Abstract
Background
COVID-19 vaccination has been advocated as the most effective way to curb the pandemic. But with its inequitable distribution and slow rollout, especially in low- to middle- income countries, it will still take a long time before herd immunity is achieved. Alternative measures must therefore be explored to bolster current COVID-19 vaccination efforts. In particular, the Bacille Calmette-Guerin vaccine has been studied extensively as to its proposed conferment of non-specific immunity against different infections, including COVID-19. The aim of this study, therefore, is to evaluate the current evidence on the effectiveness of national BCG vaccination policies in reducing infection and mortality of COVID-19.
Methods
A systematic review was conducted between April to August 2021 following the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA-P) guidelines. Literature was retrieved from PubMed, Cochrane, HERDIN, Web of Science, EBSCO, and Western Pacific Region Index Medicus (WPRIM). Studies conducted from January 2020 to August 2021 that fell within Level 1A to 2C of the Oxford Center for Evidence-Based Medicine were included in the review. Quality assessment was performed using the appropriate Joanna Briggs Institute critical appraisal tool and a quality assessment checklist for ecological studies adapted from Betran et al.
Results
A total of 13 studies were included in this review. Nine studies reported significant association between BCG vaccination policies and COVID-19 outcomes, even when controlling for confounding variables. In addition, among other mandated vaccines, such as pneumococcal, influenza, diphtheria-tetanus-pertussis, and measles, only BCG vaccination showed significant association with decreased COVID-19 adverse outcomes. However, other factors also showed positive association with COVID-19 outcomes, particularly markers of high economic status of countries, higher median age, and greater population densities.
Conclusion
The lower incidence and mortality of COVID-19 in countries with mandated BCG vaccination may not solely be attributable to BCG vaccination policies, but there is still some evidence that demonstrates a possible protective effect. Clinical trials must be continued before recommendations of BCG vaccinations are to be used as an alternative or booster vaccine against COVID-19.
Similar content being viewed by others
Background
The Coronavirus disease 2019 (COVID-19) caused a global pandemic that brought the whole world to a halt. National governments instituted sweeping lockdowns, rigorous physical distancing, and aggressive contact tracing in an effort to stop the spread of the virus. Although most cases arise from asymptomatic individuals, severe COVID-19 proves very fatal because it has long term effects on the lungs, heart, and even the central nervous system [1]. As of January 2022, COVID-19 has now reached 379.09 million cases worldwide with 5.70 million deaths [2]. As with previous health crises such as polio and measles, one of the most effective ways to curb their spread is through vaccination. Vaccines have been shown to be the safest method to prevent COVID-19 by directly conferring immune protection to inoculated individuals as well as indirectly through herd immunity [3,4,5]. Herd immunity is defined as the indirect protection conferred by a large proportion of immune individuals to susceptible individuals against infection [4, 6, 7]. The herd immunity threshold for the initial SARS-CoV-2 virus, or the proportion of immune individuals needed to observe a decline in incidence of infection, has been estimated to be 67% of every country’s population [3, 4, 6, 7]. Due to evolving variants, the virus increased its transmissibility by more than 50%, giving rise to the need to increase the herd immunity threshold to up to 80% of the population [8].
In the hopes of permanently putting a stop to the pandemic, a multinational race began to produce vaccines capable of building herd immunity. Countries such as the United States, Russia, Germany, and China, have put forward their own vaccines which have shown varying effectiveness in preventing COVID-19 and reducing its severity and mortality. Prominent vaccines currently administered are Pfizer-BioNtech, Moderna, AstraZeneca, Gamaleya, and Sinovac with each having been granted emergency use authorization [9].
Despite this progress, there are still significant barriers that must be faced before reaching herd immunity. As of January 2022, there are 4.16 billion fully vaccinated individuals, which only comprise 52.5% of the world population even with 31.92 million vaccines administered each day [2]. These data attest that achieving herd immunity may still be far ahead. One of the most significant challenges with vaccination is vaccine inequity. New COVID-19 cases and deaths continue to rise in places with high community transmission and low vaccination coverage, which may also lead to eventual vaccine resistance [10]. Despite the high rollout of vaccines in high- and upper-middle income countries, low- and middle-income countries (LMIC) lag far behind in terms of vaccine distribution. Only 41% of people in LMICs have received at least one dose as of January 2022, compared to the 74.8% of upper-middle income countries [2, 11]. In addition to vaccine inequity, there are still issues on vaccine hesitancy and questions regarding the vaccine efficacy of current vaccines against emerging COVID-19 variants [12]. These factors may further prolong the road to herd immunity, especially in LMICs. Although COVID-19 vaccines present the most viable way to end the pandemic, other strategies have to be explored as possible adjunct measures to bolster the current vaccination effort.
