Abstract
Background
Food intake and eating behavior are two important risk factors that lead to obesity and other associated metabolic and reproductive disorders like polycystic ovarian syndrome (PCOS). Most of the phytonutrients like hydrolysable tannin (HT) have the ability to reduce the nutrient intake that might be a suitable remedy for weight management of females in their reproductive age. Therefore, the present research is aimed to find out the effect of HT on nutrient intake, weekly body weight, blood glucose, serum lipids, minerals, immunoglobulins and satiety hormones in PCOS rats.
Materials and methods
A total of forty five adult healthy female rats of 56 days old, weighed 135 ± 5 g with two consecutive estrous cycles were selected. In order to induce PCOS in rats, the intramuscular injection of 4 mg/rat/kg Estradiol- Valerate was used. After induction, a Complete Randomized Design was used to divide the rats into five equal groups (n = 9) named as Pc0, Pc0.5, Pc1, Pc1.5 and Pc2. The groups of rats were offered different doses of HT i.e. 0, 0.5, 1, 1.5 and 2 % respectively per kg body weight in solution form through oral gavage once in a day for 60 days.
Results
After the intake of different levels of HT, the statistical results had shown a significant decrease (p < 0.05) in the weekly nutrient intake, body weight, water intake, weight gain, fasting blood glucose in PCOS rats. A similar trend of decrease (p < 0.05) was noticed in serum iron, IgM, IgG, leptin, ghrelin, cholesterol, low density lipoprotein and triglycerides while a significant improvement (p < 0.05) was also observed in high density lipoprotein in the PCOS rats. However, a non-significant effect (p > 0.05) was observed on serum protein and calcium levels.
Conclusions
The study concluded that HT had a therapeutical potential to decrease the nutrient intake and its anti-nutritional property could be used as remedy for the management of body weight, hyperglycemia, dyslipidemia and cardiovascular risk factors of PCOS rats.
Similar content being viewed by others
Background
Overweight and obesity are major problems of both developed and under developed countries which is characterized by an over intake of calories along with low energy expenditure. These are mainly ascribed to behavioral, environmental, genetic factors and sedentary life style [1]. These factors are also considered as the main causes for the progression of hormonal and metabolic abnormalities which are collectively called as polycystic ovarian syndrome (PCOS). About 40–80 % of females with these conditions are reported to be overweight [2]. The clinical features of PCOS are hyper-anderogenism while many other associated risk factors are insulin resistance, hyperglycemia, abnormal lipids and incidence of cardiovascular diseases (CVD) [3]. A number of pharmaceutical products are used for the treatment of PCOS which have shown deleterious side effects [4]. However, many previous research trials had also shown that different medicinal plants and their extracts were consumed to fight against these health disorders. The hydrolysable tannin (HT) is one such important phyto-nutrient or plants secondary metabolite which has been used to modulate the metabolic disorders through various mechanisms [5]. Structurally the HT contains a molecule of carbohydrate with D-glucose in its central core. The hydrolysable tannins are further classified into gallic acid and ellagic acid which posses different biological activities. The consumption of these compounds has both harmful and beneficial effects on the nutrition of ruminants and monogastric animals, depending on their types and level of intake [6, 7]. The HT has shown many health promoting properties like anti-oxidants, anti-diabetic, anti-obesity, improvement of serum lipids and the reduction of the CVD risk factors [8]. However, the HT also exerts anti-nutritional effects on nutrient feed intake and its digestibility which results in weight loss. Some studies also reported that HT had shown no effect on feed intake and nutrients absorption, but observed negative effects on iron absorption [9, 10]. The present study is therefore, focused to find an alternative therapy in order to reduce body weight of PCOS patients. Many medicinal plants and herbal therapies had already been used for the treatment of PCOS. The goal of this study was to find the effect of healthy and anti-nutritional properties of HT on weight management, abnormal blood biochemical profile and other associated metabolic risk factors in induced PCOS rats.
Methods
Adult female Albino Wistar rats of about 56 days old were purchased from Pharmacology Department, Government College University Faisalabad (GCUF) Pakistan. A total of 45 female rats were recruited with 4 to 5 days regular estrous cycle. They were kept at 25 ± 10 C with 45 to 55 % relative humidity and (12 h) dark/light cycle in the animal house of Pharmacology Department, GCUF Pakistan. The study was approved by the Directorate of Advanced Studies and the Animal Ethical Committee of GCUF had permitted for all the due animal procedures by following the instructions of Laboratory Animal Care (NIH Publications No. 8023, reviewed 1978). All the rats were offered isocaloric and isonitrogenous diet.
