Abstract
Purpose
Crohn’s disease (CD) consists of a state of chronic inflammation that can affect the entire length of the gastrointestinal tract, from the mouth to the anus. Symptoms include diarrhea, abdominal pain, and fever. Corticosteroid therapy has been widely used as the main therapeutic option to induce clinical remission and minimize the deleterious effects of inflammation. However, patients who make continuous use of corticosteroids are likely to develop Cushing’s syndrome. Exclusive enteral nutrition (EEN) is considered the first-line treatment for inducing clinical remission in children and adolescents with active Crohn’s disease. This study aimed to evaluate the use of EEN in inducing remission of CD in children and adolescents and discuss the impact of the EEN on patients’ growth, clinical and endoscopic remission, nutritional aspects, differences between formulas, and the impact on the gut microbiota.
Methods
This is a narrative review. A search for scientific articles was carried out in the PUBMED database, the SciELO electronic library, and the LILACS databases.
Results
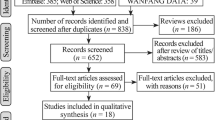
Five randomized clinical trials, 7 non-randomized clinical trials, and 5 retrospective studies with document analysis were included. In total, the studies covered 660 children and adolescents, with different degrees of initial CD activity. All 17 articles evaluated had, as a common result, a success of EEN to induce remission of active Crohn’s disease.
Conclusion
EEN is an effective therapy for inducing clinical remission in pediatric patients with active CD, and it is associated with an improvement in the quality of life in this population.
Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Abbreviations
- CD:
-
Crohn’s disease
- EEN:
-
Exclusive enteral nutrition
- GIT:
-
Gastrointestinal tract
- PCDAI:
-
Pediatrics Crohn’s Disease Activity Index
- CS:
-
Corticosteroids
- EN:
-
Enteral nutrition
- PEN:
-
Partial enteral nutritional
References
Santos GM, Silva LR, Santana GO. Repercussões nutricionais em crianças e adolescentes na presença de doenças inflamatórias intestinais. Revista Paulista de Pediatria. 2014;32(4):403–11. https://doi.org/10.1590/S0103-05822014000400018.
Forbes A, Escher J, Hébuterne X, Klek SM, Krznaric Z, Schneider S, et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36:321–47. https://doi.org/10.1016/j.clnu.2016.12.027.
Santos LAA, Dorna MS, Vulcano DSB, Augusti L, Franzoni LC, Gondo FF, Romeiro FG, Sassaki LY. Terapia nutricional nas doenças inflamatórias intestinais: artigo de revisão. Nutrire Rev Soc Bras Aliment Nutr. 2015;383–396 https://doi.org/10.4322/2316-7874.56714
Lafferty L, Tuohy M, Carey A, Sugrue S, Hurley M, Hussey S. Outcomes of exclusive enteral nutrition in paediatric Crohn’s disease. Eur J Clin Nutr. 2016;71(2):185–91. https://doi.org/10.1038/ejcn.2016.210.
Al Nabhani Z, Dietrich G, Hugot Jp, Barreau F. Nod2: the intestinal gate keeper. PLoS Pathog. 2017;13(3):e1006177. https://doi.org/10.1371/journal.ppat.1006177.g001
Sidiq T, Yoshihama S, Downs I, Kobayashi KS. Nod2: a critical regulator of ileal microbiota and Crohn’s disease. Front Immunol. 2016;7:367. https://doi.org/10.3389/fimmu.2016.00367.
Hou JK, Abraham B, El-serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106(4):563–73. https://doi.org/10.1038/ajg.2011.44.
Turner J. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. https://doi.org/10.1038/nri2653.
Yu Y, Chen KC, Chen J. Exclusive enteral nutrition versus corticosteroids for treatment of pediatric Crohn’s disease: a meta-analysis. World J Pediatr Fev. 2019;15(1):26–36. https://doi.org/10.1007/s12519-018-0204-0.
