Abstract
Background
Nasal obstruction is a common problem in patients with obstructive sleep apnea (OSA). Systematic evaluation of nasal obstruction remains challenging due to the high number of variables and factors that contribute to nasal obstruction. Nasal examination by means of anterior rhinoscopy is limited to the evaluation of anterior septal deviation, internal nasal valve angle, and inferior turbinate size, but obstruction due to posterior septal deviation and nasal polyposis may go undiagnosed. The primary objective of this study was to determine the incidence of posterior nasal obstruction in OSA patients. Specifically, we were interested in other causes of posterior nasal obstruction that were difficult to assess by anterior rhinoscopy examination alone, and that required nasal endoscopy for identification.
Methods
This is a retrospective case series study. Flexible fiberoptic examination of the nasal cavity was performed on 274 consecutive OSA patients evaluated at the Stanford Sleep Surgery Clinic. Examination video files were recorded and later reviewed and scored by a single investigator blinded to the patients’ subjective nasal complaints. Anatomic features that contribute to posterior nasal obstruction were noted.
Results
Posterior septal deviation was the most common incidental finding in OSA patients with posterior nasal obstruction. Other causes included nasal polyposis, nasal mucosal inflammation, and purulent mucosal discharge. In total, there were 73/274 (26.6%) patients for whom nasal endoscopy provided findings that directed management.
Conclusion
Nasal endoscopy provides additional diagnostic information in a significant number of OSA patients who complain of nasal obstruction. Our findings suggest the use of nasal endoscopy for OSA patients who complain of nasal obstruction or CPAP intolerance, despite unremarkable anterior rhinoscopy examination.
Similar content being viewed by others
Background
Obstructive sleep apnea (OSA) is a disorder caused by the repetitive collapse of the upper airway during sleep resulting in either partial or complete airflow obstruction (Strollo and Rogers 1996). Nasal obstruction is related to OSA in several ways: 1) reduces airflow through the collapsible airway, therefore increasing upper airway resistance, 2) forces patients to become oral breathers during sleep, which leads to narrowing of the airway, and 3) interferes with the nasal reflexes that stimulate ventilation (de Sousa Michels et al. 2014; Georgalas 2011). The nose also serves as a major conduit for the treatment of OSA with continuous positive airway pressure (CPAP) therapy (Georgalas 2011; Stepnowsky and Moore 2003; Ebben et al. 2012). Nasal obstruction can therefore interfere with the medical treatment of OSA.
For OSA patients, nasal obstruction may be treated with the goal of reducing snoring and airway collapse, or to improve CPAP tolerance. Data on OSA patients treated for nasal obstruction alone has shown consistent improvement in subjective symptoms such as daytime somnolence and snoring despite minimal change in their sleep study results (Bican et al. 2010). Nasal surgery alone has also been shown to significantly impact CPAP tolerance and adherence (Poirier et al. 2014; Powell et al. 2001).
Systematic evaluation of nasal obstruction remains challenging due to the high number of factors that contribute to nasal obstruction. Nasal examination by anterior rhinoscopy allows evaluation of anterior septal deviation, internal nasal valve angle, and inferior turbinate size. Frequently, this limited examination of the anterior nasal cavity does not correlate with patient symptoms. Patients may complain of nasal obstruction despite no signs of objective anatomical abnormalities in the nasal cavity when examined with anterior rhinoscopy alone. Other etiologies for nasal obstruction such as posterior septal deviation or chronic sinusitis with or without polyposis may go undiagnosed. Structural and inflammatory problems often coexist and need to be addressed concurrently in order to reestablish normal nasal function (Rotenberg and Pang 2015; El Rassi et al. 2015).
We therefore aimed to evaluate different causes of posterior nasal cavity obstruction that are difficult, if not impossible, to asses by anterior rhinoscopy. The high incidence of posterior nasal cavity obstruction in this study suggests the use of nasal endoscopy in all OSA patients who also complain of nasal obstruction or CPAP intolerance.
