Abstract
Background
The emergence of insecticide resistance in Aedes mosquitoes could undermine efforts to control arboviruses. The present study aims to assess in some communes of Southern Benin, the susceptibility level of Aedes aegypti (Linnaeus, 1762) and Aedes albopictus (Skuse, 1894) to insecticides commonly used in public health, as well as mechanisms involved.
Methods
Females Ae. albopictus and Ae. aegypti collected in Ifangni, Porto-Novo, Avrankou, Adjarra and Kétou from June 2021 to October 2022, were exposed to: deltamethrin 0.05%, permethrin 0.75%, alpha-cypermethrin 0.05%, pirimiphos methyl 0.25% and bendiocarb 0.1%, following the standard WHO susceptibility tube test protocol. In some sites, pre-exposure to the synergist PBO was used to verify if pyrethroid resistance of populations of Aedes was mediated by oxidases.
Results
Full susceptibility to deltamethrin and permethrin was observed in all tested populations of Ae. albopictus. However, with alphacypermethrin, a suspected resistance was observed in Adjarra (94.67%), Ifangni (93%) and Porto-Novo (94%), and a resistance in Avrankou (83%). The PBO-alphacypermethrin tests performed, led to a full susceptibility (100%) in all four sites, which confirms the full involvement of oxidases in resistance of all tested populations of Ae. albopictus to alphacypermethrin. At the opposite, Aedes aegypti was either resistant or suspected of being resistant to all tested pyrethroids in all four sites, except in Ifangni where a full susceptibility to alphacypermethrin was observed. The full susceptibility of Ae. aegypti to bendiocarb and pirimiphos-methyl in all communes suggests that these two insecticides can be good candidates for an effective control of pyrethroid-resistant Aedes vector populations. Use of permethrin and deltamethrin could also be considered for controlling populations of Ae. albopictus.
Conclusion
Results of the present study will help guide strategy to implement for an effective control of Aedes vector populations in Benin.
Similar content being viewed by others
Background
Aedes aegypti is the main indigenous vector of arboviruses in Africa [1]. In Benin, it remains abundant in both the south and the north parts of the country [2, 3]. Recent studies have reported the presence of Aedes albopictus, a vector native to Asia, in Southern Benin [4, 5]. Indeed this mosquito species is known to be invasive, and was involved in several cases of arbovirus epidemics [6, 7]. Dengue which was considered more prevalent in Asia and Latin America, has now spread to several West African countries [8], where all four serotypes of the virus are actively circulating [9]. This worrying situation deserves more attention, as severe forms of the disease are often found in areas where more than two serotypes of the virus coexist [10, 11].
Several cases of dengue fever have been diagnosed in European tourists returning from Benin in recent years [12,13,14]. Indeed, those travelers have tested positive for DENV-1, a virus serotype native to Asia [14]. Moreover, DENV-3 was recently detected in a sample of Aedes aegypti from Porto-Novo, the political capital of Benin [15].
Several factors make Benin a country likely to experience an arbovirus epidemic. These factors include: the high demographic growth, the development of trade of second hand cars and tires, the development of tourism, the presence of two major vectors of arboviruses, the growing urbanization of the country, and its proximity with Nigeria, an endemic country with which it shares a 773 km border. Except for yellow fever for which an effective vaccine is available, the other arboviruses have no curative or preventive treatment [16]. As a result, vector control appears to be the only way to control the disease transmission [17]. This control could be through the use of chemicals for killing immature stages or adult mosquitoes, the destruction of vector breeding sites resulting in the reduction of their density, and the sensitization of the population on good practices for water conservation and sanitation. Even with a strong community engagement, it is quite difficult to identify and destroy all breeding sites in the environment. Therefore, chemical control through the implementation of spatial spraying, and use of long lasting insecticidal nets (LLINs) may be the most effective way to control dengue vectors. Several studies have recently reported resistance of Aedes aegypti to pyrethroids and carbamates in Africa and Asia [18,19,20]. Ae. aegypti is well adapted to urban habitats and, therefore, is generally more susceptible to insecticide exposure and resistance than Ae. Albopictus[21].In West Africa, most of the available data on resistance of Aedes mosquitoes to insecticides, are for Ae. aegypti only, despite the reported introduction of Ae. albopictus in Nigeria since 1971. Recently, 238 and 380 confirmed cases of dengue fever have been recorded in Senegal and Côte d'Ivoire, respectively [22, 23]. To plan an effective and sustainable control strategy against arbovirus vectors in Benin, there is an urgent need to determine the susceptibility level of Ae. albopictus to insecticides commonly used in public health, and update data on the resistance status of Ae. aegypti. The present study aims to assess the resistance profile of these two main vectors of arboviruses, in Benin.