Induction of polycystic (PCOS)
Before the start of the induction procedure of PCOS, the initial body weight (weighing Balance) and blood glucose (Accu-check Glucometer, Byer) levels of the female rats were taken while at fasting. For PCOS induction (rats with 2 consecutive estrous cycle; 4 to 5 days) estradiol valerate (Progynova, Bayer Pharmaceutical Co Ltd) tablets were crushed 4 mg/rat/kg and then dissolved in distilled water using Vortex Mixture and were given through intramuscular injection in a single dose [11, 12]. The rats were then observed daily through visual method for the detection of irregular estrous cycles [13]. Other signs of PCOS were also observed on weekly basis which included body weight changes and fasting blood glucose level greater than 200 mg/dl [14, 15].
Vaginal smear
In order to confirm the PCOS in rats, a vaginal smear test was performed at the 6th week of the estradiol injection. The estradiol treated groups had shown irregular estrous cycles which were remained in the same phase for four to five days as shown in Fig. 1 [16, 17].
A vaginal smear test for the regular estrous cycle of the control rats (Nc) had shown a Pro-estrous phase (round and nucleated cells), estrous phase (cornified squamous epithelial cells), metestrous phase (cornified squamous epithelial cells and predominant leukocytes) and di-estrous phase (epithelial cells and dominant leukocytes) as shown in Fig. 2.
Experimental design
On 45th day of PCOS confirmation, all the 45 female rats were completely randomized into five equal groups (n = 9) and each treatment was repeated 3 times to make 15 experimental units, each of which has three rats. The groups were named according to the HT dose levels i.e. Pc0.5: group with 0.5 % HT; Pc1: group with 1 % HT; Pc1.5: group with 1.5 % HT; Pc2: group with 2 % HT; Pc0: group with 0 % HT and was considered control while Nc (healthy reference values) was the healthy rats group from previous trial [18]. Identification marks were given on the tail of each rat using permanent ink markers of different colors. These four levels were calculated according to the method described by Erhirhie [19]. According to the OECD’S guide lines the following formulas were used for volume selection:
The required dose of HT for each rat was calculated by using following formula:
Then the calculated doses in mg were dissolved in warm distilled water to prepare their required concentrated solutions and then given through oral gavage/rat/kg body weight/day for two months [20].
Nutrient feed analysis
The daily feed intake of each rat was recorded in the last 7 days of the experiment after 60 days of treatment with HT. Split and left over feed samples were pooled and stored in a tight plastic jar at -20oC for analysis. To calculate the daily amount of water drank per experimental rat the following formula was used: Initial water (ml) – left over water (ml) [21]. The proximate analysis of feed was done according to the Official Method of Analysis. The feed samples were dried at 65oC for 48 h in a hot air oven to determine the moisture contents and then by applying formula we measured dry matter: DM % = 100 - Moisture %. The feed samples were burnt in Muffle Furnace at 550oC for 4 h for ash determination; crude fat was determined with petroleum ether extraction method (PEE); for crude fiber determination, the feed samples were boiled in H2SO4 and then with NaOH [22]. Kjeldahl Method was used for determining the crude protein while carbohydrates were determined with deduction method by Adenike [23] % Carbohydrates (NFE) [100 - (% moisture + % crude fat + % crude fiber + % crude protein + % ash).
Blood sampling
The rats were sacrificed early morning at 8:00 o’clock using chloroform anesthesia after twelve hours overnight fast. The jugular vein of the rats was cut with sharp blade and the blood was collected directly into the labeled test tubes and allowed to clot. Serum had been collected after centrifugation at 5000 rpm for 20 min and stored at -20 ºC for biochemical analysis.
Biochemical analysis
Serum cholesterol and triglycerides were analyzed spectrometrically; Serum HDL was determined enzymatically; Serum iron was determined by photometric colorimetric test method by using Kits of Human Diagnostic worldwide, Netherland; serum LDL was measured by subtracting the average cholesterol and cholesterol in the supernatant (Dia-Sys Diagnostic System GmbH, Germany); Serum calcium was determined by Arsenazo III Colorimetric method [24]. Serum leptin and ghrelin were analyzed by enzyme-linked immune-sorbent assay ELIZA (Elabscience Biotechnology Inc. Corporate USA); Biuret Method was used to detect Serum protein [25] (Human Diagnostic worldwide, Netherland); Serum IgG and IgM were assessed by Bindarid Radial Immune-Diffusion (RID) kit method (The Binding Site Ltd., Birmingham, UK).
