Abstract
Vitamin D (VD) is a secosteroid hormone that is mainly synthesized in the skin upon exposure to UVB radiation. VD is widely known for its role in calcium metabolism; however, multiple endocrine, paracrine and autocrine functions of VD have been described, including a prominent role on carcinogenesis. In recent years, multiple associations between VD deficiency and different types of cancer have been described, supported by evidence of anti-proliferative, anti-angiogenic, pro-apoptotic, cell-differentiating and anti-invasive effects of this hormone. An immunomodulatory role of VD associated to cancer microenvironment has also been suggested. Regarding skin cancer, it has been shown that VD inhibits tumor development in basal cell carcinoma, squamous cell carcinoma, and melanoma in vitro. Some studies have suggested that lower VD levels may be a risk factor for skin cancer, while others have shown the opposite; there is also preliminary evidence on the role of VD supplementation for the prevention of melanoma in vivo. In this review, we explore the mechanisms of VD effects on carcinogenesis and the available scientific evidence of the interplay between VD and the genesis of both non-melanoma and melanoma skin cancer.
Similar content being viewed by others
Background
Vitamin D (VD) is a secosteroid hormone that is mainly synthesized in the skin from cholesterol precursors upon exposure to UVB radiation or to a lesser degree acquired from dietary sources. Widely known for its role in calcium metabolism, multiple other endocrine, paracrine and autocrine functions of VD have been described [1], with increasing emphasis on the role in modulating the innate and acquired immunity.
In recent years, a plethora of articles have been published associating lower VD levels with the development of different cancer types (Table 1); additionally, a lower survival rate has been shown in patients with lower VD levels in some studies [2, 3]. Unfortunately, only few reports have found an impact of VD supplementation on the subsequent risk of cancer. The role of VD on the occurrence and prognosis of skin cancer has increasingly been studied, but current evidence is still controversial.
The objective of this review was to explore the emerging role of VD as potential factor for carcinogenesis, mainly focusing on its role in melanoma and non-melanoma skin cancer.
Vitamin D metabolism
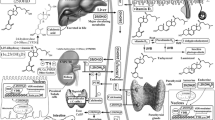
VD is mainly synthesized in the skin (90%) and to a lesser extent obtained from dietary sources (10%) [1]. After exposure to UVB radiation (λ280-320 nm) a photochemical reaction converts 7-dehydrocholesterol to vitamin D3 (VD3) by epidermal basal keratinocytes [4]. VD3, as well as VD2 obtained from diet, is released to the extracellular space and then to the bloodstream, where it is captured by VD binding protein (DBP) [4]. Subsequently, VD is hydroxylyzed in hepatocytes where the hydroxylase CYP2R1 produces 25(OH)D, the major and more stable metabolite of VD commonly used to evaluate VD plasmatic status [5]. Finally, in the kidney 25(OH)D is converted to the active form 1,25(OH) 2D3 (calcitriol) by CYP27B1, which is the active form of VD. Due to its short half-life of the latter, it is not routinely measured [6]. This two enzymatic processes can also be locally made in the epidermis by keratinocytes and melanocytes [7].
A novel pathway of VD metabolism has been recently discovered, in which VD3 is directly metabolized by the hydroxylase CYP11A1 producing a number of different derivatives from VD3, some of which can be metabolized by CYP27B, producing different but active metabolites, mainly 20(OH) D3. This new pathway has been found on the placenta, adrenal glands and epidermal keratinocytes, and may play an important role in carcinogenesis due to antiproliferative and prodifferentiation effects [8].
Vitamin D levels
VD serum levels vary widely by latitude, season, age, body mass index, use of sunblock and skin pigmentation (as melanin absorbs UVB radiation inhibiting VD3 synthesis in the skin) [9, 10]. Despite VD3 can be obtained from diet (about 10% of VD), its metabolic process is not as efficient as skin VD3 synthesis (90% of VD levels): [11] VD3 from skin lasts 2-3 times longer in the serum than dietary VD3 [12]. Moreover, dietary VD3 binds only in a 60% to DBP while the remainder is rapidly cleared; in contrast up to 100% of skin synthetized VD3 can be bound to DBP. In addition, the rest of dietary VD3 is eliminated rapidly through urine [11].
The mechanism of obtaining VD3 from sunlight is also efficient: A 15 min sunlight exposure of arms and legs in a sunny day is sufficient and produces 2000-4000 UI of VD for fair-skinned Central Europeans [13] (this estimates vary according to season, latitude and ethnics) [1, 14, 15].