Methods
Study area
The study was carried out in the urban and peri-urban areas of 6 communes belonging to two departments in Southern Benin. These are the communes of Avrankou, Adjara, and Porto-Novo in the department of Ouémé and the communes of Kétou, Ifangni and Pobè in the department of Plateau (Fig. 1). These communes that mostly neighbor Nigeria, were surveyed because of the presence recently reported of Ae. albopictus and Ae. aegypti [6]. The whole study area is characterized by a subequatorial climate with two rainy seasons (March to July, and September to November) and two dry seasons (November to February and July to August) with rainfall ranging from 1200 to 1500 mm/year. There are three groups of ecosystems, namely, coastal plain ecosystems, bar land plateau ecosystems and Lama depression clay ecosystems. Long-lasting insecticidal nets (LLINs) distributed every three years throughout the country, and repellents are the main vector control tools used in the study area.
Mosquito collection
Three sampling techniques were used to collect immature stages and adults of Aedes spp. from July 2021 to October 2022. Irrespective of the collection method, the morphological identification of adult mosquitoes was performed using the taxonomic keys of Edwards [24] and Huang & Rueda [25].
Larvae collection
Immature stages of Aedes spp. were sampled from various breeding sites (abandoned jars, tires, cans, and other containers) using the dipping technique. They were filtered and stored in labeled jars, and then transported to the insectary of the Centre de Recherche Entomologique de Cotonou (CREC) for rearing until adulthood.
Ovitrapping method
The ovitraps were used as part of the present study. They were made of polyethylene bottles which were filled with 50 cl of water. A hardboard plate (5 cm by 20 cm) immersed into the water, served as support for the eggs laid. A total of 12 ovitraps were set per site. These traps were hung on a tree/wall at a height of 1.5 m from the ground, using a nail and a metal string, and left for one week in the domestic or peri-domestic environment. To avoid egg-hatching, the inspection of traps occurred on a daily basis. They were removed after 7 days, and the eggs laid on the hardboard plates were brought back to the insectary and put in water. After the hatching, the larvae were reared until adult stage.
Human landing catches
This method was used to collect adults of Aedes spp. during the day. In all study communes, mosquito collections were conducted both indoors and outdoors, with a first group of collectors that worked from 7:00 am to 1:00 pm and which was replaced by a second group from 1:00 pm to 6:00 pm. At each collection point, a volunteer with bare-legged and barefoot serving as bait, collected mosquitoes using hemolysis tubes and a flashlight. The specimens of Aedes spp. that were collected, were released in cages and transported to the insectary.
Biological materials
Females Ae. aegypti field-collected as larvae, were used for the WHO susceptibility tube testing.
For Ae. albopictus, mosquitoes from the three collection methods were reared at the insectary, in order to have a sufficient number of individuals of F1 generation. Only the females mosquito of this generation, were used for the WHO susceptibility tube testing.