Methods
This was a retrospective case series of 274 consecutive OSA patients examined using flexible fiberoptic examination at the Stanford Sleep Surgery Clinic. The protocol for this study was approved by the Institutional Review Board and Hospital Research Ethics Committee of Stanford University. Examination video files were recorded, reviewed, and then scored by a single investigator blinded to the patients’ subjective nasal complaints. Presence of posterior septal deviation, nasal crusting, erythema, swelling, scar band, purulent drainage, thick mucus, and nasal polyposis was noted.
Results
Demographic data of the subjects are summarized in Table 1. The mean age was 42.1 +/− 14.8 years and the mean BMI 27.5 +/− 5.7 kg/m2. All patients had a positive diagnosis for OSA with a mean Apnea-Hypopnea Index (AHI) of 31.6 +/− 25.3 events/hr, Apnea Index of 7.5 +/− 15.4 events/hr, Oxygen Desaturation Index of 15.4 +/− 22.0 events/hr, and Lowest Oxygenation Saturation of 86.7 +/− 6.6%. Majority of the patients complained of excessive daytime somnolence with a mean Epworth Sleepiness Scale Score of 10.1 +/− 5.2 (mean +/− SD).
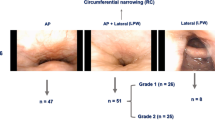
Table 2 shows the different causes of incidental posterior nasal obstruction that were identified in this patient population. Posterior nasoseptal deviation was the most common cause of posterior nasal obstruction (55/274, 20.0%). The majority presented with unilateral obstruction, although there was one case with bilateral nasoseptal deviation (Fig. 1). There were also 5 cases of combined anterior and posterior septal deviation (5/274, 1.8%).
A significant number of patients also had inflammatory problems leading to nasal obstruction (Fig. 2). The most common inflammatory problem identified was nasal polyposis (11/274, 4.0%), followed by edematous nasal mucosa inflammation (2/274, 0.7%), and purulent mucosal discharge (1/274, 0.36%). In total, there were 73/274 (26.6%) patients for whom nasal endoscopy provided findings that directed management.
Discussion
Nasal obstruction can be caused by structural abnormalities (e.g. deviated nasal septum, enlarged turbinates and nasal valve collapse) or by inflammatory mucosal disease (rhinitis, chronic rhinosinusitis with or without nasal polyps) (Lee et al. 2013; Prasad et al. 2013). Correction of nasal obstruction is unquestionably a priority in the management of OSA patients, regardless of whether it leads to an improvement in OSA severity based on objective polysomnography respiratory parameters. There is extensive evidence showing that nasal obstruction not only decreases quality of life, but that it also contributes to snoring, plays an important role in the pathophysiologic mechanisms leading to OSA, and represents an obstacle for effective treatment with CPAP therapy in OSA patients (de Sousa Michels et al. 2014; Bican et al. 2010). Currently, nasal examination of OSA patient in most medical practices is limited to anterior rhinoscopy, which fails to identify other sites and sources contributing to nasal obstruction.
There are several mechanisms by which nasal obstruction contributes to the pathogenesis of OSA. Following the Sterling resistor model, elevated nasal resistance increases negative pressure in the oropharyngeal airway downstream, thus contributing to airway collapse (Smith et al. 1988; Park 1993). Increased nasal resistance also results in compensatory oral breathing, which leads to an unstable airway with increased total resistance (Phillips 2006; Akbay et al. 2013). Finally, decreased nasal airflow blunts the activation of the nasal-ventilatory reflex important in the maintenance of adequate muscle tone, breathing frequency, and minute lung ventilation (Mcnicholas; Douglas et al. 1983). One of the priorities in the management of OSA patients should be the re-establishment of efficient nasal breathing.
Mouth breathing is a problem oftentimes ignored in the management of OSA. Oral breathing resulting from nasal obstruction may lead to a closed cycle where nasal respiration ends up becoming worse due to profound anatomic derangement. Continuous oral breathing often leads to a transverse maxillary deficiency that deepens the palatal arch. The high arched palate may compress the septum in a cranio-caudal orientation, thus resulting in a displaced septum (Akbay et al. 2013). Most of the posterior septal deviation that cannot be visualized using anterior rhinoscopy alone are not from traumatic insult, but from pressure exerted by a high arched palate during active craniofacial skeletal development. Since many OSA patients present with a high arched palate, we infer that many of these patients would present with posterior septal deviations.