One such strategy that has been heavily studied is the possible role of Bacille Calmette-Guerin vaccine, otherwise known as BCG, against COVID-19. BCG is a live attenuated vaccine derived from Mycobacterium bovis and is the only vaccine approved for use in the prevention of pulmonary and extra-pulmonary tuberculosis [13]. Aside from tuberculosis, studies have proposed that BCG provides non-specific immunity against non-tuberculous infections and some immunotherapeutic benefit to malignancies such as non-muscle invasive bladder cancer [13, 14].
This could potentially explain the delayed COVID-19 spread in low-income countries, where BCG vaccination is currently mandated [15]. Some properties of BCG vaccines can also support COVID-19 vaccine design, such as its established safety profile, easy accessibility, and better public acceptance [16,17,18]. Based on these factors, it could be used as a model for future vaccine development [13, 14, 16,17,18] The aim of this study, therefore, is to evaluate the current evidence on the effectiveness of national BCG vaccination policies in reducing infection and mortality of COVID-19 and discuss the continued importance of BCG vaccines.
Methods
Study design
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-P) as outlined by Shamseer et al. [19]. This protocol was published previously [20] and was registered with the International Prospective Register for Systematic Reviews (PROSPERO ID: CRD40221244060, Additional file 1). Ethics approval was not required due to the nature of the study.
Study setting
This study reviewed literature that reported on BCG vaccination policies and its association with COVID-19 severity and mortality. Literature was classified according to BCG vaccination policies of countries according to the BCG World Atlas, Our World in Data, and the World Health Organization (WHO).
Study period
Studies included in the review were those published between January 2020, which was when COVID-19 was initially reported to the WHO, until July 2021. Literature search was conducted from April to August 2021.
Search strategy
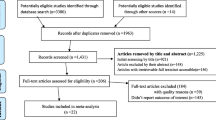
Search terms were generated using the Population/Intervention/Comparison/Outcomes (PICO) approach, guided by the research question “What is the effectiveness of national BCG vaccination policies in reducing infection and severity of COVID-19 in their native population?” Databases included PubMed, Cochrane, HERDIN, Web of Science, EBSCO, and Western Pacific Region Index Medicus (WPRIM). The search terms used are indicated in Table 1. Snowball search was employed by manual review of citations and reference sections of the studies for additional relevant articles [21]. Figure 1 shows the study selection flow chart following the PRISMA-P guidelines.
Inclusion and exclusion criteria
Articles included studies classified under Level 1A to 2C of the Oxford Center for Evidence-Based Medicine (2009) [22]. This included longitudinal studies, randomized controlled trials, ecological studies, and systematic reviews. Descriptive studies, commentaries, editorials, ongoing research, unpublished studies, and other working papers were excluded from the study. Articles not written in English, those published prior to January 2020, or those unavailable in full text were excluded. Search results were tabulated in Google Sheets for duplicate removal. JCO, MS, CE, JT, and MJMP individually screened the studies for eligibility. Any disagreements were resolved via consensus or discussion (Additional file 2).
Studies that met the inclusion criteria were assessed by two authors regarding methodological quality using the Joanna Briggs Institute critical appraisal instruments appropriate for the study design. A quality assessment checklist adapted from Betran et al. was used to appraise ecological studies (Additional file 3) [23]. The researchers agreed on a cut-off score of 70% of the total items of the quality assessment tools to be included in the review [24]. Disagreements between two reviewers were resolved by having a third reviewer assess the study. Further disagreements were resolved through discussion and consensus (Additional file 4).
Data extraction and synthesis
Extracted data from the selected studies were tabulated via Google sheets. Data included the authors, type of study, month and year of publication, population (countries included for ecological studies), source of BCG vaccination policy status, source of COVID-19 data, outcomes and confounding factors included, and the results of the study. JCO and CTE checked the accuracy of the recorded data.
Data synthesis consisted of comparing and contrasting the results of the outcomes and was interpreted using descriptive and inferential statistics applicable in the studies. Results of confounders were also discussed to better understand if the association between BCG vaccination policies and COVID-19 infection and severity was affected by other factors.