Insecticide susceptibility tests
WHO susceptibility tube tests were performed according to the WHO protocol [26], using non-blood-fed females Ae. aegypti (F0) and Ae. albopictus (F1), aged 2–5 days. These mosquitoes were exposed to the following products:
-
-deltamethrin (0.05%), permethrin (0.75%), and alphacypermethrin (0.05%);
-
-alphacypermethrin (0.05%) + PBO (4%).
Batches of 20–25 mosquitoes were introduced into each tube carpeted with an insecticide-treated paper for a 1-h exposure. The number of mosquitoes knocked down by the insecticide at different time intervals (5, 10, 15, 20, 30, 45, 60 min) was recorded. A batch of 20–25 mosquitoes exposed to an insecticide-free paper was used as a control.
The PBO synergist is an inhibitor of oxidases [27]. It helped assessing the involvement of oxidases in the pyrethroid resistance observed in the populations of Aedes. The PBO-pyrethroid, and pyrethroid-only tests were performed simultaneously on the same mosquito populations. After 60 min of exposure, mosquitoes were transferred into observation tubes and kept at 25 °C and 80% humidity, with free access to a 10% sweetened juice. Mortality after 24 h was determined according to the WHO protocol.
Biochemical analyses
Thirty females Ae. aegypti from each district, aged 2–5 days, and non-previously exposed to any insecticide, were used for biochemical analyses. These were performed to compare the expression level of detoxification enzymes (mixed function oxidases, non-specific esterases and glutathione S-transferases) of different populations of Aedes aegypti to the reference susceptible strain (Rockefeller), following the protocol described by Hemingway et al. [28].
Data analysis
Mortality rates from the susceptibility tube tests were interpreted according to the WHO protocol [26]. When a mosquito population had a mortality rate between 98 and 100%, it was considered susceptible. When mortality was between 90 and 97%, the population was suspected of resistance. Below a mortality rate of 90%, the population was said resistant. The 24-h mortality rates and the 60-min knockdown rates were compared using the Chi-square test for comparison of proportions. A linear regression with analysis of variance was used to assess the variation in enzyme activity for each mosquito population. The Mann–Whitney U test was used to compare the enzyme activity between the field mosquito populations and the susceptible laboratory strain (Rockefeller). Statistical analyses were performed using R 3.3.2 software [29].
Results
Mortality and knockdown rates
In total, 3517 females Aedes (1787 Ae. aegypti, and 1730 Ae. albopictus), were bioassayed.
Table 1 shows the pyrethroid-induced mortalities in the different Aedes mosquito populations. After 60 min of exposure to the different insecticides, the knockdown rates observed in Ae. albopictus was very high in all communes, ranging between 94 and 100% (Table 1). A 100% mortality rate was observed in the different populations of Ae. albopictus exposed to deltamethrin and permethrin, which indicates a full susceptibility of these populations to the two tested pyrethroids (Table 1). Similarly, the population of Ae. albopictus from Kétou showed full susceptibility to alphacypermethrin (100%). However, with the same product, a suspected resistance was observed in Adjarra (94.67% [86.19–98.27]), Ifangni (94.38% [86.78–97.91]) and Porto-Novo (95.7% [88.73–98.61]), and a resistance in Avrankou (83% [73.89–89.50]) (Table 1).
In the populations of Ae. aegypti, a different trend was observed, with much more variable 60-min knockdown rates. Indeed, irrespective of the pyrethroid insecticide, these rates ranged from 63.51% [51.45–74.16] in Porto-Novo to 100% [95.29–100] in Ifangni (Table 2). 24-h post-exposure, populations of Ae. aegypti from Avrankou (91% [83.16–95.54]), Adjarra (93.75% [85.38–97.67]), Porto-Novo (93.51% [84.89–97.58]) and Kétou (95.65% [88. 61–98.59]) showed a suspected resistance to alpha-cypermethrin (0.05%), while the one of Ifangni displayed a full susceptibility to the same insecticide (Table 2). With deltamethrin (0.05%), a suspected resistance was observed in Avrankou (95% [88. 17–98.14]), Adjarra (95.29% [87.73–98.48]), Ifangni (90.91% [83.00–95.49]), and Kétou (93.87% [86.62–97.48]), while there was a resistance to the same product in Porto-Novo (63.51% [51.45–74.16]). Furthermore, resistance to permethrin was observed in all tested populations of Ae aegypti, except for the Ifangni strain that displayed a suspected resistance (97% [90.84–99.22]) (Table 2).