It is common to see OSA patients with impaired nasal breathing to also present with inflammatory mucosal disease. It is estimated that 58% of OSA patients are affected by rhinitis (Gelardi et al. 2012). Over 70% of patients with chronic rhinosinusitis (CRS) report poor sleep quality, and the degree of sleep disturbance correlates with decreased overall quality of life (QOL) (Rotenberg and Pang 2015). Sleep impairment in CRS exerts a greater relative influence on the decision to pursue endoscopic sinus surgery (ESS) when compared to rhinologic specific symptom domains (El Rassi et al. 2015).
A possible explanation for this is that a night-time under-ventilated nose (due to apnea) may be at increased risk of infections and inflammation. The findings from Gelardi et al. support this theory. They found that regular CPAP treatment induces a significant reduction of cell infiltration (neutrophils, eosinophils, lymphocytes, and muciparous cells), which is not seen in non-treated patients. This supports the theory that increased nasal ventilation, in some cases secondary to CPAP use, helps reduce some of the enzymes (ex. elastasis) responsible for the production of free radicals that cause cell damage and mucosal inflammation (Gelardi et al. 2012).
Nasal surgery has not been correlated with significant improvement in post-operative Apnea-Hypopnea Index (AHI). However, ample evidence supports the re-establishment of nasal patency in OSA patients. First, decreased nasal resistance helps reduce CPAP pressures and improves its tolerance (Poirier et al. 2014). Other studies have also shown an improvement in overall sleep architecture with increases in non-REM stage 3 and 4, and REM sleep (Sériès and St Pierre 1992). Finally, nasal surgery is known to have a positive effect in the snoring complaints of OSA patients (Fairbanks 1984).
Conclusions
The observations made in this study support the fact that a significant number of OSA patients with normal anterior rhinoscopy examination may still have other etiology of nasal obstruction that can be visualized by nasal endoscopy. These findings, and the implications of nasal obstruction in the pathogenesis and treatment of OSA, warrant the use of routine nasal endoscopy in this population. We propose that OSA patients complaining of nasal obstruction or CPAP intolerance need to be offered nasal endoscopic evaluation to further define clinical strategies for treatment.
Abbreviations
- AHI:
-
Apnea-Hypopnea Index
- CPAP:
-
Continuous positive airway pressure
- CRS:
-
Chronic rhinosinusitis
- ESS:
-
Endoscopic sinus surgery
- OSA:
-
Obstructive sleep apnea
- QOL:
-
Quality of life
References
Akbay E, Cokkeser Y, Yilmaz O, Cevik C. The relationship between posterior septum deviation and depth of maxillopalatal arch. Auris Nasus Larynx. 2013;40(3):286–90.
Bican A, Kahraman A, Bora I, Kahveci R, Hakyemez B. What is the efficacy of nasal surgery in patients with obstructive sleep apnea syndrome? J Craniofac Surg. 2010;21(6):1801–6.
de Sousa Michels D, da Mota Silveira Rodrigues A, Nakanishi M, Sampaio ALL, Venosa AR. Nasal involvement in obstructive sleep apnea syndrome. Int J Otolaryngol. 2014;2014(2014):717419.
Douglas NJ, White DP, Weil JV, Zwillich CW. Effect of breathing route on ventilation and ventilatory drive. Respir Physiol. 1983;51:209–18.
Ebben MR, Oyegbile T, Pollak CP. The efficacy of three different mask styles on a PAP titration night. Sleep Med. 2012;13(6):645–9.
El Rassi E, Mace JC, Steele TO, Alt JA, Smith TL. Improvements in sleep-related symptoms after endoscopic sinus surgery in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015;00:1–9.
Fairbanks DN. Snoring: surgical vs. nonsurgical management. Laryngoscope. 1984;94(9):1188–92.
Gelardi M, Carbonara G, Maffezzoni E, Marvisi M, Quaranta N, Ferri R. Regular CPAP utilization reduces nasal inflammation assessed by nasal cytology in obstructive sleep apnea syndrome. Sleep Med. 2012;13(7):859–63.
Georgalas C. The role of the nose in snoring and obstructive sleep apnoea: an update. Eur Arch Oto-Rhino-Laryngology. 2011;268(9):1365–73.