Results
A total of 220 studies were identified on initial literature search. After screening for duplicates and eligibility, 38 studies were sought for retrieval. Upon quality assessment, 13 studies were included in the final review. Figure 1 shows the PRISMA flow diagram depicting the literature search. Of the 13 studies, 11 were ecological and 2 were cohorts. Table 2 shows the characteristics of the studies included in the review. Studies were further categorized into those that used statistical correlation tests (e.g., Spearman, Pearson, etc.) and those that used regression analysis.
Correlation between BCG vaccination and COVID-19 morbidity and mortality
Studies that used statistical correlation methods were synthesized together to assess the correlation between BCG policies and COVID-19 outcomes (Table 3). Four studies were included in this assessment. Statistical tests used by reviewed studies were either Spearman rho [28, 29] or Pearson [30, 37]. Two of the studies showed no significant correlation between mandatory BCG vaccination and COVID-19 outcomes while two studies showed strong correlation between BCG vaccination and decreased COVID-19 outcomes.
Association between BCG vaccination and COVID-19 morbidity and mortality
To assess for overall cause-and-effect, studies that used regression analysis were grouped together and compared. Table 4 presents the findings of the studies included. Of the 12 studies that used regression analysis, only 3 studies did not find a significant association between BCG vaccination and COVID-19 outcomes [26, 27, 35]. One study found a significant association between BCG vaccination and COVID-19 deaths per million but found no significant association between BCG vaccination and COVID-19 deaths per case [29]. Figures 2 and 3 show the magnitude of association between BCG vaccination policies and COVID-19 morbidity and mortality. These studies indicate the large magnitude of association between vaccination policies and COVID-19 outcomes, which may demonstrate the protective effect of such policies.
Effects of confounding variables
Even after controlling for confounding variables, most studies still found a significant association between BCG vaccination policies and decreased COVID-19 outcomes. The list of confounding variables identified per study is presented in Table 3. Positive associations were consistently observed between demographic variables, such as higher median age [29,30,31, 37] proportion of population > 65 years [25, 27, 28, 34], population density [25, 30, 31, 34], and COVID-19 outcomes. Evidence of a country’s higher economic status, namely high gross domestic product (GDP), high gross national income (GNI), and high World Bank classification, was consistently associated with higher COVID-19 incidence and mortality [28,29,30, 34, 35]. ‘Historic colonization status,’ an arbitrary variable classifying countries as to their history of participation in colonization efforts in the late 1400 s, was also used as a marker for advanced economies and was strongly associated with COVID-19 outcomes [29].
Some studies also observed relationships between other mandated vaccination programs and COVID-19 outcomes. Diphtheria, tetanus, and pertussis (DTP) coverage was not seen to be significant in decreasing COVID-19 outcomes [30, 35]. In two studies, measles vaccination coverage either showed a significant albeit weaker association to decreased COVID-19 outcomes compared to BCG or no association at all [32, 34, 35]. Pneumococcal, meningococcal, and influenza vaccinations were also assessed and were shown to have no significant effect on COVID-19 outcomes [36]. Lastly, oral polio vaccination showed no significant association to COVID-19 outcomes [34].
Discussion
Summary of the findings
Overall, most studies indicate an inverse association between BCG vaccination policies and COVID-19 incidence and mortality. However, this review presents a different finding from the study done by Ricco et al. early on in the pandemic, which previously concluded that there is no sound evidence to recommend BCG vaccination for the prevention of COVID-19 [38]. Findings may result from the inclusion of more recent studies on the topic, including those from early to mid-2021 [30, 32, 34, 35]. For one, Ricco et al. report that only two out of the 14 studies reviewed controlled for confounding variables. Meanwhile, most studies included in this review controlled for some or all confounding variables reported. In addition, Ricco et al. point out that COVID-19 outcomes highly depend on the reliability of reporting COVID-19 cases and deaths. Although consistent with other studies [29, 39], this may have been a more significant factor in the early phases of the pandemic because of its more erratic and uncertain course at the time [40]. As the pandemic continued, more robust testing and reporting strategies were implemented [30, 41], thus increasing the raw data quality.