Vitamin D effects
Once VD is synthesized it has two main actions: First, it binds to the nuclear VD receptor (VDR), which then binds to its co-receptor RXR and form a transcription factor which activates the expression of more than 3000 genes [16]. Second, in the cell membrane VD can bind to a binding protein (PDIA3), where it regulates intracellular calcium concentration [16, 17]. The VDR is an ubiquitous receptor that is expressed by most bodily tissues including intestine, skeletal system, bone marrow, breast, prostate, brain, muscle, immune cells, keratinocytes, melanocytes and neoplastic cells [16, 18]. In the following sections, we will focus on VD's immunologic and anti-carcinogenic effects (Table 2).
Immunologic effect of VD
VD has multiple immunologic effects that result in the ability to promote protective immunity and maintain tolerance [9]. Multiple cross-sectional and epidemiological studies have shown that low levels of VD are associated with an increased risk of respiratory tract infections, tuberculosis, bacterial vaginosis and even HIV infection, among others [19–22].
It is also well studied that low VD levels are associated with higher incidence and severity of several autoimmune diseases such as lupus, rheumatoid arthritis, multiple sclerosis, diabetes and inflammatory bowel disease [9]. VD plays a key role in the innate immune system antimicrobial response. VD directly enhances the transcription of cathelicidin and β-defensin, antimicrobial peptides that actively participate in immunity of skin [23].
VD also acts in B cells (inhibiting proliferation and differentiation of B cells and immunoglobulin secretion); [24] T cells (inhibiting proliferation, and hampering T cell effector responses and increasing T regulatory cells) [9, 25, 26], thus acting in the adaptive immune system, suppressing inflammatory cytokines (IL-17, IL-21) and increasing regulatory cytokine IL-10 [9]. However, effects of VD on the adaptive immune system are complex, as it also causes increases in Th1 immunity by stimulating the transcription of IFNγ, thus having antimicrobial effects. Finally, VD acts in monocytes and dendritic cells suppressing production of important inflammatory cytokines (IL-1, IL-6, IL-8, IL-12 and TNFα) in the former and inhibiting maturation and differentiation in the latter [27, 28].
Role of VD in carcinogenesis modulation
The multiple associations between VD deficiency and higher incidence and mortality due to cancer are of increasing concern, supported by the anti-proliferative, anti-angiogenic, pro-apoptotic, cell-differentiating and anti-invasive effects of this hormone [29–31]. An association has been suggested between low serum VD levels and increased incidence of cancer and mortality, thus outstanding a possible prophylactic role of VD, among many others [29, 32].
Several prospective cohort studies have demonstrated that higher levels of VD are correlated with decreased risk of diagnosis of breast [33], colon [34], renal [35], hepatic [36] and tobacco-related cancers [3, 37]. Low VD serum levels also decrease specific cancer overall survival in breast, colon, hepatic, and bladder carcinomas [3]. However, when adjusting for other prognostic criteria, the association disappeared [3]. Recent studies show that patients with colon cancer and high levels of VD have lower risk of death irrespective of tumor stage [38]. In hepatic carcinoma, a prospective study demonstrated that low VD serum levels correlated with worse cirrhosis level, worse tumor stage and mortality risk [39]. Finally, the WHO in postmenopausal women demonstrated that VD and calcium supplementation reduced the risk of all cancers [40]. However new prospective cohort studies with post diagnostic VD supplementation or high baseline VD serum levels, have failed in showing improvement of all-cause or disease-specific mortality in colon, breast or prostate cancer patients [3, 41]. Therefore, the role of VD as a prophylactic therapy in multiple cancers is still a matter of debate; despite multiple studies showing preliminary evidence of VD as a risk factor for the carcinogenesis and survival in cancer patients.
The role of DBP in cancer is a new focus of research. A recent meta-analysis found a borderline but not significant association between high levels of DBP and decreased cancer risk (OR 0,57 95%CI 0,57-1) [42]. No studies on melanoma or skin cancer have been conducted to evaluate the role of DBP in this population.
Vitamin D and skin cancer
VD immunologic and carcinogenic role in skin cancer is a current point of research and may lead to important changes in management strategies of skin cancers [29].
