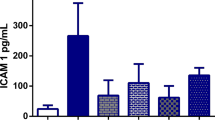
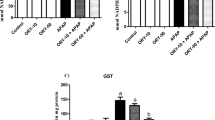
Abstract
Purpose
Available epidemiological reports have indicated an increase in the incidence of liver damage globally. Monosodium glutamate (MSG) is a widely used food additive in many processed foods which has been associated with tissue injury. However, there is a paucity of information on its effect on hepatic injury. Therefore, the present study examined the possible influence of high intake of MSG on lipopolysaccharide (LPS)-induced liver injury.
Methods
Twenty-eight healthy male Wistar rats were used for the study. MSG (1500 mg/kg) was administered orally for 14 days while LPS (0.25 mg/kg) was administered intraperitoneally for 7 days.
Results
Administration of MSG to rats with hepatocyte injury intensified liver damage with a marked increase in the activities of serum aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase. Moreover, MSG aggravated (P < 0.05) LPS-mediated hepatic oxidative stress as evidenced by a marked decrease in enzymatic and non-enzymatic antioxidants with a concomitant increase in lipid peroxidation. The increase in inflammatory biomarkers, in addition to histological damage in the liver, was aggravated following the administration of MSG to rats with liver injury.
Conclusion
Taken together, MSG exacerbated liver injury via mechanisms relating to increased oxidative stress and inflammation.
Graphical abstract






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2018;70(1):151–71. https://doi.org/10.1016/j.jhep.2018.09.014.
Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System, Mortality 1999–2020 on CDC WONDER Online Database, released in 2021. http://wonder.cdc.gov/ucd-icd10.html. Accessed 22 Oct 2022.
Josephs MD, Bahjat FR, Fukuzuka K, Ksontini R, Solorzano CC, Edwards CK, Tannahill CL, MacKay SL, Copeland EM, Moldawer LL. Lipopolysaccharide and D-galactosamine-induced hepatic injury is mediated by TNF-alpha and not by Fas ligand. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1196–201.
Ajuwon OR, Oguntibeju OO, Marnewick JL. Amelioration of lipopolysaccharide-induced liver injury by aqueous rooibos (Aspalathus linearis) extract via inhibition of pro-inflammatory cytokines and oxidative stress. BMC Complement Altern Med. 2014;14:392. https://doi.org/10.1186/1472-6882-14-392.
Huang G, Ge Y, Gui Z, Zhu M, Liu J, Wang H. Toxicity of Melastoma dodecandrum Lour. and its effects on lipopolysaccharide induced inflammation and oxidative stress. Exp Ther Med. 2021;22:807. https://doi.org/10.3892/etm.2021.10239.
Enomoto N, Ikejima K, Bradford BU, Rivera CA, Kono H, Goto M, Yamashina S, Schemmer P, Kitamura T, Oide H, et al. Role of Kupffer cells and gut-derived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;15:D20–5.
Enomoto N, Schemmer P, Ikejima K, Takei Y, Sato N, Brenner DA, Thurman RG. Long-term alcohol exposure changes sensitivity of rat Kupffer cells to lipopolysaccharide. Alcohol Clin Exp Res. 2001;25:1360–7.
Uesugi T, Froh M, Arteel GE, Bradford BU, Wheeler MD, Gäbele E, Isayama F, Thurman RG. Role of lipopolysaccharide-binding protein in early alcohol-induced liver injury in mice. J Immunol. 2002;168:2963–9.
Zhao P, Piao X, Pan L, Zeng Z, Li Q, Xu X, Wang H. Forsythia suspensa extract attenuates lipopolysaccharide-induced inflammatory liver injury in rats via promoting antioxidant defense mechanisms. Anim Sci J. 2016;88(6):873–81. https://doi.org/10.1111/asj.12717.
Mallis RJ, Buss JE, Thomas JA. Oxidative modification of H-ras: S-thiolation and S-nitrosylation of reactive cysteines. Biochem J. 2001;355:145–53.
Cadenas S, Cadenas AM. Fighting the stranger-antioxidant protection against endotoxin toxicity. Toxicology. 2002;180:45–63.
Colletti LM, Green M. Lung and liver injury following hepatic ischemia/reperfusion in the rat is increased by exogenous lipopolysaccharide which also increases hepatic TNF production in vivo and in vitro. Shock. 2001;16:312–9.
Liu Y, Li F, Zhang L, Wu J, Wang Y, Yu H. Taurine alleviates lipopolysaccharide-induced liver injury by anti-inflammation and antioxidants in rats. Mol Med Rep. 2017;16:6512–7. https://doi.org/10.3892/mmr.2017.7414.
Yang Y, Zhao J, Song X, Li L, Li F, Shang J, Wang W. Amygdalin reduces lipopolysaccharide-induced chronic liver injury in rats by down-regulating PI3K/AKT, JAK2/STAT3 and NF-κB signalling pathways. Artif Cells Nanomed Biotechnol. 2019;47:2688–97. https://doi.org/10.1080/21691401.2019.1634084.