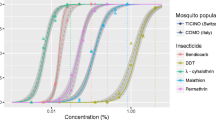
Figure 2 shows the mortality induced by bendiocarb and pirimiphos methyl. Overall, all tested populations of Ae. aegypti displayed a full susceptibility (mortality rates ≥ 98%) with these two products.
The PBO-alphacypermethrin tests performed with the four field strains of Ae. albopictus led to a mortality rate = 100% and higher than the one for the insecticide alone, indicating a full involvement of oxidases and esterases in alphacypermethrin resistance (Fig. 3).
Expression of oxidases, esterases and GSTs in Aedes aegypti
Figure 4 shows the mean levels of enzyme activities in the field populations of Ae. aegypti, and in the reference susceptible strain (Rockefeller).
The results show an overexpression of GSTs in the population of Ae. aegypti from Pobè (P < 0.0001) compared to the Rockefeller susceptible strain (Fig. 4A). A significantly elevated oxidase activity was observed in the populations of Ae. aegypti from Pobè and Ifangni, compared with that of the Rockefeller susceptible strain (P > 0.001) (Fig. 4B). In contrast, no overexpression of the α and β esterase activity was seen in all the tested populations compared with the Rockefeller susceptible strain (Fig. 4C, D).
Discussion
Studies on insecticide resistance profile have long been performed in Benin in Anopheles vectors of malaria, but very little is done on Aedes responsible for arboviruses, even though the country is close to countries Nigeria, Ghana, Cote d’Ivoire, Burkina-Faso that in recent years have become dengue endemic. Recently, Ae. albopictus, a highly invasive Asian mosquito, has entered Benin and lives in sympatry with Aedes aegypti in urban and peri-urban areas [4]. Both species are described as the main vectors of arboviruses [3, 4]. The present study provides knowledge on their insecticide resistance profile, which will help to better orient the vector control strategy to implement in case of a dengue epidemic.
Findings of the present study showed full phenotypic susceptibility of the different populations of Ae. albopictus tested to permethrin and deltamethrin, despite the widespread resistance observed of Anopheles vectors and Ae. aegypti to these two pyrethroids in Benin [4, 30]. This could be because permethrin and deltamethrin are among the type II pyrethroids with high toxicity. However, with alpha-cypermethrin, Ae. albopictus was resistant in Avrankou, and suspected of resistance in Adjarra, Porto-Novo and Ifangni. This shows the beginning of the emergence of resistance of Ae. albopictus to alphacypermethrin, a type I pyrethroid with a relatively low toxicity compared to type IIs. Previous studies had already revealed a relative susceptibility of this mosquito species to pyrethroids [21]. This onset of resistance of Ae. albopictus to pyrethroids was reported by Ngo et al. [31] and Yougang et al. [32] in the cities of Douala and Yaoundé in Cameroon, respectively. According to these authors, this rapid expansion of resistance in Ae. albopictus could result from domestic or organic pollutants, as this species is widely distributed in water tanks, spare tires and discarded containers, which are widely distributed in cultivated agricultural sites [33]. It is also possible that the susceptibility of this species has been affected by the increased use of repellent insecticides in peri-urban settings. However, this resistance of Ae. albopictus to alpha-cypermethrin was reversed by the synergist PBO in all communes, confirming the involvement of oxidases in pyrethroid resistance in the localities studied [34]. The deployment of next-generation LLINs combining the PBO synergist with a pyrethroid insecticide could be considered an alternative option for an effective control of populations of Ae albopictus. At the opposite, Ae. aegyptiwas found to be much more resistant or suspected of being resistant to pyrethroids. Similar observations were previously made in Cameroon [32]. This supports the hypothesis of an occurrence of a strong