Abstract
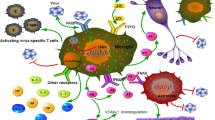
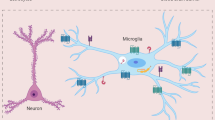
Neuroinflammation can be induced under several conditions including pathogen infection such as virus. As the main immune cells in brain, microglia activation plays a pivotal role in neuroinflammation by responding to the invading pathogens (viral DNA/RNA) through toll-like receptors. Chronic activation of microglia caused by sustained viral infection will lead to persistent release of pro-inflammatory molecules, which is different from their beneficial functions under physiological conditions. Sustained exposure of neurons to the inflammatory condition can result in neuronal dysfunction as well as cell degeneration that contribute to the pathogenesis of several neurological disorders. This review proposed that during sustained infection, viral DNA/RNA activated microglia through TLRs, inducing persistent inflammatory response that causes long term, mild but irreversible changes, which ultimately contribute to the neuronal dysfunction or cell degeneration.
Similar content being viewed by others
Role of microglia in neuroinflammation
Microglia are the main immune cells that are widely spread in the central nervous system (CNS), which are originally derived from the mesodermal yolk sac at the developmental stage [14]. In the normal condition, microglia display as ramified phenotype with a high number of processes which moves and interacts with adjacent blood vessels, neurons as well as astrocytes, that are important to maintain CNS homeostasis and neuronal plasticity [25]. Upon infection or other neural injuries, microglia response to the invading pathogens (exogenous protein and RNAs) or misfolded proteins, retracting their process and change into a reactive-like morphology with hypertrophy of the cell body [50, 58]. Those activated microglia migrate rapidly to brain lesion areas and devour potential invaders or degenerated cells. Along with astrocyte, reactive microglia play a pivotal role in neuroinflammation, releasing several pro-inflammatory cytokines and chemokines, which further recruit more microglia and macrophages to remove cell debris during neural injury [15, 48]. The release of these pro-inflammatory factors including the tumor necrosis factor-α (TNFα) and interleukin-1β (IL-1β), free radicals such as nitric oxide (NO) and superoxide is initially as a defensive strategy of the immune system [17, 57]. However, sustained exposure of neurons to these inflammatory factors can result in neuronal dysfunction as well as cell degeneration that contribute to the pathogenesis of aging related neurodegenerative diseases [26, 51]. The double-edged effects of microglia in brain during disease condition may be explained by the M1/M2 phenotype of the cells. M1 polarization is considered to be pro-inflammatory while the M2 polarization has anti-inflammatory effect that facilitates tissue recovery [51]. Long term and intensive stress condition will lead to sustained activation of microglia that represent neurotoxic M1 phenotype that has detrimental effects to the pathogenesis of neurological diseases [6, 23].
Recently, it is observed that there are microglia mediated synaptic structure loss in several neurological disease model including Alzheimer’s disease, multiple sclerosis and lupus, which further emphasizes the significance of microglia in the pathogenesis of CNS disorders [2, 20, 36, 42]. It is originally suggested microglial cells as important players in pruning excessive synapses during early development in CNS, which is an essential process for brain maturation [16, 38]. Among these studies, the best characterized are the complement cascade regarding C1q and C3 pathway since these KO mice showed significant defects in synapse clearance and neural circuits refinement [16]. Although the same molecular pathway is activated in the progression of these diseases, it is still not clear whether such synaptic clearance in disease condition is similar to the physiological spine pruning during development stage. Neuroinflammation is likely to be involved in the process as it also leads to the dysregulation of microglial phagocytosis.
Toll like receptors in microglia mediated neuroinflammation
The microglial cell surface expresses several important proteins including transporters, channels and receptors, in which there are a class of pattern recognition receptors (PRR) – Toll like receptors (TLRs) [3, 4]. Toll was first shown with important function in Drosophila’s immunity to fungal invading [31]. Afterwards, TLRs were found in mammalian cell that is responsible for the initiation of innate immune to infection. For instance, activation of TLR4 induces pro-inflammatory cytokines such as IL-1, IL-6, and IL-8 in human monocytes in NF-κB dependent manner [35]. Abnormal activation of TLRs may lead to devastating results ranging from sustained inflammation to diseases of autoimmunity [40]. In microglia, there are constitutively expression of TLR3, TLR7 and TLR9 [12]. Although the basal level of these membrane protein is relatively low, their expression is strongly upregulated after viral infection [39, 45].