Tong F, Shen W, Song P, Song J, Hu Y, Liu F, Meng Z, Liu J. Dexmedetomidine attenuates lipopolysaccharide-induced acute liver injury in rats by inhibiting caveolin-1 downstream signaling pathway. Biosci Rep. 2021;41:BSR20204279. https://doi.org/10.1042/BSR20204279.
Foran L, Blackburn K, Kulesza RJ. Auditory hindbrain atrophy and anomalous calcium binding protein expression after neonatal exposure to monosodium glutamate. Neuroscience. 2017;344:406–17. https://doi.org/10.1016/j.neuroscience.2017.01.004.
Zanfirescu A, Ungurianu AA, Tsatsakis M, Nitulescu GM, Kouretas D, Veskoukis A, Tsoukalas D, Engin AB, Aschner M, Margină D. A review of the alleged health hazards of monosodium glutamate. Compr Rev Food Sci Food Saf. 2019;18:1111–34.
Kazmi Z, Fatima I, Perveen S, et al. Monosodium glutamate: review on clinical reports. Int J Food Prop. 2017;20:1807–15.
Tomé D. The roles of dietary glutamate in the intestine. Ann Nutr Metab. 2018;5:15–20. https://doi.org/10.1159/000494777.
Onaolapo AY, Onaolapo OJ. Dietary glutamate and the brain: in the footprints of a Jekyll and Hyde molecule. Neurotoxicology. 2020;80:93–104. https://doi.org/10.1016/j.neuro.2020.07.001.
Chakraborty SP. Patho-physiological and toxicological aspects of monosodium glutamate. Toxicol Mech Methods. 2019;29:389–96.
Abd-Elkareem M, Soliman M, Abd El-Rahman MAM, Khalil NSA. The protective effect of Nigella sativa seeds against monosodium glutamate-induced hepatic dysfunction in rats. Toxicol Rep. 2022;9:147–53.
Tilson HA, Jaspers RMA, Kornet LMW, van Ree JM (eds) Introduction to neurobehavioral toxicology: food and environment (1st ed.). CRC Press.1998. https://doi.org/10.1201/9781439805985.
Elbassuoni EA, Ragy MM, Ahmed SM. Evidence of the protective effect of L-arginine and vitamin D against monosodium glutamate-induced liver and kidney dysfunction in rats. Biomed Pharmacother. 2018;108:799–808.
Abu-Taweel GM, Zyadah MA, Ajarem JS, Ahmad M. Cognitive and biochemical effects of monosodium glutamate and aspartame, administered individually and in combination in male albino mice. Neurotoxicol Teratol. 2014;42:60–7.
Eid RA, Al-Shraim M, Zaki MS, Kamar SS, Abdel Latif NS, Negm S, Al-Ani B, Haidara MA. Vitamin E protects against monosodium glutamate-induced acute liver injury and hepatocyte ultrastructural alterations in rats. Ultrastruct Pathol. 2019;43:199–208.
Quines CB, Jardim NS, Araujo PCO, Cechella JL, Prado VC, Nogueira CW. Resistance training restores metabolic alterations induced by monosodium glutamate in a sex-dependent manner in male and female rats. J Cell Biochem. 2019;120:13426–40.
Tawfik MS, Al-Badr N. Adverse effects of monosodium glutamate on liver and kidney functions in adult rats and potential protective effect of vitamins C and E. Food Nutr Sci. 2012;3:5. https://doi.org/10.4236/fns.2012.35089.
Banerjee A, Mukherjee S, Maji BK. Efficacy of Coccinia grandis against monosodium glutamate induced hepatocardiac anomalies by inhibiting NF-kB and caspase 3 mediated signalling in rat model. Hum Exp Toxicol. 2021;40:1825–51. https://doi.org/10.1177/09603271211010895.
Banerjee A, Das D, Paul R, et al. Mechanistic study of attenuation of monosodium glutamate mixed high lipid diet induced systemic damage in rats by Coccinia grandis. Sci Rep. 2020;10:15443.
Arya A, Al-Obaidi MMJ, Karim RB, Taha H, Khan AK, Shahid N, et al. Extract of Woodfordia fruticosa flowers ameliorates hyperglycemia, oxidative stress and improves β-cell function in streptozotocin–nicotinamide induced diabetic rats. J Ethnopharmacol. 2015;175:229–40.
Nagababu E, Rifkind JM, Boindala S, Nakka L. Assessment of antioxidant activity of eugenol in vitro and in vivo. Methods Mol Biol. 2010;610:165–80.
Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196:143–51.
Misra HP, Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5.
Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferase; the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9.
Beutler E. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8.
Suzuki K, Ota H, Sasagawa S, Sakatani T, Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem. 1983;132:345–52.
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8.