Keratinocytes produce VD, but can also synthesize locally the active 1,25(OH) 2D form that acts in a autocrine and paracrine fashion. Skin VD synthesis is regulated according to serum calcitriol levels [29]. VD synthesis also varies by the degree of keratinocyte differentiation. Moreover, keratinocytes also express VDR, therefore they respond to calcitriol, which is known as one of the most potent regulator of epidermal differentiation. Other important function of VD is its capacity to induce formation of intercellular junctions by Protein kinase C (PKC) activation [29, 43]. It has been shown that intracellular junctions are closely associated with carcinogenesis, tumor progression and metastasis [29]. In fact, the expression of E-cadherin and B-catenin -important proteins of the intracellular junctions- are decreased in skin tumors: basal cell carcinoma (BCC), squamous cell carcinoma (SCC) and in melanoma [29, 43, 44]. Another role that has been postulated of VD in the skin is its capacity to prevent DNA-damage produced by UVB radiation [45].
It has been shown that VD inhibits Hedgehog signaling pathway, suppressing tumor development in BCC [46]. VDR-knockout mice have increased risk of BCC development after exposure to a carcinogen [47], and VD topical application inhibits BCC cell proliferation in vivo and in vitro [48]. VD has also a role in SCC carcinogenesis, as it inhibits SCC cells growth in vivo and in vitro [49]. Nested- case control studies in elderly men have shown lower incidence of non-melanoma skin cancer (NMSC) in patients with highest serum levels of 25(OH) D [50] and higher levels of 25(OH) D were associated with longer overall survival and fewer metastases [11]. Despite these findings, studies to assess the effect of VD supplementation or topical application in BCC and SCC on humans are lacking.
VD and melanoma
Vitamin D effects in vitro and in vivo
Since the 1970’s there has been evidence of an effect of VD in both melanocytes and melanoma cells. The first finding was that VD stimulates melanogenesis as it promotes tyrosinase synthesis [51]. Then in the 80’s, Colston et al. [52] discovered that VDR was found in melanoma cells, and that VD dramatically reduced cell proliferation, in a dose-response manner. 1,25(OH)2D causes arrest at the G1/S phase in tumor cell cycle, mainly by inhibiting cyclin D1 and an upregulation of p27 and p21 [53].
New findings were made showing that VD inhibits proliferation, induces differentiation of melanoma cells, and inhibits angiogenesis and invasion of melanoma cells [29] with a protective antineoplastic effect. In fact, Gupta et al. demonstrated that VD had an important role in the activation of the DNA repair mechanism after UV radiation, both in keratinocytes and in melanoma cells [54].
The effect of VD in melanoma -as in other cancers- depends of VD serum levels, DBP and the expression of VDR in tissue and its polymorphisms. Studies analyzing the association of genes coding for VD metabolizing enzymes (1 alpha-hydroxylase [CYP27B1] and 1,25(OH) (2)D-24hydroxylase [CYP24A]), and DBP found no association between polymorphisms in these genes and melanoma risk [55].
Melanoma and VDR polymorphisms
The VDR gene contains >1000 polymorphic sites [30]. The most studied in skin cancer are TaqI, BsmI, ApaI and FokI [29, 56]. FokI and BsmI have been identified as high risk alleles, and TaqI allele as a protective polymorphism in melanoma [56]. In addition, the rs4516035 allele has been reported to be a melanoma risk allele [57]. For BsmI bb genotype, recent studies show a significant association with frequency of melanoma, Breslow thickness, and even with the risk of multiple primary melanomas [58].
Melanoma and VDR expression
Recent studies have shown a negative correlation between immunohistochemical VDR expression and progression of pigmented skin lesions, as VDR immunoreactivity was strongest in normal skin > nevi > melanoma = metastasis, for both nuclear and cytoplasmic expression [59]. However, the stain graduation was more pronounced in nuclear staining. The authors explain this alterations alluding that for VD normal regulatory actions, both compartments- nuclear and cytoplasmic- are relevant. However, as nuclear VDR expression and activation leads to activation of target genes, the reduction of nuclear VDR expression could contribute to the escape of melanoma cells from homeostatic surveillance and growth control. Thus, reduction of nuclear VDR can induce some malignant features of melanoma like unlimited replicative potential or the ability to invade the dermis [56].