Statistical analysis
Statistical presentation of data, as Mean ± SEM and differences of significance were calculated by using Analysis of Variance (ANOVA) IBM SPSS statistics 21 (USA) at p value < 0.05.
Results
Composition of diet on dry matter basis (%)
The rats were fed ad libitum diet as per AIN-93 guidelines [26]. However, on the dry matter basis, the composition of the diet contained 88.9 % dry matter, 8.3 % ash, 5.5 % crude fat, 6.5 % crude fiber and 18.6 % crude protein contents and 50 % nitrogen free extract.
Feed conversion ratio and nutrient intake (%)
The results regarding feed conversion ratio (FCR) and nutrient intake in different HT fed groups of PCOS rats are presented in Figs. 3 and 4. A significant decreased (p < 0.05) in weekly nutrient intake of all treatment groups was noticed as compared to Pc0 as shown in Fig. 3. The statistically maximum increase was observed in FCR of about 54.24 % in Pc2, 42.39 % in Pc1.5, 42.04 % in Pc1 while Pc0.5 had shown 10.14 % increase as compared with Pc0 and Nc [18] as shown in Fig. 4.
Weekly body weight, weight gain (g), blood glucose (mg/dl) and Water intake (ml)
The weekly body weight (BW), weight gain (BWG), fasting blood glucose and water intake of Pc0 and after 2 months intervention with HT in Pc0.5, Pc1, Pc1.5 and Pc2 are presented in Figs. 4 and 5. A significant increase (p < 0.05) in weekly BW, BWG, fasting blood glucose levels and water intake were noticed in Pc0 as compared with Nc [18, 27]. However, a significant reduction (p < 0.05) trend was observed in all treatment groups i.e. weekly BW, BWG and blood glucose as shown in Fig. 4. While the Pc2 had shown 5.87 % reduction in weekly BW, 90 % in BWG and 36.54 % in blood glucose level as compared with Pc0. A significant increase was observed in water intake in Pc0 as compared to Nc [27] as shown in Fig. 5. A significant decrease (p < 0.05) was observed in water intake of Pc1.5 and Pc2 as compared to Pc1, Pc0.5 and Pc0.
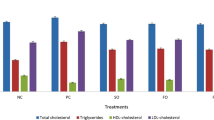
Serum lipids (mg/dl)
The statistical results regarding serum cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL) and triglycerides (TG) are shown in Table 1. A significant increase (p < 0.05) was observed in serum cholesterol, LDL and TG; while a highly significant decrease (p < 0.05) was observed in serum HDL of Pc0 as compared to Nc (healthy rats reference values) [18]. After dietary intervention with HT, the serum cholesterol levels were decreased significantly (P < 0.05) in Pc1 (145.37 mg/dl), Pc1.5 (139.18 mg/dl) and Pc2 (144.19 mg/dl) as compared to Pc0.5 (150.22 mg/dl) and Pc0 (152.77 mg/dl) as shown in Table 1. The statistical results of serum HDL had shown 20.83 % improvement in Pc2, 18.51 % in Pc1.5, 14.18 % in Pc1 and 7.60 % in Pc0.5 as compared to Pc0. The results of Pc1.5 and PC2 had shown maximum reduction in serum LDL levels which was about 18 and 15.8 % respectively as compared to Pc0. The levels of serum TG had shown 12.29 % reduction in Pc1 and 11.52 % in Pc1.5 as compared to Pc0.5, Pc2 and Pc0.