Watford M. Glutamine and glutamate metabolism across the liver sinusoid. J Nutr. 2000;4:983S-987S.
Beheshti F, Norouzi F, Abareshi A, Khazaei M, Alikhani V, Moussavi S, Biglari G, Soukhtanloo M, Hosseini M. Nigella sativa prevented liver and renal tissue damage in lipopolysaccharide-treated rat. Saudi J Kidney Dis Transpl. 2018;29:554–66.
Boutry C, Bos C, Matsumoto H, Even P, Azzout-Marniche D, Tome D, Blachier F. Effects of monosodium glutamate supplementation on glutamine metabolism in adult rats. Front Biosci. 2011;3:279–90.
Davies KJ. Protein damage and degradation by oxygen radicals I General aspects. J Biol Chem. 1987;262:9895–901.
Klein T, Neuhaus K, Reutter F, Nüsing RM. Generation of 8-epi-prostaglandin F(2alpha) in isolated rat kidney glomeruli by a radical-independent mechanism. Br J Pharmacol. 2001;133:643–50.
Sewerynek E, Melchiorri D, Chen L, Reiter RJ. Melatonin reduces both basal and bacterial lipopolysaccharide-induced lipid peroxidation in vitro. Free Radic Biol Med. 1995;19:903–9.
Banerjee A, Mukherjee S, Maji BK. Monosodium glutamate causes hepato-cardiac derangement in male rats. Hum Exp Toxicol. 2021;40:S359–69. https://doi.org/10.1177/09603271211049550.
Liu J, Du S, Kong Q, Zhang X, Jiang S, Cao X et al. HSPA12A attenuates lipopolysaccharide-induced liver injury through inhibiting caspase-11-mediated hepatocyte pyroptosis via PGC-1α-dependent acyloxyacyl hydrolase expression. Cell Death Differ. 2020;27(9):2651–2667. https://doi.org/10.1038/s41418-020-0536-x.
Shukry M, El-Shehawi MA, El-Kholy WM, Elsisy RA, Hamoda HS, Tohamy HG, Abumandour MM, Farrag FA. Ameliorative effect of graviola (Annona muricata) on mono sodium glutamate-induced hepatic injury in rats: antioxidant, apoptotic, anti-inflammatory, lipogenesis markers and histopathological studies. Animals. 2020;10:1996. https://doi.org/10.3390/ani10111996.
Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27–42.
Hamza AA, Mohamed MG, Lashin FM, Amin A. Dandelion prevents liver fibrosis, inflammatory response, and oxidative stress in rats. J Basic Appl Zool. 2020;81:43.
Andreasen AS, Krabbe KS, Krogh-Madsen R, Taudorf S, Pedersen BK, Moller K. Human Endotoxemia as a model of systemic inflammation. Curr Med Chem. 2008;15:1697–705.
Steib CJ. Kupffer cell activation and portal hypertension. Gut. 2011;60:1307–8.
Chiao CW, Lee SS, Wu CC, Su MJ. Thaliporphine increases survival rate and attenuates multiple organ injury in LPS-induced endotoxaemia. Naunyn-Schmiedebergs Arch Pharmacol. 2005;371:34–43.
Nakagawa H, Maeda S. Molecular mechanisms of liver injury and hepatocarcinogenesis: focusing on the role of stress-activated MAPK. Pathol Res Int. 2012;2012:172894. https://doi.org/10.1155/2012/172894.
Zhu DY, Li R, Liu GQ, Hua WY. Tumor necrosis factor alpha enhances the cytotoxicity induced by nitric oxide in cultured cerebral endothelial cells. Life Sci. 2000;66:1325–35.
Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424.
Aratani Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys. 2018;640:47–52.
Ndrepepa G. Myeloperoxidase - A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin Chim Acta. 2019;493:36–51.
Acknowledgements
The authors acknowledge the technical assistance of Dr Aina of the histopathology unit, University of Ibadan for tissue processing. This study was done using the authors’ personal funds without a specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors. The graphical abstract figure was created with Biorender (https://biorender.com).
Author information
Authors and Affiliations
Contributions
Folake O. Asejeje: conceptualization, methodology, investigation, writing-original draft, writing – review & editing. Gladys O. Gabriel: methodology, investigation. Michael A. Abiola: methodology, data curation and analysis, writing-original draft, writing – review & editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The experimental procedure for this study was accepted and approved by the Research Ethics Committee of Ajayi Crowther University (FNS/ERC/2021/081 M). The animal handling and treatment protocol followed standard procedures in strict compliance with the “Principle of Laboratory Animal Care” (NIH Publication No. 85–23).
Conflict of interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asejeje, F.O., Gabriel, G.O. & Abiola, M.A. Monosodium glutamate aggravates lipopolysaccharide-induced liver injury via inflammation and oxidative stress in rats. Nutrire 48, 5 (2023). https://doi.org/10.1186/s41110-023-00188-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-023-00188-w