Among these TLRs, TLR3 responses to viral double-stranded RNA (dsRNA) as well as its synthetic analog, polyinosine-deoxycytidylic acid (poly(I:C)) [32]. In contrast, TLR7 is found to response to single-stranded RNA (ssRNA) in cells after virus infection while TLR9 recognizes DNA with unmethylated CpG motifs from bacteria and virus [7, 28]. The existence of these TLRs in cell surface sensitized response of microglia to exogenous pathogens, including exRNA and exDNA, leading to the neuroinflammation after infection. However, the effect of persistent infection on microglia activation as well as potential neuronal dysfunction needs further investigation.
TLR3 is the first identified PRR that recognize to the viral pathogens by glial cells, which also respond to poly (I:C) producing proinflammatory factor such TNF-α and IL-6 [10, 24]. In addition, administration of poly (I:C) in brain results in microglia activation that ultimately cause neuronal damage, while such neural injury is remarkably alleviated in TLR3 deficient mice [52]. There are substantial evidences that Poly(I:C) not only regulates the gene expression but also modulates immune response in microglia, which further affects neuronal function during stress condition [11, 32]. Furthermore, the expression of TLR3 is strongly upregulated at the very beginning of viral infection, indicting the important role of TLR3 in viral RNA response [33]. When binding to the dsRNA produced by viral during infection, TLR3 dimerized and activated transcription factors including nuclear factor-κB (NF-κB), that further modulate the cytokine production and induce viral associated encephalitis [24]. In line with this finding, TLR3 absence can protect the mice from viral induced neuroinflammation by reducing microglia activation [56]. Nevertheless, it is also reported that TLR7 expression increased in the brains of RABV infected mice [29]. Activation of microglia through TLR7 results in the production of inflammatory factors including TNF-α, IL-1β, IL-6, and IL-12 as well as several chemokines such as CCL2, CCL3 and CSCL1 [28]. These results suggest the positive role of TLRs in mediating neuroinflammatory response after viral infection. Although deficiency of TLRs signaling reduced glial cells activation and inflammatory response in CNS, the following consequences after viral infection is much more complicated. Mice lack TLR7 signaling have defects in both the development of peripheral immunity and viral clearance in the CNS in RABV model [29]. In contrast, in HSV-1 mice model, absence of TLR2 expression lead to alleviated CNS inflammation and increased rate of survival. Microglia without TLR2 expression demonstrated less neuronal toxicity after HSV-1 infection [1].
Viral infection and neurological disorders
A wide range of viruses may cause immediate or delayed neurological manifestations in humans and animals. Infection by neurotropic viruses as well as the consequent immune response can lead to irreversible disruption of functional structure in CNS, which frequently cause significant clinical phenotype [21, 22, 60]. In addition to the immediate and direct effects, there are several neurological diseases that are considered to be long term and delayed virus-induced disorders: multiple sclerosis, Guillain–Barré syndrome and encephalitis lethargica, which are usually associated with autoimmune response. Varicella zoster virus infections in immunocompromised patients may induce multifocal encephalitis, cerebral infarcts and macrophage-rich demyelinating “multiple sclerosis-like” periventricular cerebral and spinal cord lesions [44]. In several West Nile virus (WNV) infection cases, muscle weakness with a similar presentation to Guillain–Barré syndrome are reported [27]. These viral infection-induced symptoms mentioned above are relatively easy to identified. However, there are mild, long term but irreversible change after viral infection that may possibly be neglected, playing critical roles in those slow progressive neurological disorders during developmental stage or aging such as Autism spectrum disorders (ASD) and Alzheimer’s disease (AD) [9, 19].