Serum minerals, satiety hormones and immunoglobulin’s
The statistical results regarding serum protein (g/dl) calcium (mg/dl), iron (ug/dl), leptin, ghrelin (ng/ml), IgG and IgM (g/l) in Pc0 and after the treatment with HT in Pc0.5, Pc1, Pc1.5 and Pc2 of PCOS rats are presented in Table 2. The serum protein, calcium and ghrelin levels had shown non-significant effects (p > 0.05) while a significant increase was observed in serum iron, leptin, IgG and IgM in Pc0 as compared to Nc (healthy rats reference value) [18]. After two months of treatment with HT, a non-significant effect (P > 0.05) was observed in the serum calcium levels in all the treatment groups as compared to Pc0. The statistical results of serum iron had shown 5.98 % reduction in Pc1 and 4.79 % in Pc2 as compared to Pc1.5, Pc0.5 and Pc0. The statistical results regarding serum leptin and ghrelin are shown in Table 2. Levels of serum leptin had shown significant reduction which was about 14 % in Pc2, Pc1.5 and Pc1 as compared to Pc0.5 and Pc0. Level of serum ghrelin was reduced significantly (p < 0.05) in Pc1.5 (174.78ng/ml) as compared with Pc1 (174.89ng/ml), Pc2 (174.85ng/ml), Pc0.05 (174.97ng/ml) and Pc0 (174.98ng/ml) as shown in Table 2. A non significant effect (p > 0.05) was observed on serum protein while the serum IgG level had shown significant decrease, represented as 10 % in Pc1.5 and 14 % in Pc2 as compared to Pc0.5, Pc1 and Pc0. The statistical data of serum IgM levels had shown 19.1 % reduction in Pc2 and 11.1 % in Pc1.5 as compared to Pc1, Pc0.5 and Pc0.
Discussion
The phenolic compound, hydrolysable tannin had shown anti-obesity, anti-diabetic and antioxidant activities [28]. So the present study was conducted to further explore its therapeutical effects on PCOS rat’s model. Abnormal reproductive hormones and insulin resistance had found in PCOS patients and were responsible for the development of adiposity and imbalance in energy homeostasis and weight gain [29, 30]. The first finding of the present trial was the increase in nutrient intake, WBW and WBWG of PCOS control rats and the standard treatments involved changes in eating habits and weight reduction [31]. So in the present study, HT helped to decrease WBW and WBWG and nutrient intake of the PCOS rats [6]. Another finding of this study was the improvement in the percentage of FCR in 1.5 and 2 % HT fed PCOS rats which were also reported previously [18]. The proposed mechanism of weight reduction and the improvement of FCR were due to the reason that HT had the anti-nutritional properties which were further responsible for poor macronutrients utilization of feed which resulted in weight loss and increased FCR [32]. However, the decrease in nutrient intake might be due to the reason that HT had slowed down the process of digestion and resulted satiety signals generated as a feedback to the nerves that were involved in intake control center of the rats and decreased the feed intake and body weight. The present study had also shown the increased levels of serum leptin, but showed no effect on serum ghrelin levels in the control group of the PCOS rats and these results also supported the previous findings [33]. Leptin and ghrelin were the two important hormones which controlled the intake and maintained energy homeostasis of the body while increased level of serum leptin indicated the condition of leptin resistance in PCOS rats which was further associated with increased intake [34]; while the treatment with HT improved the leptin resistance and also decreased the serum ghrelin in the present trial which might be due to the decrease in BW and nutrient intake. In the present trial, hyperglycemia was observed in the PCOS rats which was also confirmed previously [35] but the ingestion of 1 %, 1.5 and 2 % HT reduced the blood glucose levels in PCOS rats, and this possible effect might be due to the fact that HT helped to enhance the glucose transport through insulin mediated signaling pathways in adipocytes which as a result reduced the blood glucose level [35]. In the pathogenesis of PCOS, insulin resistance was considered the major initiative element of the oxidative stress (OS) which further contributed to hyperandrogenism [14]. Hyperglycemia and increased androgen levels were further responsible to decrease in the serum HDL level, increased serum LDL, hypercholesterolemia and hyper-triglyceridemia, which were also reported previously [4] and the present study had also found the same results. But after the intake of HT, a decrease was observed in serum cholesterol, LDL, triglycerides and improvement in serum HDL in all treated groups. The improvement in the serum lipids in the PCOS rats might possibly be due to the increase in peripheral insulin sensitivity to rat’s adipose tissues which inhibited the lipogenesis