Furthermore, the same authors found that in melanoma, VDR cytoplasmic and nuclear immunoreactivity was lower in more aggressive nodular melanomas than in superficial melanomas. Moreover, in the melanoma cohort, the nuclear and cytoplasmic VDR immunoreactivity was associated with longer overall survival. A further study by the same authors showed that VDR cytoplasmic and nuclear expression in melanoma was inversely correlated with Breslow thickness, Clark’s level and pTNM AJCC staging [60] [57]. In addition, VDR expression was decreased in melanomas with melanization and with ulceration, but increased in those melanomas with high tumor infiltrating lymphocytes (TIL) expression. This last association could be explained because active lymphocytes could express VD and that way induce an antineoplastic activity [60]. Finally, VDR expression affected the survival of patients with melanoma [60]. Our own unpublished studies showed differential immunohistochemical expression of VDR in melanoma and nevi showing some differences with data reported in the literature (Fig. 1, panel).
VDR immunohistochemical expression in melanoma and dermal nevus. a cytoplasmic and nuclear staining for VDR in dermal nevus, AEC, 400x; b negative VDR staining in melanoma in situ, AEC, 400x; c positive VDR nuclear staining in melanoma in situ, AEC, 400x; d positive VDR nuclear staining in invasive melanoma, AEC, 400x
Serum VD levels and melanoma
Since the last decade, multiple studies have tried to evaluate the association between VD serum levels and melanoma risk and/or prognosis. However, the results have been contradictory. These discrepancies may be explained mainly because of multiple methodological differences between studies: differences in adjustment of VD by known confounding factors; differences in the assay used to determine serum 25(OH)D levels; in the cutoff point of normal-deficiency levels of VD between studies; and differences in the time of the year (seasonality) and time from diagnosis of the melanoma when VD serum levels where obtained, as some studies used 25(OH)D levels obtained even years after diagnosis of melanoma (and could reflect the increased sun awareness and protection after melanoma diagnosis).
In an effort to determine the real role of VD in melanoma pathogenesis, Caini et al. [61] made a meta-analysis and analyzed the effect of 25(OH)D baseline serum levels and the risk of melanoma genesis and of melanoma prognostic factors. They included 4 studies (392 melanoma cases), and found a borderline trend (though not significant) between lower 25(OH)D and melanoma risk. However, they did found that lower VD serum levels were inversely correlated with melanoma thickness.
Other studies have been conducted to evaluate VD levels and melanoma. Firstly, in their revision of the Leeds Melanoma Cohort with 2182 patients followed for 7 years, Newton-Bishop et al. [62] analyzed the relationship between known systemic markers of inflammation (smoking, body mass index (BMI), low 25(OH)D serum levels, and use of NSAIDs) with melanoma risk with tumor ulceration and melanoma specific survival (MSS). They found that deficient VD serum levels and smoking were significantly associated with ulceration. When multivariate analysis was made (adjusted by sex, age, tumor location and Breslow thickness) deficient 25(OH)D serum levels (<20 mmol/L vs 20-60nmol/L = HR 1,79; p = 0,009) and smoking duration significantly decreased MSS. The authors suggested, based on their results, correction of VD deficiency and smoking cessation might be of benefit for melanoma prognosis.
A cross-sectional Australian study with 100 patients with recently diagnosed melanoma and VD levels obtained at the moment of diagnosis, evaluated baseline 25(OH)D levels and melanoma prognostic factors. After a multivariate analysis adjusted for confounding factors, they found a significant association between VD deficiency (defined as <50mmol/L by the Australian Health survey) and Breslow thickness, a known factor of poorer prognosis. In fact, they found a four-fold increase in the odds of higher Breslow thickness (>0,75 mm) in association with low VD levels [63]. There was no association of 25(OH)D levels and Clark level or mitotic activity.
Saiag et al. [3] reported a prospective-cohort study with 1171 patients with melanoma followed for a mean of 4.5 years, and studied VD serum levels at diagnosis and during follow-up, in order to see if increased VD levels during follow-up improved prognosis. After adjustment of 25(OH)D levels (by age, sex, BMI, and month of blood drawn), 25(OH)D baseline levels were significantly inversely correlated with poor prognostic factors such as AJCC stage, Breslow’s thickness, and ulceration. When evaluating 25(OH)D baseline levels adjusted by prognostic factors (AJCC staging) the associations with melanoma relapse or prognosis disappeared, suggesting that VD levels were not an independent prognostic marker of melanoma. Then, they analyzed the data of 856 patients that had at least two values of VD serum levels measured, allowing calculation of an annual trend of 25(OH)D variation levels. The annual variation of 25(OH)D over time and prognosis showed a U-shape in Kaplan Meier curve, meaning that any change above or under 0.5 nmol/L per year was significantly associated with worse prognosis. Authors could explain the relationship between decreased annual trend of 25(OH)D levels and worse prognosis, assuming tumor inflammation and lower sun exposure as the main causes. But they could not explain how higher levels of VD decreased prognosis.