Nonspecific protective effect of BCG vaccination
Numerous studies have proposed the nonspecific immune effect of BCG on unrelated infections as a possible mechanism to explain its protective effect against COVID-19. BCG has long been reported to grant "trained immunity," a nonspecific innate immune response resulting from epigenetic reprogramming. Through epigenetic reprogramming, BCG modifies human monocytes leading to increased cytokine production. This epigenetic programming explains BCG's ability to confer protection against unrelated infections [42, 43]. In addition, possible long-term effects are brought about by heterologous T helper 1 (Th1) and Th17 immune responses [43] and more rapid seroconversion from enhanced leukocyte response and robust cytokine response to unrelated pathogens. This long-term effect may explain the protection BCG confers on individuals years after vaccination, which is significant since policies usually mandate its inoculation immediately after birth.
Studies included in this review have demonstrated this nonspecific protective effect. Rivas et al. explored the serologic effects of previous BCG vaccination on COVID-19 [36]. The study demonstrated that a history of BCG vaccination in healthcare workers lowers the incidence of clinically or laboratory-confirmed diagnosis of COVID-19. The study also demonstrated that prior BCG vaccination was associated with lower seroconversion and anti-SARS-CoV-2 IgG index even when controlled for age and sex [36]. These lower serological features are still consistent even in the presence of co-morbidities, even though these conditions increase the likelihood of acquiring COVID-19. This study, therefore, gives credence to the supposed nonspecific protection afforded by prior inoculation with BCG.
Compared to other vaccines, only BCG has consistently shown a robust significant association in decreasing COVID-19 outcomes. Studies reported on DTP, meningococcal, pneumococcal, measles, influenza, and oral polio against COVID-19 outcomes have failed to produce the same protective effect compared to BCG [30, 32, 34,35,36]. Results may further illustrate that BCG vaccination alone could confer nonspecific immunity, thereby reducing incidence and mortality related to COVID-19. This stresses the importance of BCG vaccines, not only for its potential against COVID-19, but also against other infectious diseases and non-communicable diseases such as malignancies [13]. Additional studies on BCG must be done to augment these findings, not only to help eradicate tuberculosis but also to increase its potential as a model for future vaccine design [13, 17, 18].
Confounding variables and lower COVID-19 adverse outcomes
This study is the first to synthesize the effects of the common confounding variables reported among studies on BCG and COVID-19 adverse outcomes. Although most of the studies controlled for these variables, their effect on a higher incidence of COVID-19 may still have played a role in the perceived effectiveness of BCG policies against COVID-19.
Markers of the high economic status of a country (e.g., GDP, GNI, World Bank classification) have consistently been associated with increased COVID-19 incidence and mortality [28,29,30, 34, 35]. Those higher-income countries are also likely to have phased out their mandated BCG policies [27, 28, 35]. However, it is essential to note that these higher-income countries are likely to be more developed and may have better healthcare infrastructure, thus enabling adequate testing and contact tracing measures [29, 30, 32]. Consequently, this increases the recorded COVID-19 adverse outcomes in these countries compared to those of lower-income countries, which may suffer from underreporting of cases. This may have contributed to the relatively decreased incidence in countries with a current mandated BCG policy [29, 30] and may therefore underscore the protective effect of BCG against COVID-19 outcomes.
Studies have also reported positive associations between specific population demographics and COVID-19 adverse outcomes. For example, countries with higher median age and population over 65 have shown an increase in COVID-19 incidence and mortality [25, 27,28,29,30,31, 34, 36]. These two factors show that a higher proportion of older residents in these countries may have increased COVID-19 adverse outcomes resulting in more susceptibility to acquiring COVID-19 and exhibiting severe manifestations leading to death [44]. Furthermore, in relation to this study, an older-aged population also reflects these countries' higher economic status, which may have also contributed to the increased incidence and mortality to COVID-19 in these high-income countries [28, 30].
Significance of the findings
This review was able to collate results from various papers about the effectiveness of BCG vaccine policies with COVID-19. Unlike the previous review by Ricco et al., the current study was able to find evidence that supports the protective effect of such policies against COVID-19. The study was also able to account for the influence of various confounding variables in the association. However, due to most studies being ecological or observational, actual cause-and-effect between BCG and COVID-19 incidence and mortality cannot be ultimately concluded. The studies reviewed also point out the possibility that the supposed protective effect may have merely been due to the effect of other variables.