Verburgt CM, Ghiboub M, Benninga MA, de Jonge WJ, Van Limbergen JE, Nutritional therapy strategies in pediatric Crohn’s disease. Nutrients. 2021;13:212 https://doi.org/10.3390/nu13010212
Steiner S, Noe J, Denne S. Corticosteroids increase protein breakdown and loss in newly diagnosed pediatric Crohn disease. Pediatr Res. 2011;70:484–8. https://doi.org/10.1203/PDR.0b013e31822f5886.
Cardoso MGC, Prates SMS, Anastácia LR. Fórmulas para nutrição enteral padrão e modificada disponíveis no Brasil: Levantamento e classificação. Braspen J. 2018;33(4):402–17.
Levine A, Wine E, Assa A, Sigall Boneh R, Shaoul R, Kori M, Cohen S, et al. Crohn ’s disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology. 2019;157(2):440-450.e8. https://doi.org/10.1053/j.gastro.2019.04.021.
Gavin J, Ashton JJ, Heather N, Marino LV, Beattie RM. Nutritional support in paediatric Crohn’s disease: outcome at 12 months. Acta Pediatr. 2018;107:156–62. https://doi.org/10.1111/apa.14075.
Turner D, Griffiths AM, Walters TD, Seah T, Markowitz J, Pfefferkorn M, Keljo D, Otley A, Leleiko NS, Mack D, Hyams J, Levine A. Appraisal of the pediatric Crohn’s disease activity index on four prospectively collected datasets: recommended cutoff values and clinimetric properties. Am J Gastroenterol. 2010;105(9):2085–92. https://doi.org/10.1038/ajg.2010.143.
Verburgt CM, Ghiboub M, Benninga MA, de Jonge WJ, Van Limbergen JE. Nutritional therapy strategies in pediatric Crohn’s disease. Nutrients. 2021;13:212. https://doi.org/10.3390/nu13010212
Souza GN, Draghi PF, Yonamine GH. Terapia nutricional oral e enteral nas doenças inflamatórias intestinais em crianças e adolescentes: uma revisão na literatura. Revista Paulista de Pediatria, v. 38, 2020. São Paulo. https://doi.org/10.1590/1984-0462/2020/38/2019032
Rother ET. Revisão sistemática X revisão narrativa. Acta Paulista de Enfermagem. 2007;20(2). https://doi.org/10.1590/S0103-21002007000200001
Elias CSR, Silva LA, Martins MTSL, Ramos NAPR, Souza, MGG, Hipolito R. Quando chega o fim? Uma revisão narrativa sobre terminalidade do período escolar para alunos deficientes mentais. SMAD: Revista Electrónica em Salud Mental, Alcohol y Drogas, 2012; 1(8):48–53.
Grover Z, Muir R, Lewindon P. Exclusive enteral nutrition induces early clinical, mucosal and transmural remission in paediatric Crohn’s disease. J Gastroenterol. 2013;49(4):638–45. https://doi.org/10.1007/s00535-013-0815-0.
Navas-López VM, Blasco-Alonso J, Lacasa Maseri S, Girón Fernández-Crehuet F, Serrano Nieto MJ, Vicioso Recio MI, Sierra Salinas C. La nutrición enteral exclusiva continua siendo el tratamiento de primera línea en la enfermedad de Crohn pediátrica en la era de los biológicos An Pediatr (Barc). 2015;83(1):47–54. https://doi.org/10.1016/j.anpedi.2014.02.027. Spanish.
Day AS, Whitten KE, Lemberg DA, Clarkson C. Vitug-Sales M, Jackson R, Bohane TD. Exclusive enteral feeding as primary therapy for Crohn’s disease in Australian children and adolescents: a feasible and effective approach. J Gastroenterol Hepatol. 2006;21(10):1609–1614. https://doi.org/10.1111/j.1440-1746.2006.04294.x
De Bie C, Kindermann A, Escher J. Use of exclusive enteral nutrition in paediatric Crohn’s disease in The Netherlands. J Crohn’s Colitis. 2013;7(4):263–70. https://doi.org/10.1016/j.crohns.2012.07.001.