ASD are considered as developmental neural disorders without a definitive etiology, which is characterized by impairments in two core domains: social-communication and restricted and repetitive patterns of behavior, interest or activities [5]. Congenital cytomegalovirus (CMV) infection are reported to be associated with the onset of ASD, in which virus infection may cause abnormality in brain development and neuronal connectivity. Ivan et al. investigated the prevalence of congenital CMV infection in children with ASD and demonstrated that the infection rate was 10-fold higher in ASD population than in healthy control at birth [13]. There is also demonstration that valnoctamide inhibits CMV infection during developmental stage and improved neurobehavioral dysfunctions and alleviated brain abnormalities indicating the pivotal role of CMV infection in the pathogenesis of ASD [41]. Deficit in dendritic spine pruning is reported in ASD patients and changes in synaptic structure are detected in multiple ASD model mice [59]. Postnatal synaptic development in brain is a dynamic process regulated by both synapse formation and elimination. Microglia plays the major work in dendritic spine pruning both in physiological and pathological condition [16]. Therefore, it is possible that congenital CMV infection alters microglial cells function which ultimately results in the abnormalities in dendritic spine development. In line with this, it is demonstrated that targeting microglia alleviates the neurodevelopmental defects after CMV infection [8].
Alzheimer’s disease (AD) is one of the most popular neurodegenerative diseases in aged people that characterized by the progressive loss of memory and cognitive dysfunction [30]. The pathological hallmark of AD are amyloid plaques composed of β-amyloid (Aβ) protein, neurofibrillary tangles with hyperphosphorylated tau protein, as well as sustained neuroinflammation [47]. Circumstantial evidences suggest the association between cognitive decline and the levels of cytokines in AD patients at all stages, targeting this process in AD may contribute to the diagnostic as well as therapeutic purposes [46]. Various pathogens are indicated to contribute to the pathogenesis of AD, including Herpes simplex virus type 1 (HSV-1), Cytomegalovirus, and Chlamydophila pneumoniae [34, 49]. However, a consistent association with specific viral species has not been identified until recently, a multiscale analysis of independent AD cohort showed that AD patients have increased human herpesvirus 6A (HHV-6A) and human herpesvirus 7 (HHV-7) infection rate [43]. These pathogens usually cause persistent infection that may lead to chronic inflammation, in which pathogen DNA and RNA increase the level of pro-inflammatory factors by activating glial cells in the CNS, destroying neuron directly or indirectly. Especially in aging brain, microglia are primed that will response more quickly and robustly to the invading pathogen such as DNA/RNA from virus, causing more inflammatory molecules production and dysregulated phagocytosis [18]. Nevertheless, the blood brain barrier integrity is compromised during aging, resulting in increased risk of pathogen (viral RNA/DNA) infection in brain [37, 53]. In the meantime, the aging brain is usually characterized by increased neuroinflammation and declined cognition, which is at least partially due to the sustained exposure to exogenous pathogen DNA/RNA in brain [54]. Notably, it is reported that during WNV-induced memory impairment, there is remarkable synapses loss which is driven by complement-microglial axis. This study identifies a potential mechanism underlying virus induced memory loss, in which microglia plays a pivotal role [55].
Conclusion
Taken together, we proposed that during infection, exogenous DNA/RNA activated microglia through TLRs, which induces inflammatory response with dysregulated cytokine production as well as phagocytosis, that further destroy the structure of neural connectivity and neuronal survival, leading to the neuronal dysfunction or neurodegeneration.
Abbreviations
- ASD:
-
Autism spectrum disorders
- CCL:
-
Chemokine (C-C motif) ligand
- CMV:
-
Congenital cytomegalovirus
- CNS:
-
Central nervous system
- HHV-6A:
-
Human herpesvirus 6A
- HHV-7:
-
Human herpesvirus 7
- HSV-1:
-
Herpes simplex virus 1
- IL-1β:
-
Interleukin-1β
- NF-κB:
-
Nuclear factor-κB
- NO:
-
Nitric oxide
- poly(I:C):
-
Polyinosine-deoxycytidylic acid
- PRR:
-
Pattern recognition receptors
- RABV:
-
Rabies virus
- TLRs:
-
toll-like receptors
- TNFα:
-
Tumor necrosis factor-α
References
Aravalli RN, Hu S, Lokensgard JR. Toll-like receptor 2 signaling is a mediator of apoptosis in herpes simplex virus-infected microglia. J Neuroinflammation. 2007;4:11.
Bialas AR, Presumey J, Das A, van der Poel CE, Lapchak PH, Mesin L, Victora G, Tsokos GC, Mawrin C, Herbst R, Carroll MC. Microglia-dependent synapse loss in type I interferon-mediated lupus. Nature. 2017;546:539–43.