This study, therefore, highlights the importance of conducting clinical trials to determine the effectiveness of an intervention adequately. Short papers and case studies on the topic have shown conflicting results, demonstrating either no protective effect [45, 46] or some effect such as lower hospitalization rates [47]. Studies on animal models have also been performed which may provide physiologic evidence for the perceived protective effect [48]. Recently completed randomized controlled trials are now attesting to the protective effect of BCG, supporting the findings of this study [49,50,51]. In contrast, some early clinical trials show no effect of BCG vaccination on COVID-19 infection [52, 53]. Because of the conflicting nature of the results, these data should be interpreted with utmost prudence [54]. It is therefore still recommended that further studies be continued to map the true effect of BCG vaccination against COVID-19, especially with the rise of variants and the possibility of waning immunity with the current vaccines [55]. Studies have now even shifted to explore the possibility of using BCG as a booster for current COVID-19 vaccines [56]. Further studies to be performed at the molecular or cellular level may also provide further basis for this protective effect. These can help in conclusively establishing a link between BCG vaccination and lower COVID-19 adverse outcomes and may also aid in the production of future vaccines.
Limitations and recommendations
This study has several limitations. For one, our database was only limited to open access journals and those that were published in English. Moreover, our study data gathering was only limited until August 2021. With the COVID-19 situation continuously evolving and with even more papers being published regarding this disease, recent breakthroughs published after the said date might not have been included in this study. We recommend future researchers to include recent data and information not covered in this study.
Conclusion
Even though the lower incidence and mortality due to COVID-19 may not merely be attributable to BCG vaccination policies, there is still a large amount of evidence that shows their possible protective effect. Only actual clinical trials, which are just recently being completed, may truly be able to elucidate the actual effect of BCG vaccination against COVID-19. Therefore, before recommendations can be made regarding the use of BCG vaccination as an adjunct solution to the pandemic, further studies must be done to adequately report its efficacy, including further review of these clinical trials. Lastly, further research to improve BCG vaccines must be continued to strengthen its effectiveness in not only eliminating tuberculosis but also in aid of reducing the morbidity and mortality of other communicable and non-communicable diseases.
Abbreviations
- BCG:
-
Bacille Calmette-Guerin vaccine
- COVID-19:
-
Coronavirus disease 2019
- DTP:
-
Diphtheria-tetanus-pertussis vaccine
- GDP:
-
Gross domestic product
- GNI:
-
Gross national income
- HAQI:
-
Healthcare Access and Quality Index
- IgG:
-
Immunoglobulin G
- LMIC:
-
Low- and middle-income country
- MCV:
-
Measles-containing vaccine
- PCV:
-
Pneumococcal conjugate vaccine
- PRISMA-P:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocol
- Th1:
-
T helper 1 cell
- Th17:
-
T helper 17 cell
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- WHO:
-
World Health Organization
- WPRIM:
-
Western Pacific Region Index Medicus
References
Nuzzo D, Picone P. Potential neurological effects of severe COVID-19 infection. Neurosci Res. 2020;158:1–5.
Ritchie H, Mathieu E, Rodés-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, Hasell J, Macdonald B, Beltekian D, Roser M. Coronavirus pandemic (COVID-19). Published online at OurWorldInData.org. Retrieved from https://ourworldindata.org/coronavirus [Online Resource].
Fontanet A, Cauchemez S. COVID-19 herd immunity: where are we? Nat Rev Immunol. 2020;20(10):583–4.
Omer SB, Yildirim I, Forman HP. Herd immunity and implications for SARS-CoV-2 control. JAMA. 2020;324(20):2095–6.
Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21(2):83–100 (Erratum in: Nat Rev Immunol. 2021 Jan 5).
Randolph HE, Barreiro LB. Herd immunity: understanding COVID-19. Immunity. 2020;52(5):737–41.
McDermott A. Core concept: herd immunity is an important-and often misunderstood-public health phenomenon. Proc Natl Acad Sci U S A. 2021;118(21):e2107692118.
Lippi G, Henry BM. How will emerging SARS-CoV-2 variants impact herd immunity? Ann Transl Med. 2021;9(7):585. https://doi.org/10.21037/atm-21-893.
The COVID-19 vaccine race [Internet]. Gavi, the Vaccine Alliance. 2022 [cited 2022Apr1]. Available from: https://www.gavi.org/vaccineswork/covid-19-vaccine-race.
Rella SA, Kulikova YA, Dermitzakis ET, Kondrashov FA. Rates of SARS-CoV-2 transmission and vaccination impact the fate of vaccine-resistant strains. Sci Rep. 2021;11(1):15729.
Asundi A, O’Leary C, Bhadelia N. Global COVID-19 vaccine inequity: the scope, the impact, and the challenges. Cell Host Microbe. 2021;29(7):1036–9.