insecticide resistance selection in Ae. aegypti relative to Aedes albopictus. It is likely that these species exhibit different biting and resting behaviors, which could explain variable insecticide exposure. Indeed, Ae. aegypti has been frequently collected indoors in urban environments, while Ae. albopictus is much more sampled outdoors in a peri-urban environment. This particular behavior of Ae. aegypti could expose it more to indoor insecticide-based interventions such as insecticide sprays, aerosols, or LLINs to prevent nuisance in urban settings [35,36,37,38]. This high resistance of Ae. aegypti compared to Ae. albopictus has been reported in different epidemiological settings in Central and West African countries [39,40,41] such as Benin [3]. Reduced susceptibility of Aedes mosquitoes to pyrethroids is widespread and has been reported in several countries [17]. Our results corroborate previous studies from Thailand that reported high resistance to deltamethrin and permethrin in Ae. aegypti [42, 43]. Our biochemical data revealed the overexpression of MFOs and GSTs in populations of Ae. aegypti from Ifangin and Pobè. Oxidases are involved in the detoxification of pyrethroids in mosquitoes [44, 45]. The enormous quantities of insecticides of the same class used in agriculture could be at the origin of this overproduction of oxidases. The glutathione-S-transferase activity observed in our vector populations confirms the strong resistance to DDT observed by Yadouleton et al. [2] in Ae. aegypti in Benin. High GST expression may be due to overexpression of the GST2 gene [45]. In contrast, the low esterase activity observed would certainly be because the vectors from Ifangni and Pobè have not been or are weakly exposed to carbamates and organophosphates. However, a full susceptibility of populations of Ae. aegypti to bendiocarb and pyrimiphos-methyl was observed in all the surveyed communes. This can be explained by the low esterase activity observed in Ifangni and Pobè, where the use of pyrimiphos methyl and carbamates is low. It would be also interesting to characterize the Kdr and Ace-1R resistance mechanisms in populations of Aedes vectors, as they are commonly found in Anopheles mosquitoes. Carbamates and organophosphates could also be considered a good alternative for the control of arbovirus vectors in Benin in case of epidemics. Though our findings suggest that permethrin and deltamethrin could also be used, there is a growing concern that Ae. albopictus develop resistance to these pyrethroid insecticides. Ae. aegypti may develop other resistance mechanisms, such as behavioral or physiological one, to circumvent the modes of action of these insecticides. It would be interesting to investigate all the resistance mechanisms in Ae. aegypti to establish its full resistance profile to make good vector control decisions.
Conclusion
The present study showed full susceptibility of Ae. albopictus to deltamethrin and permethrin but widespread resistance to pyrethroids in Ae. aegypti in the study area. This suggests that these insecticides (deltamethrin and permethrin) can be used in arboviruses control programs in sites where Ae. albopictus is reported as the main vector. Given pyrethroids resistance observed in Ae. aegypti, and the emergence alpha-cypermethrin resistance in Ae. albopictus, rational use of insecticides, especially pyrethroids, should be encouraged to help reduce insecticide pressure. The synergist PBO increased the mortality of Ae. albopictus to alpha-cypermethrin, showing the involvement of oxidases. However, full susceptibility of Ae. aegypti to bendiocarb and pirimiphos methyl was observed in all study communes.
Given the increasing number of dengue cases in neighboring countries, strategies to improve vector control and prevent the spread of the diseases in Benin are urgently needed.
Availability of data and materials
The datasets that were analyzed in this study are available from the corresponding author and the lead author.