Buchanan E, Gaunt WW, Cardigan T, Garrick V, McGrogan P, Russell RK. The use of exclusive enteral nutrition for induction of remission in children with Crohn’s disease demonstrates that disease phenotype does not influence clinical remission. Aliment Pharmacol Ther. 2009;30(5):501–7. https://doi.org/10.1111/j.1365-2036.2009.04067.x.
Bannerjee K, Camacho-Hübner C, Babinska K, Dryhurst KM, Edwards R, Savage MO, Sanderson IR, Croft NM. Anti-inflammatory and growth-stimulating effects precede nutritional restitution during enteral feeding in Crohn disease. J Pediatr Gastroenterol Nutr. 2004;38(3):270–5. https://doi.org/10.1097/00005176-200403000-00007.
Johnson T, Macdonald S, Hill SM, Thomas A, Murphy MS. Treatment of active Crohn’s disease in children using partial enteral nutrition with liquid formula: a randomised controlled trial. Gut. 2006;55(3):356–61. https://doi.org/10.1136/gut.2004.062554.
Borrelli O, Cordischi L, Cirulli M, Paganelli M, Labalestra V, Uccini S, Russo PM, Cucchiara S. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clin Gastroenterol Hepatol. 2006;4(6):744–53. https://doi.org/10.1016/j.cgh.2006.03.010.
Connors J, Basseri S, Grant A, Giffin N, Mahdi G, Noble A, Rashid M, Otley A, Van Limbergen J. Exclusive enteral nutrition therapy in paediatric Crohn’s disease results in long-term avoidance of corticosteroids: results of a propensity-score matched cohort analysis. J Crohns Colitis. 2017;11(9):1063–70. https://doi.org/10.1093/ecco-jcc/jjx060.
Berni Canani R, Terrin G, Borrelli O, Romano MT, Manguso F, Coruzzo A, D’Armiento F, Romeo EF, Cucchiara S. Short- and long-term therapeutic efficacy of nutritional therapy and corticosteroids in paediatric Crohn’s disease. Dig Liver Dis. 2006;38(6):381–7. https://doi.org/10.1016/j.dld.2005.10.005.
Pigneur B, Lepage P, Mondot S, Schmitz J, Goulet O, Doré J, Ruemmele FM. Mucosal healing and bacterial composition in response to enteral nutrition vs steroid-based induction therapy—a randomised prospective clinical trial in children with Crohn’s disease. J Crohns Colitis. 2019;13(7):846–55. https://doi.org/10.1093/ecco-jcc/jjy207.
Akobeng AK, Miller V, Stanton J, Elbadri AM, Thomas AG. Double-blind randomized controlled trial of glutamine-enriched polymeric diet in the treatment of active Crohn’s disease. J Pediatr Gastroenterol Nutr. 2000;30(1):78–84. https://doi.org/10.1097/00005176-200001000-00022.
Fell JM, Paintin M, Arnaud-Battandier F, Beattie RM, Hollis A, Kitching P, Donnet-Hughes A, MacDonald TT, Walker-Smith JA. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment Pharmacol Ther. 2000;14(3):281–9. https://doi.org/10.1046/j.1365-2036.2000.00707.x.
Ludvigsson JF, Krantz M. Bodin L, Stenhammar L, Lindquist B Elemental versus polymeric enteral nutrition in paediatric Crohn’s disease: a multicentre randomized controlled trial. Acta Paediatr. 2004;93(3):327–35. https://doi.org/10.1111/j.1651-2227.2004.tb02956.x.
Lionetti P, Callegari ML, Ferrari S, Cavicchi MC, Pozzi E, de Martino M, Morelli L. Enteral nutrition and microflora in pediatric Crohn’s disease. J Parenter Enteral Nutr. 2005;29(4 supl):S173–S178. https://doi.org/10.1177/01486071050290s4s173.
Kaakoush NO, Day AS, Leach ST, Lemberg DA, Nielsen S, Mitchell HM. Effect of exclusive enteral nutrition on the microbiota of children with newly diagnosed Crohn’s disease. Clin Transl Gastroenterol. 2015;6(1):e71–e71. https://doi.org/10.1038/ctg.2014.21.