Kaplan RM, Milstein A. Influence of a COVID-19 vaccine’s effectiveness and safety profile on vaccination acceptance. Proc Natl Acad Sci U S A. 2021;118(10):e2021726118.
Lange C, Aaby P, Behr MA, Donald PR, Kaufmann SHE, Netea MG, Mandalakas AM. 100 years of mycobacterium bovis bacille Calmette-Guérin. Lancet Infect Dis. 2022;22(1):e2–12.
Haddad-Boubaker S, Othman H, Touati R, Ayouni K, Lakhal M, Ben Mustapha I, Ghedira K, Kharrat M, Triki H. In silico comparative study of SARS-CoV-2 proteins and antigenic proteins in BCG, OPV, MMR and other vaccines: evidence of a possible putative protective effect. BMC Bioinform. 2021;22(1):163.
Malik YS, Ansari MI, Ganesh B, Sircar S, Bhat S, Pande T, Vinodhkumar OR, Kumar P, Iqbal Yatoo M, Tiwari R, Touil N, Patel SK, Pathak M, Sharun K, Dhama K. BCG vaccine: a hope to control COVID-19 pandemic amid crisis. Hum Vaccin Immunother. 2020;16(12):2954–62.
Gong W, Aspatwar A, Wang S, Parkkila S, Wu X. COVID-19 pandemic: SARS-CoV-2 specific vaccines and challenges, protection via BCG trained immunity, and clinical trials. Expert Rev Vaccines. 2021;20(7):857–80.
Angelidou A, Diray-Arce J, Conti MG, Smolen KK, van Haren SD, Dowling DJ, Husson RN, Levy O. BCG as a case study for precision vaccine development: lessons from vaccine heterogeneity, trained immunity, and immune ontogeny. Front Microbiol. 2020;11:332.
Covián C, Fernández-Fierro A, Retamal-Díaz A, Díaz FE, Vasquez AE, Lay MK, Riedel CA, González PA, Bueno SM, Kalergis AM. BCG-induced cross-protection and development of trained immunity: implication for vaccine design. Front Immunol. 2019;10:2806.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
Obnial JC, Suzuki M, Escuadra CJ, Austria JT, Ponce MJ, Sia NE, Lapenas T, Gatpandan-Bergantin MR, Cunanan E. Effectiveness of Bacille Calmette-Guerin vaccination policies in reducing infection and severity of COVID-19: a systematic review protocol. J Med Univ Santo Tomas. 2022;6(1):823–9.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. https://doi.org/10.1136/bmj.n160.
Phillips B, Bal Cl, Sackett D, Badenoch D, Straus S, Haynes B, Dawes M, Howick J. Levels of evidence (March 2009). Oxford Centre for Evidence-Based Medicine. [cited 2 Jun 2021]. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009.
Betran AP, Torloni MR, Zhang J, et al. What is the optimal rate of caesarean section at population level? A systematic review of ecologic studies. Reprod Health. 2015;12:57.
Aromataris E, Munn Z. JBI manual for evidence synthesis. JBI. https://synthesismanual.jbi.global (2020).
Escobar LE, Molina-Cruz A, Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc Natl Acad Sci USA. 2020;117(30):17720–6.
de Chaisemartin C, de Chaisemartin L. Bacille Calmette-Guérin vaccination in infancy does not protect against coronavirus disease 2019 (COVID-19): evidence from a natural experiment in Sweden. Clin Infect Dis. 2021;72(10):e501–5.
Chimoyi L, Velen K, Churchyard GJ, Wallis R, Lewis JJ, Charalambous S. An ecological study to evaluate the association of Bacillus Calmette-Guerin (BCG) vaccination on cases of SARS-CoV2 infection and mortality from COVID-19. PLoS ONE. 2020;15(12):e0243707.
Wickramasinghe D, Wickramasinghe N, Kamburugamuwa SA, Arambepola C, Samarasekera DN. Correlation between immunity from BCG and the morbidity and mortality of COVID-19. Trop Dis Travel Med Vaccines. 2020;6:17.
Szigeti R, Kellermayer D, Trakimas G, Kellermayer R. BCG epidemiology supports its protection against COVID-19? A word of caution. PLoS ONE. 2020;15(10):e0240203.
Li WX. Worldwide inverse correlation between Bacille Calmette-Guérin (BCG) immunization and COVID-19 mortality. Infection. 2021;49(3):463–73.
Berg MK, Yu Q, Salvador CE, Melani I, Kitayama S. Mandated Bacillus Calmette-Guérin (BCG) vaccination predicts flattened curves for the spread of COVID-19. Sci Adv. 2020;6(32):eabc1463.