Abbreviations
- CREC:
-
Centre de recherche entomologique de cotonou
- WHO:
-
World Health Organization
- LLINs:
-
Long-lasting insecticidal nets
- GST:
-
Glutathione-S-transferase
- DDT:
-
Dichlorodiphenylchloroethane
- MFO:
-
Mixed function oxygenases
- PBO:
-
Piperonyl butoxide
- HLC:
-
Human landing catches
References
Frédéric S, Elysée N, Jean CT, Didier F. Geographic distribution and breeding site preference of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) in Cameroon, Central Africa. J Med Entomol. 2005;42:726–31.
Anges Y, Gado D, Ahadji-Dabla KM, Ramziyath A, Carine T, Achaz A, et al. Evaluation du comportement trophique de Aedes aegypti dans la ville de cotonou au sud du Bénin. Eur Sci J. 2018;14:70–9.
Padonou GG, Ossè R, Salako AS, Aikpon R, Sovi A, Kpanou C, et al. Entomological evaluation of the risk of dengue epidemic in the commune of Abomey-Calavi, Benin. Trop Med Health. 2020;48(1):1–9.
Yadouleton A, Hounkanrin G, Tchibozo C, Bialonski A, Schmidt-Chanasit J, Jöst H. First detection of the invasive mosquito vector Aedes albopictus (Diptera: Culicidae) in Benin, West Africa, 2021. J Med Entomol. 2022;59(3):1090–4.
Padonou GG, Konkon KA, Salako SA, Zougbédji MD, Ossé R, Tokponnon F, et al. Distribution and abundance of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Benin, West Africa. In press.
Kraemer MUG, Reiner RC, Brady OJ, Messina JP, Gilbert M, Pigott DM, et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol. 2019;4:854–63.
Abdalgader T, Banerjee M, Zhang L. Spatially weak synchronization of spreading pattern between Aedes albopictus and dengue fever. Ecol Modell. 2022;473:110123.
Stoler J, Dashti R, Anto F, Fobil JN, Awandare GA. Deconstructing malaria: West Africa as the next front for dengue fever surveillance and control. Acta Trop. 2014;134:58–65.
Tinto B, Kania D, Kagone TS, Dicko A, Traore I, de Rekeneire N, et al. Circulation du virus de la dengue en Afrique de l’Ouest-Une problématique émergente de santé publique. Médecine/sciences. 2022;38(2):152–8.
Gubler DJ. Epidemic Dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10(2):100–3.
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7.
Gautret P, Botelho-Nevers E, Charrel RN, Parola P. Dengue virus infections in travellers returning from Benin to France, July-August 2010. Euro Surveill. 2010;15:36.
Eckerle I, Kapaun A, Junghanss T, Schnitzler P, Drosten C, Jänisch T. Dengue virus serotype 3 infection in a traveler returning from West Africa in Germany. Emerg Infect Dis. 2015;21(1):175.
Fourié T, Luciani L, Amrane S, Zandotti C, Leparc-Goffart I, Ninove L, et al. Dengue virus type 1 infection in traveler returning from Benin to France, 2019. Emerg Infect Dis. 2020;26(8):1946–9.
Tchibozo C, Hounkanrin G, Yadouleton A, et al. Surveillance of arthropod-borne viruses in Benin, West Africa–2021: detection of dengue virus 3 in Aedes aegypti (Diptera: Culicidae). Military Med Res. 2022;9:64.
Londono-Renteria B, Troupin A, Colpitts TM. Arbovirosis and potential transmission blocking vaccines. Parasit Vectors. 2016;9:516.
Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, et al. Contemporary status of insecticide resistance in major Aedes vectors of human-infecting arboviruses. PLoS Negl Trop Dis. 2017;11(7): e0005625.
Sene NM, Mavridis K, Ndiaye EH, Diagne CT, Gaye A, Ngom EHM, et al. Insecticide resistance status and mechanisms in Aedes aegypti populations from Senegal. PLoS Negl Trop Dis. 2021;15(5): e0009393.