Tjellstrom B, Hogberg L, Stenhammar L, Magnusson KE, Midtvedt T, Norin E, Sundqvist T. Effect of exclusive enteral nutrition on gut microflora function in children with Crohn’s disease. Scand J Gastroenterol. 2012;47(12):1454–9. https://doi.org/10.3109/00365521.2012.703234.
Issa M, Binion DG. Bowel Rest and Nutrition Therapy in the Management of Active Crohn’s Disease. Nutr Clin Pract. 2008;23(3):299–308. https://doi.org/10.1177/0884533608318675.
Agin M, Yucel A, Gumus M, Yuksekkaya HA, Tumgor G. The Effect of Enteral Nutrition Support Rich in TGF-β in the Treatment of Inflammatory Bowel Disease in Childhood. Medicina (Kaunas). 2019;55(10):620. https://doi.org/10.3390/medicina55100620.
Vinolo MAR. Efeito dos ácidos graxos de cadeia curta sobre neutrófilos. 2010. Tese (Doutorado em Fisiologia Humana) - Instituto de Ciências Biomédicas, University of São Paulo, São Paulo. 2010. https://doi.org/10.11606/T.42.2010.tde-10012011-152253
Neurath MF. Host-microbiota interactions in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17(2):76–7. https://doi.org/10.1038/s41575-019-0248-1.
Yilmaz B, Juillerat P, Øyås O, Ramon C, Bravo FD, Franc Y, Fournier N, Michetti P, Mueller C, Geuking M, Pittet VEH, Maillard MH, Rogler G; Swiss IBD Cohort Investigators, Wiest R, Stelling J, Macpherson AJ. Microbial network disturbances in relapsing refractory Crohn’s disease. Nat Med. 2019;25(2):323–336. https://doi.org/10.1038/s41591-018-0308-z.
Sanz Y, Romaní-Perez M, Benítez-Páez A, Portune KJ, Brigidi P, Rampelli S, Dinan T, Stanton C, Delzenne N, Blachier F, Neyrinck AM, Beaumont M, Olivares M, Holzer P, Günther K, Wolters M, Ahrens W, Claus SP, Campoy C, Murphy R, Sadler C, Fernández L, Kamp JV. Towards microbiome-informed dietary recommendations for promoting metabolic and mental health: Opinion papers of the MyNewGut project. Clin Nutr. 2018;37(6 Pt A):2191–2197. https://doi.org/10.1016/j.clnu.2018.07.007.
Duncan H, Buchanan E, Cardigan T, Garrick V, Curtis L, McGrogan P, Barclay A, Russell RK. A retrospective study showing maintenance treatment options for paediatric CD in the first year following diagnosis after induction of remission with EEN: supplemental enteral nutrition is better than nothing! BMC Gastroenterol. 2014;14:50. https://doi.org/10.1186/1471-230x-14-50.
Afzal NA, Van Der Zaag-Loonen HJ, Arnaud-Battandier FS. Davies S. Murch B, Derkx R. Heuschkel, Fell JMN. Improvement in quality of life of children with acute Crohn’s disease does not parallel mucosal healing after treatment with exclusive enteral nutrition. Aliment Pharmacol Ther. 2004;20(2):167–72. https://doi.org/10.1111/j.1365-2036.2004.02002.x.
Cordeiro AM, Oliveira GM, Renteria JM, Guimarães CA. Revisão sistemática: uma revisão narrativa. Rev Col Bras Cir. 2007;34(6):428–31. https://doi.org/10.1590/S0100-69912007000600012.
Galvão CM. Níveis de evidência. Acta Paulista de Enfermagem. 2006;19(2):5. https://doi.org/10.1590/S0103-21002006000200001.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
M.M.R. was responsible for the study concept. H.C.V. performed the search of the studies and the initial manuscript preparation. H.C.V. and M.M.R. were responsible for the study analyses, design, and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vidal, H.C., Rogero, M.M. The use of exclusive enteral nutritional therapy in children and adolescents with active Crohn’s disease: an integrative review. Nutrire 47, 25 (2022). https://doi.org/10.1186/s41110-022-00177-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-022-00177-5