Vitamin D supplementation and melanoma risk and progression
There have been few studies evaluating the real usefulness of VD supplementation and how it may affect melanoma progression. Most of them have been based in cohorts that studied supplementation for other diseases and therefore the VD dosage and the adjustment by confounding factors was not accurate.
Interestingly, some studies support VD supplementation to prevent melanoma genesis. A case control study by Millen et al. [64] conducted in 503 patients and 565 controls, found a reduced risk of melanoma in patients with dietary VD intake. However, VD exposure data were collected after melanoma diagnosis, which make the results susceptible to recall bias.
With the scarce evidence available to date, supplementation with VD is not currently supported. A small case control study with 165 melanoma patients and 209 controls found no association between VD intake or supplementation and further melanoma risk [65]. In contrast, another prospective study found an increased risk between cod-liver oil consumption (600-1000 UI VD/teaspoon) and melanoma risk among women, but not men [66].
The first important epidemiological study was reported with indirect data obtained by questionnaires. The Vitamins and Lifestyle Cohort prospective study included 68,611 participants, who were assessed for VD dietary intake and/or supplementation and its association with melanoma risk [67]. Data was extracted through a baseline questionnaire of the last decade VD intake and/or supplementation. After a year of follow-up, 455 incident melanomas were identified. They found no association between VD supplementation and/or dietary intake and melanoma risk. However, this study did not measured serum VD levels nor adjusted results by other confounding risk factors.
Another indirect evidence arises from the post-hoc analysis of the WHI CaD trial [68], which included 36282 participants who were randomly assigned to receive Calcium 100mg - VD 400 UI/daily or placebo. After a mean 7-year follow up by annual questionnaire reporting of melanoma or NMSC, the analysis found no association between VD supplementation and a reduced risk of NMSC or melanoma. However, in the group of women with previous history of NMSC (a known risk factor for melanoma) [69] there was a 57% of fewer melanomas in the group who received supplementation versus placebo (HR 0.43; p = 0.026). The authors postulated that the probable main cause of lack of association between supplementation and reduced risk is that the supplementation was inadequate, as it was lower than the recommended 600 UI/d by the Institute of Medicine consensus statement, and as it only raises serum VD levels by 4ng/ml, an inadequate dose to observe clinical differences in skin cancer [68].
Consequently with these data and based in its recently systematic reviews, the US Preventive Services Task Force concluded that the current available data were insufficient to recommend VD screening in clinical practice or to assess a benefit of supplemental VD intake for the primary prevention of cancer [70].
There are ongoing clinical pilot trials evaluating VD supplementation with VD and cancer risk [71], and a specific placebo controlled pilot trial evaluating high dose VD supplementation in patients with advanced melanoma, evaluating safety of dosage and progression free survival, with promising outcomes [72].
Conclusions and future directions
For many years dermatologists have recommended patients simply avoiding sun exposure pragmatically in order to prevent the appearance of skin cancer. Recent evidence suggests a much more complex issue than initially thought and that carcinogenesis is not as simple as “more sun exposure, more skin cancer”. As VD levels are mainly regulated by sun exposure, it seems that as with any other hormone, a delicate equilibrium governs over the presence of pathology or a homeostatic healthy environment. More studies are needed in order to give patients solid evidence based recommendations of optimal 25(OH)D levels, VD supplementation, and sun exposure.
Abbreviations
- AJCC:
-
American joint committee on cancer
- BCC:
-
Basal cell carcinoma
- BMI:
-
Body mass index
- HIV:
-
Human Immunodeficiency virus
- IL-1:
-
IL-6, IL-8, IL-10, IL-12, IL-17, IL-21, Interleukins: 1,6,8,10,12,17,21.