Jangir PK, Prasad A. Spatial distribution of insecticide resistance and susceptibility in Aedes aegypti and Aedes albopictus in India. Int J Trop Insect Sci. 2022;42:1019–44.
Toé HK, Zongo S, Moussa W, Guelbeogo BK, Mafalda V, Madou T, et al. Multiple insecticide resistance and first evidence of V410L kdr mutation in Aedes (Stegomyia) aegypti (Linnaeus) from Burkina Faso. Med Vet Entomol. 2022;36(3):309–19.
Vontas J, Kioulos E, Pavlidi N, Morou E, della Torre A, Ranson H. Insecticide resistance in the major dengue vectors Aedes albopictus and Aedes aegypti. Pest Biochem Physiol. 2012;104(2):126–31.
Dieng I, Barry MA, Talla C, Sow B, Faye O, Diagne MM, et al. Analysis of a dengue virus outbreak in Rosso, Senegal 2021. Trop Med Infect Dis. 2022;7(12):420.
European Centre for Disease Prevention and Control. Dengue worldwide overview. 2022.
Edwards FW. Mosquitoes of the Ethiopian region. III. Culicine adults and pupae. London: British Museum (Natural History); 1941. p. 499.
Huang YMIN, Leopoldo MR. Pictorial keys to the sections, groups, and species of the Aedes (Finlaya) in the Afrotropical Region (Diptera: Culicidae) Zootaxa. 2017; 4221:131–141.
World Health Organization. Testing procedures for monitoring insecticide resistance in malaria vector mosquitoes. Geneva: World Health Organization; 2013.
Khot AC, Bingham G, Field LM, Moores GD. A new assay reveals esterase blockade by piperonyl butoxide. Pest Manag Sci. 2008;64(11):1139–42.
Hemingway J, Hawkes N, Prapanthadara L, Jayawardenal KGI, Ranson H. The role of gene splicing, gene amplification and regulation in mosquito insecticide resistance. Philos Trans R Soc Lond B Biol Sci. 1998;353:1695–9.
R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2011.
Kpanou CD, Sagbohan HW, Dagnon F, Padonou GG, Ossè R, Salako AS, et al. Characterization of the resistance profile (intensity and mechanisms) of Anopheles gambiae in three communes of northern Benin, West Africa. Malar J. 2021;20(1):1–2.
Ngo Hondt OE, Ngo Hiol JV, Tonga C, Ga FD, Kekeunou S. Competitive adaptation of Aedes albopictus, Skuse 1894 in the presence of Aedes aegypti Linné 1862 in temporary larvae breeding sites and in the context of pyrethroids resistance in Douala (Cameroon). Bull Ent Soc Amer. 2020;113(2):79–87.
Yougang AP, Kamgang B, Tedjou AN, Wilson-Bahun TA, Njiokou F, Wondji CS. Nationalwide profiling of insecticide resistance in Aedes albopictus (Diptera : Culicidae) in Cameroon. PLoS ONE. 2020;15(6): e0234572.
Wilke ABB, Vasquez C, Carvajal A, Medina J, Chase C, Cardenas G, et al. Proliferation of Aedes aegypti in urban environments mediated by the availability of key aquatic habitats. Sci Rep. 2020;10(1):12925.
Lumjuan N, Wicheer J, Leelapat P, Choochote W, Somboon P. Identification and characterization of Aedes aegypti aldehyde dehydrogenases involved in pyrethroid metabolism. PLoS ONE. 2014;9(7): e102746.
Chen CD, Nazni WA, Lee HL, Sofian-Azirun M. Susceptibility of Aedes aegypti and Aedes albopictus to temephos in four study sites in Kuala Lumpur City Center and Selangor State, Malaysia. Trop Biomed. 2005;22(2):207–16.
Ponlawat A, Harrington LC. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol. 2005;42(5):844–9.