Bruno K, Woller SA, Miller YI, Yaksh TL, Wallace M, Beaton G, Chakravarthy K. Targeting toll-like Receptor-4 (TLR4) - emerging therapeutic target for persistent pain states. Pain. 2018;159(10):1908–1915.
Caplan IF, Maguire-Zeiss KA. Toll-like receptor 2 signaling and current approaches for therapeutic modulation in Synucleinopathies. Front Pharmacol. 2018;9:417.
Cattane N, Richetto J, Cattaneoa A. Prenatal exposure to environmental insults and enhanced risk of developing schizophrenia and autism Spectrum disorder: focus on biological pathways and epigenetic mechanisms. Neurosci Biobehav Rev. 2018.
Cheon SY, Kim EJ, Kim JM, Kam EH, Ko BW, Koo BN. Regulation of microglia and macrophage polarization via apoptosis signal-regulating kinase 1 silencing after ischemic/hypoxic injury. Front Mol Neurosci. 2017;10:261.
Chinnery HR, McLenachan S, Binz N, Sun Y, Forrester JV, Degli-Esposti MA, Pearlman E, McMenamin PG. TLR9 ligand CpG-ODN applied to the injured mouse cornea elicits retinal inflammation. Am J Pathol. 2012;180:209–20.
Cloarec R, Bauer S, Teissier N, Schaller F, Luche H, Courtens S, Salmi M, Pauly V, Bois E, Pallesi-Pocachard E, et al. In utero Administration of Drugs Targeting Microglia Improves the neurodevelopmental outcome following cytomegalovirus infection of the rat fetal brain. Front Cell Neurosci. 2018;12:55.
Costa AS, Agostini S, Guerini FR, Mancuso R, Zanzottera M, Ripamonti E, Racca V, Nemni R, Clerici M. Modulation of immune responses to herpes simplex virus type 1 by IFNL3 and IRF7 polymorphisms: a study in Alzheimer's disease. J Alzheimers Dis. 2017;60:1055–63.
Das A, Chai JC, Kim SH, Lee YS, Park KS, Jung KH, Chai YG. Transcriptome sequencing of microglial cells stimulated with TLR3 and TLR4 ligands. BMC Genomics. 2015;16:517.
Field R, Campion S, Warren C, Murray C, Cunningham C. Systemic challenge with the TLR3 agonist poly I:C induces amplified IFNalpha/beta and IL-1beta responses in the diseased brain and exacerbates chronic neurodegeneration. Brain Behav Immun. 2010;24:996–1007.
Gambuzza ME, Sofo V, Salmeri FM, Soraci L, Marino S, Bramanti P. Toll-like receptors in Alzheimer's disease: a therapeutic perspective. CNS Neurol Disord Drug Targets. 2014;13:1542–58.
Gentile I, Zappulo E, Riccio MP, Binda S, Bubba L, Pellegrinelli L, Scognamiglio D, Operto F, Margari L, Borgia G, Bravaccio C. Prevalence of congenital cytomegalovirus infection assessed through viral genome detection in dried blood spots in children with autism Spectrum disorders. In Vivo. 2017;31:467–73.
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–5.
Greenmyer JR, Gaultney RA, Brissette CA, Watt JA. Primary human microglia are Phagocytically active and respond to Borrelia burgdorferi with upregulation of chemokines and cytokines. Front Microbiol. 2018;9:811.
Harry GJ. Microglia during development and aging. Pharmacol Ther. 2013;139:313–26.
Hernandez-Romero MC, Delgado-Cortes MJ, Sarmiento M, de Pablos RM, Espinosa-Oliva AM, Arguelles S, Bandez MJ, Villaran RF, Maurino R, Santiago M, et al. Peripheral inflammation increases the deleterious effect of CNS inflammation on the nigrostriatal dopaminergic system. Neurotoxicology. 2012;33:347–60.
Holtman IR, Raj DD, Miller JA, Schaafsma W, Yin Z, Brouwer N, Wes PD, Moller T, Orre M, Kamphuis W, et al. Induction of a common microglia gene expression signature by aging and neurodegenerative conditions: a co-expression meta-analysis. Acta Neuropathol Commun. 2015;3:31.
Honda T, Sofuku K, Matsunaga H, Tachibana M, Mohri I, Taniike M, Tomonaga K. Prevalence of antibodies against Borna disease virus proteins in Japanese children with autism spectrum disorder. Microbiol Immunol. 2018. https://doi.org/10.1111/1348-0421.12603.
Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–6.
Hosseini S, Wilk E, Michaelsen-Preusse K, Gerhauser I, Baumgartner W, Geffers R, Schughart K, Korte M. Long-term Neuroinflammation induced by influenza a virus infection and the impact on hippocampal neuron morphology and function. J Neurosci. 2018;38:3060–80.
Hsu DC, Sunyakumthorn P, Wegner M, Schuetz A, Silsorn D, Estes JD, Deleage C, Tomusange K, Lakhashe SK, Ruprecht RM, et al. Central nervous system inflammation and infection during early, nonaccelerated simian-human immunodeficiency virus infection in rhesus macaques. J Virol. 2018;92. https://doi.org/10.1128/JVI.00222-18.
Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron. 2017;95:1246–65.
Jiang R, Ye J, Zhu B, Song Y, Chen H, Cao S. Roles of TLR3 and RIG-I in mediating the inflammatory response in mouse microglia following Japanese encephalitis virus infection. J Immunol Res. 2014;2014:787023.
Larson TA. Sex steroids, adult neurogenesis, and inflammation in CNS homeostasis, degeneration, and repair. Front Endocrinol (Lausanne). 2018;9:205.
Le W, Rowe D, Xie W, Ortiz I, He Y, Appel SH. Microglial activation and dopaminergic cell injury: an in vitro model relevant to Parkinson's disease. J Neurosci. 2001;21:8447–55.
Leis AA, Stokic DS. Neuromuscular manifestations of west nile virus infection. Front Neurol. 2012;3:37.
Lewis SD, Butchi NB, Khaleduzzaman M, Morgan TW, Du M, Pourciau S, Baker DG, Akira S, Peterson KE. Toll-like receptor 7 is not necessary for retroviral neuropathogenesis but does contribute to virus-induced neuroinflammation. J Neuro-Oncol. 2008;14:492–502.
Li J, Faber M, Dietzschold B, Hooper DC. The role of toll-like receptors in the induction of immune responses during rabies virus infection. Adv Virus Res. 2011;79:115–26.
Li L, Zhang X, Yang D, Luo G, Chen S, Le W. Hypoxia increases Abeta generation by altering beta- and gamma-cleavage of APP. Neurobiol Aging. 2009;30:1091–8.
Lindsay SA, Lin SJH, Wasserman SA. Short-form Bomanins mediate humoral immunity in Drosophila. J Innate Immun. 2018:1–9.
Marinelli C, Di Liddo R, Facci L, Bertalot T, Conconi MT, Zusso M, Skaper SD, Giusti P. Ligand engagement of toll-like receptors regulates their expression in cortical microglia and astrocytes. J Neuroinflammation. 2015;12:244.
McKimmie CS, Fazakerley JK. In response to pathogens, glial cells dynamically and differentially regulate toll-like receptor gene expression. J Neuroimmunol. 2005;169:116–25.
McManus RM, Heneka MT. Role of neuroinflammation in neurodegeneration: new insights. Alzheimers Res Ther. 2017;9:14.
Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7.
Michailidou I, Willems JG, Kooi EJ, van Eden C, Gold SM, Geurts JJ, Baas F, Huitinga I, Ramaglia V. Complement C1q-C3-associated synaptic changes in multiple sclerosis hippocampus. Ann Neurol. 2015;77:1007–26.
Montgomery RR. Age-related alterations in immune responses to West Nile virus infection. Clin Exp Immunol. 2017;187:26–34.
Norris GT, Smirnov I, Filiano AJ, Shadowen HM, Cody KR, Thompson JA, Harris TH, Gaultier A, Overall CC, Kipnis J. Neuronal integrity and complement control synaptic material clearance by microglia after CNS injury. J Exp Med. 2018;215:1789–801.
Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–24.
O'Neill LA, Golenbock D, Bowie AG. The history of toll-like receptors - redefining innate immunity. Nat Rev Immunol. 2013;13:453–60.
Ornaghi S, Hsieh LS, Bordey A, Vergani P, Paidas MJ, van den Pol AN. Valnoctamide inhibits cytomegalovirus infection in developing brain and attenuates neurobehavioral dysfunctions and brain abnormalities. J Neurosci. 2017;37:6877–93.