- MSS:
-
Melanoma specific survival
- NMSC:
-
Non melanoma skin cancer
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- PKC:
-
Protein kinase C
- pTNM:
-
Pathological Tumor-node-metastasis staging
- RXR:
-
Retinoid X receptor
- SCC:
-
Squamous cell carcinoma
- TIL:
-
Tumor infiltrating lymphocytes
- TNFα:
-
Tumor necrosis factor alpha
- UV:
-
Ultraviolet radiation
- UVB:
-
Ultraviolet B radiation
- VD:
-
Vitamin D
- VD3:
-
Vitamin D3
- VDBP:
-
Vitamin D binding protein
- VDR:
-
Vitamin D receptor
- WHO:
-
World Health Organization
References
Norman AW. Sunlight, season, skin pigmentation, vitamin D, and 25-hydroxyvitamin D: integral components of the vitamin D endocrine system. Am J Clin Nutr. 1998;67:1108–10.
Fang S, Sui D, Wang Y, Liu H, Chiang YJ, Ross MI, et al. Association of Vitamin D Levels with outcome in patients with melanoma after Adjustment For C-reactive protein. J Clin Oncol. 2016;34(15):1741–7.
Saiag P, Aegerter P, Vitoux D, Lebbé C, Wolkenstein P, Dupin N, et al. Prognostic value of 25-hydroxyvitamin D3 levels at diagnosis and during follow-up in melanoma patients. J Natl Cancer Inst. 2015;107:djv264.
Bikle DD. Vitamin D, and the skin: Physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13:3–19.
Holick MF. Vitamin D: a d-lightful solution for health. J Investig Med. 2011;59:872–80.
Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science. 1997;277:1827–30.
Bikle DD, Elalieh H, Welsh J, Oh D, Cleaver J, Teichert A. Protective role of vitamin D signaling in skin cancer formation. J Steroid Biochem Mol Biol. 2013;136:271–9.
Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EK, Nguyen MN, et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012;26:3901–15.
Aranow C. Vitamin D, and the immune system. J Investig Med. 2011;59:881–6.
Nam HS, Kweon SS, Choi JS, Zmuda JM, Leung PC, Lui LY, et al. Racial/ethnic differences in bone mineral density among older women. J Bone Miner Metab. 2013;31:190–8.
Burns EM, Elmets CA, Yusuf N. Vitamin D and skin cancer. Photochem Photobiol. 2015;91:201–9.
Haddad JG, Matsuoka LY, Hollis BW, Hu YZ, Wortsman J. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest. 1993;91:2552–5.
Płudowski P, Karczmarewicz E, Bayer M, Carter G, Chlebna-Sokół D, Czech-Kowalska J, et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe - recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol Pol. 2013;64:319–27.
Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr. 1998;67:1232–6.
Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–8.
Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25:543–59.
Nemere I, Garbi N, Hammerling G, Hintze KJ. Role of the 1,25D3-MARRS receptor in the 1,25(OH)2D3-stimulated uptake of calcium and phosphate in intestinal cells. Steroids. 2012;77:897–902.
Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34.
Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–40.
Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr. 2009;139:1157–61.
Keflie TS, Nölle N, Lambert C, Nohr D, Biesalski HK. Vitamin D deficiencies among tuberculosis patients in Africa: A systematic review. Nutrition. 2015;31:1204–12.
Villamor E. A potential role for vitamin D on HIV infection? Nutr Rev. 2006;64:226–33.
Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3.
Chen S, Sims GP, Chen XX, Gu YY, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–47.
Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–80.
Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23–33.
Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, et al. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4 + Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106:3490–7.
Almerighi C, Sinistro A, Cavazza A, Ciaprini C, Rocchi G, Bergamini A. 1Alpha,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine. 2009;45:190–7.
Piotrowska A, Wierzbicka J, Zmijewski MA. Vitamin D in the skin physiology and pathology. Acta Biochim Pol. 2016;63(1):1104.
Szyszka P, Zmijewski MA, Slominski AT. New vitamin D analogs as potential therapeutics in melanoma. Expert Rev Anticancer Ther. 2012;12:585–99.
Vanoirbeek E, Krishnan A, Eelen G, Verlinden L, Bouillon R, Feldman D, et al. The anti-cancer and anti-inflammatory actions of 1,25(OH)2D3. Best Pract Res Clin Endocrinol Metab. 2011;25:593–604.
Yin L, Ordóñez-Mena JM, Chen T, Schöttker B, Arndt V, Brenner H. Circulating 25-hydroxyvitamin D serum concentration and total cancer incidence and mortality: a systematic review and meta-analysis. Prev Med. 2013;57:753–64.