or by increasing the activity of lipo-protein lipase enzyme. However, HT might also be contributed in the cholesterol biosynthesis inhibitory activities [36, 37]. Many previous studies also reported that in PCOS patients, insulin resistance was one of the reasons of serum iron over load [38] which was another finding of the present research as a high level of serum iron was observed; while no effect on serum calcium levels had observed in PCOS control rats. A study on phenolic compounds showed that these bio-nutrients had the metal chelating activities when consumed in large quantities and might affect the iron status and as a result inhibited the absorption of non-heme iron while the calcium also played the same inhibitory effects on iron absorption which as a result reduced the serum iron concentration. It was also reported previously that tannin intake might reduce the calcium absorption but showed no effect on the apparent calcium absorption rate. As a result, it had shown a non-significant effect on serum calcium level on the PCOS treated rats [18, 39]. In the line of previous findings, our results showed that the intake of HT in the PCOS treated rats induced the formation of stable iron/HT complex in the gut and thus decreased the plasma iron concentration [18]. The present study had also shown the increased serum IgG and IgM levels as also reported previously [40]; while no effect on serum total protein was observed in PCOS control group. Serum immunoglobulins were the important parts of the humoral immune system which was involved in pathogen killing activities but high levels of these immunoglobulins (IgG and IgM) were also involved for the development of cardiovascular diseases in PCOS patients [40]. In present study the dietary intervention with HT helped to decrease the serum IgG and IgM levels which might positively be associated with digestibility of protein in rats [41]. However, the lack of experimental effect of HT was observed on the serum total protein which was in agreement with the previous study [18] and showed that HT had shown non toxic effects on protein metabolism. In this study the increased water intake was also observed in PCOS rats which were also observed in various previous research trials [8]; but the experimental effect of HT was observed to decrease the water intake in rats [42]. However, this effect might be due to the role of HT to maintain the water homeostasis which helped in the prevention of water loss from body [43].
Conclusions
The hydrolysable tannin had shown a considerable reduction in the satiety hormones (leptin and ghrelin) and immunoglobulins (IgG and IgM), nutrient intake and blood glucose levels. The study concluded that HT can be used as an alternative herbal medicine for weight management, dyslipidemia, diabetes and cardiovascular risk factors associated with PCOS.
Availability of data and materials
The data used to support the findings of this study is included within the article.
Abbreviations
- PCOS:
-
Polycystic ovarian syndrome
- HT:
-
Hydrolysable tannin
- EV:
-
Estradiol valerate
- HDL:
-
High Density Lipoprotein
- LDL:
-
Low density Lipoprotein
- TG:
-
Triglycerides
- IgG:
-
Immunoglobulin G
- IgM:
-
Immunoglobulin M
- FCR:
-
Feed conversion ratio
- BW:
-
Body weight
- WBW:
-
Weekly Body weight
- WBWG:
-
Weekly body weight gain
- CVD:
-
Cardiovascular disease
- ELIZA:
-
Enzyme-linked immune-sorbent assay
- AOAC:
-
Official Method of Analysis
- H2SO4 :
-
Sulfuric Acid
- NaOH:
-
Sodium Hydro-Oxide
References
Hajivandi L, Noroozi M, Mostafavi F, Ekramzadeh M. Food habits in overweight and obese adolescent girls with polycystic ovary syndrome (PCOS): A qualitative study in Iran. BMC Pediatric. 2020;20(1):1-9.
Barber TM, Hanson P, Weickert MO, Franks S. Obesity and polycystic ovary syndrome: Implications for pathogenesis and novel management strategies. Clin Med Insights. 2019;(13):1-7.
Rashmeen S, Nisar N, Butt S. Polycystic Ovarian Syndrome and Eating Disorder: Are They Associated. Int J Adv Res. 2017;5(2):765-9.
Desai BN, Radha H, Maharjan, Laxmipriya P. Nampoothiri. Aloe barbadensis Mill. Formulation restores lipid profile to normal in a letrozole-induced polycystic ovarian syndrome rat model. Pharma Res. 2012;4:2.
Chiva-Blanch G, Badimon L. "Effects of Polyphenol Intake on Metabolic Syndrome: Current Evidences from Human Trials." Oxid Med Cell Longev. 2017;(9):1-18.
Manzoor F, Nisa MN, Hussain HA, Ahmad N, Umbreen H. Effect of Chestnut Hydrolysable Tannin on Weight Management and Ovarian Histopathology of Healthy Female Rats. J Ani Plant Sci. 2021;31(3):831–40.
Mueller-Harvey I. Analysis of hydrolysable tannins. Ani Feed Sci Tech. 2001;91:3–20.
Goyal R, Patel S. Cardioprotective effects of gallic acid in diabetes-induced myocardial dysfunction in rats. Pharm Res. 2011;3(4):239.