Lee DC, Shin JH, Kim SW, et al. Anatomical analysis of nasal obstruction: nasal cavity of patients complaining of stuffy nose. Laryngoscope. 2013;123(6):1381–4.
Mcnicholas WT, Coffey M, Boyle T. Effects of nasal airflow on breathing during sleep in normal humans. Am Rev Respir Dis. 1993;147(3):620–3.
Park SS. Flow-regulatory function of upper airway in health and disease: A unified pathogenetic view of sleep-disordered breathing. Lung. 1993;171(6):311–33.
Phillips BA. Mouth breathing compromises adherence to nasal continuous positive airway pressure therapy. Yearb Pulm Dis. 2006;2006:239–40.
Poirier J, George C, Rotenberg B. The effect of nasal surgery on nasal continuous positive airway pressure compliance. Laryngoscope. 2014;124(1):317–9.
Powell NB, Zonato AI, Weaver EM, et al. Radiofrequency treatment of turbinate hypertrophy in subjects using continuous positive airway pressure: a randomized, double-blind, placebo-controlled clinical pilot trial. Laryngoscope. 2001;111(10):1783–90.
Prasad S, Varshney S, Bist SS, Mishra S, Kabdwal N. Correlation study between nasal septal deviation and rhinosinusitis. Indian J Otolaryngol Head Neck Surg. 2013;65(4):363–6.
Rotenberg BW, Pang KP. The impact of sinus surgery on sleep outcomes. Int Forum Allergy Rhinol. 2015;5(4):329–32.
Sériès F, St Pierre SCG. Effects of surgical correction of nasal obstruction in the treatment of obstructive sleep apnea. Am Rev Respir Dis. 1992;146(5 Pt 1):1261–5.
Smith PL, Wise RA, Gold AR, Schwartz AR. Upper airway pressure-flow relationships in obstructive sleep apnea. Permut J Appl Physiol Publ. 1988;1(2):789–95.
Stepnowsky CJ, Moore PJ. Nasal CPAP treatment for obstructive sleep apnea: developing a new perspective on dosing strategies and compliance. J Psychosom Res. 2003;54(6):599–605.
Strollo Jr PJ, Rogers RM. Obstructive sleep apnea. N Engl J Med. 1996;334:99–104.
Acknowledgements
None.
Funding
None.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
All authors met the four criteria for authorship established by the International Committee of Medical Journal Editors: CT was responsible for the conception, design, analysis, and drafting the work, revising the work and reviewing the manuscript. SL, RW, and RC had substantial contributions to the acquisition of data for the work as well as drafting the work, revising the work and reviewing the manuscript. SZ had substantial contributions to data analysis, interpretation of data for the work, and revising the work critically for important intellectual content. Additionally, all authors provided final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring including the accuracy and/or integrity of the work.
Authors’ information
All authors in this manuscript, except for Dr. Ryan Williams, are fellowship trained sleep surgeons graduated from Stanford University. We believe that one of the priorities in the management of OSA patients should be the re-establishment of efficient nasal breathing. The goal of this study was to show that among OSA patients, there is a significant number presenting with other causes of nasal obstruction that are hard, if not impossible, to identify by means of anterior rhinoscopy.
Competing interests
None.
-
i.
No financial and no material support for this research and work.
-
ii.
Authors have no financial interests in any companies or other entities that have an interest in the information in the Contribution (e.g., grants, advisory boards, employment, consultancies, contracts, honoraria, royalties, expert testimony, partnerships, or stock ownership in medically‐related fields).
Consent for publication
Not applicable.
Ethics approval and consent to participate
The protocol for this study was approved by the Institutional Review Board and Hospital Research Ethics Committee of Stanford University (Protocol # 33577).
This is a retrospective case series of recorded flexible laryngoscopy examinations of patients’ airways. Therefore, consent from patients was not obtained.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Torre, C., Capasso, R., Zaghi, S. et al. High incidence of posterior nasal cavity obstruction in obstructive sleep apnea patients. Sleep Science Practice 1, 8 (2017). https://doi.org/10.1186/s41606-016-0002-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41606-016-0002-3