Kim Y, Je Y. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: a meta-analysis. Br J Cancer. 2014;110:2772–84.
Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qin H. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol. 2011;29:3775–82.
Muller DC, Fanidi A, Midttun Ø, Steffen A, Dossus L, Boutron-Ruault MC, et al. Circulating 25-hydroxyvitamin D3 in relation to renal cell carcinoma incidence and survival in the EPIC cohort. Am J Epidemiol. 2014;180:810–20.
Fedirko V, Duarte-Salles T, Bamia C, Trichopoulou A, Aleksandrova K, Trichopoulos D, et al. Prediagnostic circulating vitamin D levels and risk of hepatocellular carcinoma in European populations: a nested case-control study. Hepatology. 2014;60:1222–30.
Afzal S, Bojesen SE, Nordestgaard BG. Low plasma 25-hydroxyvitamin D and risk of tobacco-related cancer. Clin Chem. 2013;59:771–80.
Ng K, Meyerhardt JA, Wu K, Feskanich D, Hollis BW, Giovannucci EL, et al. Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. J Clin Oncol. 2008;26:2984–91.
Finkelmeier F, Kronenberger B, Köberle V, Bojunga J, Zeuzem S, Trojan J, et al. Severe 25-hydroxyvitamin D deficiency identifies a poor prognosis in patients with hepatocellular carcinoma - a prospective cohort study. Aliment Pharmacol Ther. 2014;39:1204–12.
Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–91.
Yang B, McCullough ML, Gapstur SM, Jacobs EJ, Bostick RM, Fedirko V, et al. Calcium, vitamin D, dairy products, and mortality among colorectal cancer survivors: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2014;32:2335–43.
Tagliabue E, Raimondi S, Gandini S. Meta-analysis of vitamin D-binding protein and cancer risk. Cancer Epidemiol Biomarkers Prev. 2015;24:1758–65.
Gniadecki R, Gajkowska B, Hansen M. 1,25-dihydroxyvitamin D3 stimulates the assembly of adherens junctions in keratinocytes: involvement of protein kinase C. Endocrinology. 1997;138:2241–8.
Takayama T, Shiozaki H, Shibamoto S, Oka H, Kimura Y, Tamura S, et al. Beta-catenin expression in human cancers. Am J Pathol. 1996;148:39–46.
Gordon-Thomson C, Gupta R, Tongkao-on W, Ryan A, Halliday GM, Mason RS. 1α,25 dihydroxyvitamin D3 enhances cellular defences against UV-induced oxidative and other forms of DNA damage in skin. Photochem Photobiol Sci. 2012;11:1837–47.
Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 2006;4:e232.
Zinser GM, Suckow M, Welsh J. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J Steroid Biochem Mol Biol. 2005;97:153–64.
Tang JY, So PL, Epstein EH. Novel Hedgehog pathway targets against basal cell carcinoma. Toxicol Appl Pharmacol. 2007;224:257–64.
Ellison TI, Smith MK, Gilliam AC, MacDonald PN. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J Invest Dermatol. 2008;128:2508–17.
Tang JY, Parimi N, Wu A, Boscardin WJ, Shikany JM, Chren MM, et al. Inverse association between serum 25(OH) vitamin D levels and non-melanoma skin cancer in elderly men. Cancer Causes Control. 2010;21:387–91.
Oikawa A, Nakayasu M. Stimulation of melanogenesis in cultured melanoma cells by calciferols. FEBS Lett. 1974;42:32–5.
Colston K, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108:1083–6.
Osborne JE, Hutchinson PE. Vitamin D and systemic cancer: is this relevant to malignant melanoma? Br J Dermatol. 2002;147:197–213.
Gupta R, Dixon KM, Deo SS, Holliday CJ, Slater M, Halliday GM, et al. Photoprotection by 1,25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J Invest Dermatol. 2007;127:707–15.
Schäfer A, Emmert S, Kruppa J, Schubert S, Tzvetkov M, Mössner R, et al. No association of vitamin D metabolism-related polymorphisms and melanoma risk as well as melanoma prognosis: a case-control study. Arch Dermatol Res. 2012;304:353–61.
Li C, Liu Z, Zhang Z, Strom SS, Gershenwald JE, Prieto VG, et al. Genetic variants of the vitamin D receptor gene alter risk of cutaneous melanoma. J Invest Dermatol. 2007;127:276–80.