Rezar V, Salobir J. Effects of tannin-rich sweet chestnut (Castanea sativa mill.) wood extract supplementation on nutrient utilisation and excreta dry matter content in broiler chickens. Europ Poult Sci. 2014;78:0003–9098.
Afsana K, Shiga K, Ishizuka S, Hara H. Reducing Effect of Ingesting Tannic Acid on the Absorption of Iron, but Not of Zinc, Copper and Manganese by Rats. Biosci Biotechnol Biochem. 2004;68(3):584–92.
Amini L, Tehranian N, Movahedin M, Ramezani Tehrani F, Soltanghoraee H. Polycystic Ovary Morphology (PCOM) in Estradiol Valerate Treated Mouse Model. Inter J Wom Health Reprod Sci. 2016;4(1):13–7.
Nofal EA, El-Habeby MM, El-Kholy WB, El-Akabawl GF, Faried MA. Protective role of broccoli extract on estradiol valerate-induced polycystic ovary syndrome in female rats. Eur J Anat. 2019;23:121–9.
Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse Estrous cycle identification tool and images. PLoS ONE. 2012;7(1):e35538.
Zhai H, Wu H, Xu H, Weng P, Xia F, Chen Y, Lu Y. Trace glucose and lipid metabolism in high androgen and high-fat diet induced polycystic ovary syndrome rats. Reprod Biol Endocrinol. 2012;10(1):5.
Ghasemzadeh A, Farzadi L, Khaki A, Ahmadi SK. Effect of Allium cepa seeds ethanolic extract on experimental polycystic ovary syndrome (PCOS) apoptosis induced by estradiol-valerate. Life Sci. 2013;10(4):170–5.
Tohma Y G, Onalan M, Tepeoglu N, Bayraktar E, Colak E, Ozcimen, Zeyneloglu H. Phosphodiesterase 4 inhibitor plus metformin is superior to metformin alone for the treatment of polycystic ovary syndrome: A rat model study. Experiment Therap Med. 2019;17(5):4013-22.
Manzoor F, Nisa M U, Hussain H A, Anwar H, Ahmad N, Umbreen H. Therapeutic potential of hydrolysable tannin on weight management oxidative stress and reproductive health in polycystic rats. Food Sci Technol. 2021b;(7):1-8.
Manzoor F, Nisa MU, Hussain HA, Ahmad N, Umbreen H. Effect of different levels of hydrolysable tannin intake on the reproductive hormones and serum biochemical indices in healthy female rats. Sci Rep. 2020;10(1):1-7.
Erhirhie E, Ekene NE, Ajaghaku D. Guidelines on dosage calculation and stock solution preparation in experimental animals’ studies. J Nat Sci Res. 2014;4(18):100–6.
Bonelli F, Turini L, Sarri G, Serra A, Buccioni A, Mele M. Oral administration of chestnut tannins to reduce the duration of neonatal calf diarrhea. BMC Vet Res. 2018;14(1):1-6.
Laaksonen KS, Nevalainen TO, Haasio K, Kasanen IHE, Nieminen PA, Voipio HM. Food and water intake, growth, and adiposity of Sprague-Dawley rats with diet board for 24 months. Lab Anim. 2013; 47(4):245–256.
AOAC. Official methods of analysis. Arligton. Virginia: Association of Analytical Chemists; 2000. p. 17.
Adenike K. Effect of processing on the lectin and trypsin inhibitor content of Plukenetia conophora seeds as it affects growth performance and nutrients metabolism in rat. Afri J Food Sci. 2013;7(9):306–16.
Bauer PJ. Affinity and stoichiometry of calcium binding by arsenazo III. Anal Biochem. 1981;110:61–72.
Gornall AC, Bardawill CJ, David MM. Determination of serum proteins by means of the Biuret Reaction. J Biol Chem. 1949;177:751–66.
Reeves PG, Nielsen FH, Fahey GC. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J Nutr. 1993;123(11):1939–51.
Manzoor F, Nisa MU, Hussain HA, Ahmad N, Umbreen H. Effect of chestnut hydrolysable tannin on weight management and ovarian histopathology of healthy female rats. J Anim Plant Sci. 2021;31(3):831–40.
Hsu C, Yen G. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br J Nutr. 2007;98(4):727–35.