Ogimi C, Qu P, Boeckh M, Bender Ignacio RA, Zangeneh SZ. Association between live childhood vaccines and COVID-19 outcomes: a national-level analysis. Epidemiol Infect. 2021;149:e75.
Ebina-Shibuya R, Horita N, Namkoong H, Kaneko T. Current national policies for infant universal bacille Calmette-Guérin vaccination were associated with lower mortality from coronavirus disease 2019. Clin Exp Vaccine Res. 2020;9(2):179–82.
Brooks NA, Puri A, Garg S, Nag S, Corbo J, Turabi AE, Kaka N, Zemmel RW, Hegarty PK, Kamat AM. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guerin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11(1):774.
Abdulah DM, Hassan AB. Exploration of association between respiratory vaccinations with infection and mortality rates of COVID-19. In: Disaster medicine and public health preparedness. Cambridge University Press; 2021. p. 1–16.
Rivas MN, Ebinger JE, Wu M, Sun N, Braun J, Sobhani K, Van Eyk JE, Cheng S, Arditi M. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J Clin Invest. 2021;131(2):e145157.
Klinger D, Blass I, Rappoport N, Linial M. Significantly improved COVID-19 outcomes in countries with higher BCG vaccination coverage: a multivariable analysis. Vaccines (Basel). 2020;8(3):378.
Riccò M, Gualerzi G, Ranzieri S, Bragazzi NL. Stop playing with data: there is no sound evidence that Bacille Calmette-Guérin may avoid SARS-CoV-2 infection (for now). Acta Biomed. 2020;91(2):207–13.
Lau H, Khosrawipour T, Kocbach P, Ichii H, Bania J, Khosrawipour V. Evaluating the massive underreporting and undertesting of COVID-19 cases in multiple global epicenters. Pulmonology. 2021;27(2):110–5.
Vandenberg O, Martiny D, Rochas O, van Belkum A, Kozlakidis Z. Considerations for diagnostic COVID-19 tests. Nat Rev Microbiol. 2021;19(3):171–83.
Li H, Burm SW, Hong SH, Ghayda RA, Kronbichler A, Smith L, Koyanagi A, Jacob L, Lee KH, Shin JI. A comprehensive review of coronavirus disease 2019: epidemiology, transmission, risk factors, and international responses. Yonsei Med J. 2021;62(1):1–11.
Arts RJ, Moorlag SJ, Novakovic B, Li Y, Wang SY, Oosting M, Kumar V, Xavier RJ, Wijmenga C, Joosten LA, Reusken CB, Benn CS, Aaby P, Koopmans MP, Stunnenberg HG, van Crevel R, Netea MG. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23(1):89-100.e5.
Kleinnijenhuis J, Quintin J, Preijers F, Benn CS, Joosten LA, Jacobs C, van Loenhout J, Xavier RJ, Aaby P, van der Meer JW, van Crevel R, Netea MG. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J Innate Immun. 2014;6(2):152–8.
Wu JT, Leung K, Bushman M, Kishore N, Niehus R, de Salazar PM, Cowling BJ, Lipsitch M, Leung GM. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med. 2020;26(4):506–10.
Hamiel U, Kozer E, Youngster I. SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults. JAMA. 2020;323(22):2340–1.
Bates MN, Herron TJ, Lwi SJ, Baldo JV. BCG vaccination at birth and COVID-19: a case-control study among U.S. military veterans. Hum Vaccin Immunother. 2022;18(1):1981084. https://doi.org/10.1080/21645515.2021.1981084.
Weng CH, Saal A, Butt WW, Bica N, Fisher JQ, Tao J, Chan PA. Bacillus Calmette-Guérin vaccination and clinical characteristics and outcomes of COVID-19 in Rhode Island, United States: a cohort study. Epidemiol Infect. 2020;148:e140.
Jalalizadeh M, Buosi K, Dionato FAV, Dal Col LSB, Giacomelli CF, Ferrari KL, Pagliarone AC, Leme PAF, Maia CL, Yadollahvandmiandoab R, Trinh QD, Franchini KG, Bajgelman MC, Reis LO. Randomized clinical trial of BCG vaccine in patients with convalescent COVID-19: Clinical evolution, adverse events, and humoral immune response. J Intern Med. 2022. https://doi.org/10.1111/joim.13523.