Halsall JA, Osborne JE, Epstein MP, Pringle JH, Hutchinson PE. The unfavorable effect of the A allele of the vitamin D receptor promoter polymorphism A-1012G has different mechanisms related to susceptibility and outcome of malignant melanoma. Dermatoendocrinol. 2009;1:54–7.
Mandelcorn-Monson R, Marrett L, Kricker A, Armstrong BK, Orlow I, Goumas C, et al. Sun exposure, vitamin D receptor polymorphisms FokI and BsmI and risk of multiple primary melanoma. Cancer Epidemiol. 2011;35:e105–10.
Brożyna AA, Jozwicki W, Janjetovic Z, Slominski AT. Expression of vitamin D receptor decreases during progression of pigmented skin lesions. Hum Pathol. 2011;42:618–31.
Brożyna AA, Jóźwicki W, Slominski AT. Decreased VDR expression in cutaneous melanomas as marker of tumor progression: new data and analyses. Anticancer Res. 2014;34:2735–43.
Caini S, Boniol M, Tosti G, Magi S, Medri M, Stanganelli I, et al. Vitamin D and melanoma and non-melanoma skin cancer risk and prognosis: a comprehensive review and meta-analysis. Eur J Cancer. 2014;50:2649–58.
Newton-Bishop JA, Davies JR, Latheef F, Randerson-Moor J, Chan M, Gascoyne J, et al. 25-Hydroxyvitamin D2 /D3 levels and factors associated with systemic inflammation and melanoma survival in the Leeds Melanoma Cohort. Int J Cancer. 2015;136:2890–9.
Wyatt C, Lucas RM, Hurst C, Kimlin MG. Vitamin D deficiency at melanoma diagnosis is associated with higher Breslow thickness. PLoS ONE. 2015;10:e0126394.
Millen AE, Tucker MA, Hartge P, Halpern A, Elder DE, Guerry D, et al. Diet and melanoma in a case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:1042–51.
Weinstock MA, Stampfer MJ, Lew RA, Willett WC, Sober AJ. Case-control study of melanoma and dietary vitamin D: implications for advocacy of sun protection and sunscreen use. J Invest Dermatol. 1992;98:809–11.
Veierød MB, Thelle DS, Laake P. Diet and risk of cutaneous malignant melanoma: a prospective study of 50,757 Norwegian men and women. Int J Cancer. 1997;71:600–4.
Asgari MM, Maruti SS, Kushi LH, White E. A cohort study of vitamin D intake and melanoma risk. J Invest Dermatol. 2009;129:1675–80.
Tang JY, Fu T, Leblanc E, Manson JE, Feldman D, Linos E, et al. Calcium plus vitamin D supplementation and the risk of nonmelanoma and melanoma skin cancer: post hoc analyses of the women's health initiative randomized controlled trial. J Clin Oncol. 2011;29:3078–84.
Rosenberg CA, Khandekar J, Greenland P, Rodabough RJ, McTiernan A. Cutaneous melanoma in postmenopausal women after nonmelanoma skin carcinoma: the Women's Health Initiative Observational Study. Cancer. 2006;106:654–63.
Moyer VA, Force USPST. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: U.S. Preventive services Task Force recommendation statement. Ann Intern Med. 2014;160:558–64.
Manson JE, Bassuk SS. Vitamin D research and clinical practice: at a crossroads. JAMA. 2015;313:1311–2.
Saw RP, Armstrong BK, Mason RS, Morton RL, Shannon KF, Spillane AJ, et al. Adjuvant therapy with high dose vitamin D following primary treatment of melanoma at high risk of recurrence: a placebo controlled randomised phase II trial (ANZMTG 02.09 Mel-D). BMC Cancer. 2014;14:780.
Funding
This investigation was funded in part by the Concurso de Fondos Interdepartamentales (CI05/14), Escuela de Medicina, Pontificia Universidad Católica de Chile.
Authors’ contributions
All authors contributed to the contents of this manuscript which was written by CP and CN, and reviewed and edited by all authors (CP, CN, MM, AB, SG). All authors read and approved the final manuscript.
Competing interests
The authors declares that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Del Puerto, C., Navarrete-Dechent, C., Molgó, M. et al. Vitamin D axis and its role in skin carcinogenesis: a comprehensive review. Appl Cancer Res 36, 5 (2016). https://doi.org/10.1186/s41241-016-0006-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41241-016-0006-4