Ouattara LP, Sangaré I, Namountougou M, Hien A, Ouari A, Soma DD, et al. Surveys of arbovirus vectors in four cities stretching along a railway transect of Burkina Faso: risk transmission and insecticide susceptibility status of potential vectors. Front Vet Sci. 2019;6:140.
Marcombe S, Fustec B, Cattel J, Chonephetsarath S, Thammavong P, Phommavanh N, et al. Distribution of insecticide resistance and mechanisms involved in the arbovirus vector Aedes aegypti in Laos and implication for vector control. PLoS Negl Trop Dis. 2019;13(12): e0007852.
Amelia-Yap ZH, Chen CD, Sofian-Azirun M, Low VL. Pyrethroid resistance in the dengue vector Aedes aegypti in Southeast Asia: current status and management perspectives. Parasit Vectors. 2018;11:332.
Namountougou M, Soma DD, Balboné M, Kaboré DA, Kientega M, Hien A, et al. Monitoring insecticide sensibility in Aedes Aegypti populations from the two biggest cities, Ouagadougou and Bobo-Dioulasso, in Burkina Faso: implication of metabolic resistance. Trop Med Infect Dis. 2020;5(2):84.
Leong CS, Vythilingam I, Wong ML, Sulaiman WY, Lau YL. Aedes aegypti (Linnaeus) larvae from dengue outbreak areas in Selangor exhibiting pyrethroid resistance but susceptible to organophosphates. Acta Trop. 2018;185:115–26.
Somboon P, Prapanthadara LA, Suwonkerd W. Insecticide susceptibility testing of Anopheles minimus s.l., Aedes aegypti, Aedes albopictus and Culex quinquefasciatus in northern Thailand. Southeast Asian J Trop Med. 2003;34(1):87–93.
Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988;9(3):295–318.
Ishaaya I. Detoxifying enzymes of insects: their importance in pesticide synergism and resistance. Arch Insect Biochem Physiol. 1993;22(1–2):263–76.
Djouaka R, Irving H, Tukur Z, Wondji CS. Exploring mechanisms of multiple insecticide resistance in a population of the malaria vector Anopheles funestus in Benin. PLoS ONE. 2011;6(11): e27760.
Acknowledgements
We are grateful to the Ministry of Health of Benin who financially supported this study through the national research budget. We also thank the technicians and researchers of the Centre de Recherche Entomologique de Cotonou (CREC) who provided technical support to the study both in the field and in the laboratory.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
The original study was conducted by GGP, KKA, RO, ASS, and MCA who also supplied the data. The idea for the study was conceptualized and generated by GGP, KAK, RO, ASS, LB, and MCA. Data were collected by KAK, MDZ, ASS, AS, RA, HN and HS. KAK, ASS, AS and GGP drafted the manuscript. Statistical data analysis by ASS and KAK. GGP, LB and MCA provided intellectual criticism on the content of the manuscript. All authors have read and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Ethics for approval and consent to participate
The protocol for this study was reviewed and approved by the Institutional Health Research Ethics Committee of the Centre de Recherche Entomologique de Cotonou (CIERS-CREC) of Benin in accordance with favorable ethical opinion N°06-22/CREC/CIERS-CREC/SG. The risk of mosquito collectors contracting yellow fever was minimized by identifying them on site as they already had some immunity due to their prolonged exposure to mosquitoes. They have all been vaccinated against yellow fever and are regularly monitored. In the event of a confirmed outbreak of fever, they are immediately attended to by the team doctor. Larval survey in jars, tires and domestic containers is carried out with the consent of the house owners.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Konkon, A.K., Padonou, G.G., Osse, R. et al. Insecticide resistance status of Aedes aegypti and Aedes albopictus mosquitoes in southern Benin, West Africa. Trop Med Health 51, 22 (2023). https://doi.org/10.1186/s41182-023-00514-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41182-023-00514-y