Presumey J, Bialas AR, Carroll MC. Complement system in neural synapse elimination in development and disease. Adv Immunol. 2017;135:53–79.
Readhead B, Haure-Mirande JV, Funk CC, Richards MA, Shannon P, Haroutunian V, Sano M, Liang WS, Beckmann ND, Price ND, et al. Multiscale analysis of independent Alzheimer's cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018.
Ruprecht K, Wildemann B, Jarius S. Low intrathecal antibody production despite high seroprevalence of Epstein-Barr virus in multiple sclerosis: a review of the literature. J Neurol. 2018;265:239–52.
Sabouri AH, Marcondes MC, Flynn C, Berger M, Xiao N, Fox HS, Sarvetnick NE. TLR signaling controls lethal encephalitis in WNV-infected brain. Brain Res. 2014;1574:84–95.
Shal B, Ding W, Ali H, Kim YS, Khan S. Anti-neuroinflammatory potential of natural products in attenuation of Alzheimer's disease. Front Pharmacol. 2018;9:548.
Shen W, Zhu L, Lee SR, Chung SH, Gillies MC. Involvement of NT3 and P75(NTR) in photoreceptor degeneration following selective Muller cell ablation. J Neuroinflammation. 2013;10:137.
Singh DK, Ling EA, Kaur C. Hypoxia and myelination deficits in the developing brain. Int J Dev Neurosci. 2018.
Sochocka M, Zwolinska K, Leszek J. The infectious etiology of Alzheimer's disease. Curr Neuropharmacol. 2017;15:996–1009.
Sun X, Sun J, Shao X, Feng J, Yan J, Qin Y. Inhibition of microRNA-155 modulates endotoxin tolerance by upregulating suppressor of cytokine signaling 1 in microglia. Exp Ther Med. 2018;15:4709–16.
Tang Y, Li T, Li J, Yang J, Liu H, Zhang XJ, Le W. Jmjd3 is essential for the epigenetic modulation of microglia phenotypes in the immune pathogenesis of Parkinson's disease. Cell Death Differ. 2014;21:369–80.
Town T, Jeng D, Alexopoulou L, Tan J, Flavell RA. Microglia recognize double-stranded RNA via TLR3. J Immunol. 2006;176:3804–12.
Uno M, Takano T, Yamano T, Shimada M. Age-dependent susceptibility in mumps-associated hydrocephalus: neuropathologic features and brain barriers. Acta Neuropathol. 1997;94:207–15.
Valcarcel-Ares MN, Tucsek Z, Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T, Galvan V, Ballabh P, et al. Obesity in aging exacerbates Neuroinflammation, dysregulating synaptic function-related genes and altering eicosanoid synthesis in the mouse Hippocampus: potential role in impaired synaptic plasticity and cognitive decline. J Gerontol A Biol Sci Med Sci. 2019;74(3):290–298.
Vasek MJ, Garber C, Dorsey D, Durrant DM, Bollman B, Soung A, Yu J, Perez-Torres C, Frouin A, Wilton DK, et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016;534:538–43.
Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–73.
Wilms H, Sievers J, Rickert U, Rostami-Yazdi M, Mrowietz U, Lucius R. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model of brain inflammation. J Neuroinflammation. 2010;7:30.
Zabala A, Vazquez-Villoldo N, Rissiek B, Gejo J, Martin A, Palomino A, Perez-Samartin A, Pulagam KR, Lukowiak M, Capetillo-Zarate E, et al. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol Med. 2018;10(8). https://doi.org/10.15252/emmm.201708743.
Zoghbi HY, Bear MF. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb Perspect Biol. 2012;4(3). https://doi.org/10.1101/cshperspect.a009886.
Zukor K, Wang H, Hurst BL, Siddharthan V, Van Wettere A, Pilowsky PM, Morrey JD. Phrenic nerve deficits and neurological immunopathology associated with acute West Nile virus infection in mice and hamsters. J Neuro-Oncol. 2017;23:186–204.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (31471019) and (31741053).
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
MS, WJ, DX and LL participated the discussion of manuscript, MS and LL wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, L., Mao, S., Wang, J. et al. Viral infection and neurological disorders—potential role of extracellular nucleotides in neuroinflammation. ExRNA 1, 26 (2019). https://doi.org/10.1186/s41544-019-0031-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41544-019-0031-z