Prometheus, Roche, Takeda, and UCB; research funding from AbbVie, Janssen, Pfizer, and Prometheus; and licensing fees from Takeda. She owns stock in Trellus Health. MS, LD, TL, and TH are employees and shareholders of Eli Lilly and Company. GH, LS, and DA are employees of Evidera, which was paid by Eli Lilly and Company for work in support of this article. JDL has consulted or served on an advisory board for Eli Lilly and Company, Samsung Bioepis, UCB, Bristol Myers Squibb, Nestlé Health Science, Merck, Celgene, Janssen Pharmaceuticals, Bridge Biotherapeutics, Entasis Therapeutics, AbbVie, Pfizer, Gilead, Arena Pharmaceuticals, Protagonist Therapeutics, Amgen, and Scipher Medicine. He has received research funding from Nestlé Health Science, Takeda, Janssen Pharmaceuticals, and AbbVie. He has performed legal work on behalf of generic manufacturers of ranitidine, including L. Perrigo Company, Glenmark Pharmaceuticals, Inc., Amneal Pharmaceuticals LLC, Aurobindo Pharma USA, Inc., Dr. Reddy’s Laboratories, Inc., Novitium Pharma, Ranbaxy Inc., Sun Pharmaceutical Industries, Inc., Strides Pharma, Inc., and Wockhardt USA LLC.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary Table 1. Known-groups validity based on uncollapsed PGRS scores. Supplementary Table 2. Responsiveness at week 12 based on uncollapsed PGRS score changes. Supplementary Table 3. Responsiveness at week 12 based on uncollapsed PGRC categories. Supplementary Table 4. Anchor-based analysis of meaningful change from baseline: clinical remission. Supplementary Table 5. Anchor-based analysis of urgency remission at week 12: endoscopic remission and histologic remission.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dubinsky, M.C., Shan, M., Delbecque, L. et al. Psychometric evaluation of the Urgency NRS as a new patient-reported outcome measure for patients with ulcerative colitis. J Patient Rep Outcomes 6, 114 (2022). https://doi.org/10.1186/s41687-022-00522-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41687-022-00522-2