Tsilika M, Taks E, Dolianitis K, Kotsaki A, Leventogiannis K, Damoulari C, Kostoula M, Paneta M, Adamis G, Papanikolaou I, Stamatelopoulos K, Bolanou A, Katsaros K, Delavinia C, Perdios I, Pandi A, Tsiakos K, Proios N, Kalogianni E, Delis I, Skliros E, Akinosoglou K, Perdikouli A, Poulakou G, Milionis H, Athanassopoulou E, Kalpaki E, Efstratiou L, Perraki V, Papadopoulos A, Netea MG, Giamarellos-Bourboulis EJ. ACTIVATE-2: a double-blind randomized trial of BCG vaccination against COVID-19 in individuals at risk. Front Immunol. 2022;13:873067. https://doi.org/10.3389/fimmu.2022.873067.
Dionato FAV, Jalalizadeh M, Buosi K, Visacri MB, Dal Col LSB, Giacomelli CF, Leme PAF, Maia CL, Moriel P, Reis LO. BCG vaccine safety in COVID-19 convalescent adults: BATTLE a randomized controlled trial. Vaccine. 2022;40(32):4603–8. https://doi.org/10.1016/j.vaccine.2022.06.039.
Dos Anjos LRB, da Costa AC, Cardoso ADRO, Guimarães RA, Rodrigues RL, Ribeiro KM, Borges KCM, Carvalho ACO, Dias CIS, Rezende AO, Souza CC, Ferreira RRM, Saraiva G, Barbosa LCS, Vieira TDS, Conte MB, Rabahi MF, Kipnis A, Junqueira-Kipnis AP. Efficacy and safety of BCG revaccination with M. bovis BCG Moscow to prevent COVID-19 infection in health care workers: a randomized phase II clinical trial. Front Immunol. 2022;13:841868. https://doi.org/10.3389/fimmu.2022.841868.
Ten Doesschate T, van der Vaart TW, Debisarun PA, Taks E, Moorlag SJ, Paternotte N, Boersma WG, Kuiper VP, Roukens AH, Rijnders BJ, Voss A, Veerman KM, Kerckhoffs APM, Oever JT, van Crevel R, van Nieuwkoop C, Lalmohamed A, van de Wijgert JHHM, Netea MG, Bonten MJM, van Werkhoven CH. Bacillus Calmette-Guérin vaccine to reduce healthcare worker absenteeism in COVID-19 pandemic, a randomized controlled trial. Clin Microbiol Infect. 2022. https://doi.org/10.1016/j.cmi.2022.04.009.
Upton CM, van Wijk RC, Mockeliunas L, Simonsson USH, McHarry K, van den Hoogen G, Muller C, von Delft A, van der Westhuizen HM, van Crevel R, Walzl G, Baptista PM, Peter J, Diacon AH, BCG CORONA Consortium. Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: a double-blind, randomised, controlled, phase 3 trial. EClinicalMedicine. 2022;48:101414. https://doi.org/10.1016/j.eclinm.2022.101414.
Gong W, An H, Wang J, Cheng P, Qi Y. The natural effect of BCG vaccination on COVID-19: the debate continues. Front Immunol. 2022;13:953228. https://doi.org/10.3389/fimmu.2022.953228.
Dolgin E. COVID vaccine immunity is waning - how much does that matter? Nature. 2021;597(7878):606–7.
Amirlak L, Haddad R, Hardy JD, Khaled NS, Chung MH, Amirlak B. Effectiveness of booster BCG vaccination in preventing covid-19 infection. Hum Vaccin Immunother. 2021;17:3913–5.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific funding.
Author information
Authors and Affiliations
Contributions
JCO and JTA conceived the idea. JCO, MS, JTA, MJMP, and CJE wrote the draft of the manuscript, collected data and literature with joint equal contribution by EC. EC assisted with article interpretation and language edit. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Supplementary Information
Additional file 1
. PROSPERO Registration.
Additional file 2
. Initial record screening.
Additional file 3
. Quality assessment checklist for ecological studies adapted from Betran et al.
Additional file 4
. Critical appraisal of studies using the adapted appraisal tool from Betran et al. and the JBI Critical Appraisal Instruments.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Obnial, J.C., Suzuki, M., Escuadra, C.J. et al. Effectiveness of Bacille Calmette-Guerin vaccination policies in reducing infection and mortality of COVID-19: a systematic review. glob health res policy 7, 42 (2022). https://doi.org/10.1186/s41256-022-00275-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41256-022-00275-x