Baranova A, Tran T, Afendy A, Wang L, Shamsaddini A, Mehta R, Younossi ZM. Molecular signature of adipose tissue in patients with both non-alcoholic fatty liver disease (NAFLD) and polycystic ovarian syndrome (PCOS). J Trans Med. 2013;11(1):133.
El-Gharib M, Badawy T. Correlation between insulin, leptin and polycystic ovary syndrome. J Basic Clin Reprod Sci. 2014;3(1):49.
Bency Baby T, Smitha R, Remya K, Shebina PR, Azeem AK. Polycystic ovarian syndrome: Therapeutic potential of herbal remedies- A review. Inter J Herb Med. 2016;4(5):91–6.
Idoko AS, Oladiji AT, Ilouno LE. Growth Performance of Rats Maintained on Citrullus colocynthis Seed Coat-based Diet. J Biotech Biochem. 2015;1:9–14.
Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes Rev. 2007;8(1):21–34.
Baig M, Rehman R, Tariq S, Fatima SS. Serum Leptin levels in polycystic ovary syndrome and its relationship with metabolic and hormonal profile in Pakistani females. Inter J Endocrinol. 2014;(2014):1-5.
Babby A, Elanchezhiyan C, Suhasini S, Chandirasegaran G. Antihyperglycemic Effect of Tannic acid in Streptozotocin induced Diabetic rats. Inter J Curr Res. 2014;6(3):5396–8.
Ong KC, Khoo HE, Das NP. Tannic acid inhibits insulin-stimulated lipogenesis in rat adipose tissue and insulin receptor function in vitro. Experientia. 1995;51:577–84.
Kim B, Ku CS, Pham TX, Park Y, Martin DA, Xie L, Taheri L, Lee J, Bolling BW. Alonia melanocarpa (Chokoberry) polyphenol rich extract improves antioxidant function and reduces total plasma cholesterol in apolipoprotein E knockout mice. Nutr Res. 2013;33(5):403–41.
Hossein RB, Shams S, Shariat M, Kazemi JH, Mohebi M, Haghollahi F. Evaluation of serum hepcidin and iron levels in patients with PCOS: A case-control study. J Endocrinol Invest. 2017;40:779–84.
Chang MJ, Bailey JW, Collins JL. Dietary Tannins from Cowpeas and tea transiently alter apparent calcium absorption but not absorption and utilization of protein in rats. J Nutr. 1994;124(2):283–8.
Wadood SA, Kadhum NAK, Hussien MK. Immunoglobulins IgG, IgA, IgM, complement C3 and C4 levels in sera of patients with polycystic ovary syndrome and the risk of cardiovascular diseases. Iraqi J Biotechnol. 2015;14:329–38.
Marzo F, Tosar A, Santidrian S. Effect of tannic acid on the immune response of growing chickens. J Anim Sci. 1990;68(10):3306.
Ekambaram SP, Babu KB, Perumal SS, Rajendran D. Repeated oral dose toxicity study on hydrolysable tannin rich fraction isolated from fruit pericarps of Terminalia chebula Retz in Wistar albino rats. Reg Toxicol Pharmaco. 2018;92:182–8.
Javornik A, Blazic K, sehic N, Snoj T. Long-term oral administration of sweet chestnut (Castanea sativa mill.) extract does not affect the contraction ability of isolated ileum. Acta Veterinaria Brno. 2019;88:219–23.
Acknowledgements
The authors are also highly obliged to the Library Department, Government College University Faisalabad (GCUF) and IT Department, Higher Education Commission (HEC, Islamabad) for access to journals, books and valuable database.
Funding
The research was completed by utilizing the available university resources.
Author information
Authors and Affiliations
Contributions
FM Contributed in conduction and execution of experimental work; MUN planned and supervised; HAH provided Lab assistance and supervised experimental analysis; MKK and RSA contributed to experimental measurements, discussion and interpretation of the results; NA and MI supervised and supported the data processing, edited manuscript; HU supervised and edited manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Animal Ethical Committee of Government College University, Faisalabad, Pakistan had proved this research by following Procedures of Laboratory Animal Care.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Manzoor, F., Nisa, M.U., Hussain, H.A. et al. Effect of hydrolysable tannin on nutrient intake obesity and other associated metabolic risk factors in polycystic rats. transl med commun 6, 10 (2021). https://doi.org/10.1186/s41231-021-00089-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41231-021-00089-y