Abstract
Background
l-Glutamine (l-GLN) is the most abundant amino acid in the human body. In hypercatabolic conditions, supplementation with l-GLN improves immunological function. Among the immune cells, the lymphocytes are the largest consumers of l-GLN in a catabolic disease state. Here, we evaluated the effect of l-GLN treatment on the expression of T and B cell surface molecules and secreted cytokines in cultured peripheral blood of healthy subjects.
Methods
Peripheral blood samples (N = 14) were cultured with or without l-GLN treatment and immunostained for T (anti-CD45, anti-CD3, anti-CD4, anti-CD8) and B (anti-CD19 and anti-CD20) lymphocytes at different time points over 24 h. In addition, secreted cytokines IL1-β, IL-6, IL-8, IL-10, IL-12p70, and TNF were measured at the same period with cytometric bead array.
Results
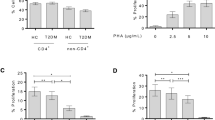
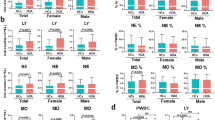
Our data showed that CD45 expression increased after 18 h in the l-GLN treatment group. On the other hand, l-GLN decreased the expression of total (CD3+) and helper (CD3+CD4+) T cells within 24 h, reducing the expression of cytotoxic cell markers (CD3+CD8+) at 12 and 18 h and of B cells (CD19+ and CD20+) at 18 h. Also, IL-1β and IL-10 cytokines increased at 18 and 24 h in the l-GLN treatment group supernatant of cultured peripheral blood.
Conclusions
The main finding of this study is that in vitro treatment with l-GLN can modulate the expression of T and B lymphocytes surface molecules and increase the secretion of IL-1β and IL-10 after 24 h.


Similar content being viewed by others
Data availability
Only if you give prior notice to the corresponding author. The datasets generated during and/or analyzed during the current study are available.
References
Wilmore DW, Shabert JK. Role of glutamine in immunologic responses. Nutrition. 1998;14:618–26. https://doi.org/10.1016/S0899-9007(98)00009-4.
Kao C, Hsu J, Bandi V, Jahoor F. Alterations in glutamine metabolism and its conversion to citrulline in sepsis. Am J Physiol Endocrinol Metab. 2013;304:E1359–64. https://doi.org/10.1152/ajpendo.00628.2012.
Newsholme EA, Crabtree B, Ardawi MS. Glutamine metabolism in lymphocytes: its biochemical, physiological and clinical importance. Q J Exp Physiol. 1985;70:473–89.
Cruzat VF, Petry ÉR, Tirapegui J. Glutamina: Aspectos bioquímicos, metabólicos, moleculares e suplementação. Rev Bras Med do Esporte. 2009;15:392–7. https://doi.org/10.1590/S1517-86922009000600015.
Smedberg M, Grass NN, Pettersson L, et al. Plasma glutamine concentration after intensive care unit discharge: an observational study. Crit Care. 2014;18:1–8. https://doi.org/10.1186/s13054-014-0677-8.
Berger MM, Binz PA, Roux C, et al.Exudative glutamine losses contribute to high needs after burn injury. J Parenter Enter Nutr. 2021;1–26.https://doi.org/10.1002/jpen.2227
Matsuyama T, Yoshinaga SK, Shibue K, Mak TW. Comorbidity-associated glutamine deficiency is a predisposition to severe COVID-19. Cell Death Differ. 2021;28:3199–213. https://doi.org/10.1038/s41418-021-00892-y.
Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48–79. https://doi.org/10.1016/j.clnu.2018.08.037.
Wischmeyer PE. The glutamine debate in surgery and critical care. Curr Opin Crit Care. 2019;25:322–8. https://doi.org/10.1097/MCC.0000000000000633.
Papanikolopoulou A, Syrigos N, Vini L, et al. Use of oral glutamine in radiation-induced adverse effects in patients with thoracic and upper aerodigestive malignancies: Results of a prospective observational study. Oncol Lett. 2022;23. https://doi.org/10.3892/ol.2021.13137.
Hörig H, Spagnoli GC, Filgueira L, et al. Exogenous glutamine requirement is confined to late events of T cell activation. J Cell Biochem. 1993;53:343–51.
Chang WK, Yang KD, Chuang H, et al. Glutamine protects activated human T cells from apoptosis by up-regulating glutathione and Bcl-2 levels. Clin Immunol. 2002;104:151–60. https://doi.org/10.1006/clim.2002.5257.
Lin MT, Kung SP, Yeh SL, et al. Glutamine-supplemented total parenteral nutrition attenuates plasma interleukin-6 in surgical patients with lower disease severity. World J Gastroenterol. 2005;11:6197–201.
Fan J, Wu L, Li G, et al. Effects of enteral nutrition with parenteral glutamine supplementation on the immunological function in septic rats. Br J Nutr. 2015;113:1712–22. https://doi.org/10.1017/S0007114515001099.
Jordan I, Balaguer M, Esteban ME, et al. Glutamine effects on heat shock protein 70 and interleukines 6 and 10: randomized trial of glutamine supplementation versus standard parenteral nutrition in critically ill children. Clin Nutr. 2016;35:34–40. https://doi.org/10.1016/j.clnu.2015.01.019.
Marino LV, Pathan N, Meyer RW, et al. An in vitro model to consider the effect of 2 mM glutamine and KNK437 on endotoxin-stimulated release of heat shock protein 70 and inflammatory mediators. Nutrition. 2016;32:375–83. https://doi.org/10.1016/j.nut.2015.09.007.
Huang J, Liu J, Chang G, et al. Glutamine supplementation attenuates the inflammation caused by LPS-induced acute lung injury in mice by regulating the TLR4/MAPK signaling pathway. Inflammation. 2021;44:2180–92. https://doi.org/10.1007/s10753-021-01491-2.
Mohajeri M, Horriatkhah E, Mohajery R. The effect of glutamine supplementation on serum levels of some inflammatory factors, oxidative stress, and appetite in COVID-19 patients: a case–control study. Inflammopharmacology. 2021;29:1769–76. https://doi.org/10.1007/s10787-021-00881-0.
Arribas-lópez E, Omorogieva O, Zand N, et al. A meta-analysis of the effect on wound healing of amino acids arginine and glutamine. Nutrients. 2021;13:2498.
Peng X, Yan H, You Z, et al. Glutamine granule-supplemented enteral nutrition maintains immunological function in severely burned patients. Burns. 2006;32:589–93. https://doi.org/10.1016/j.burns.2005.11.020.
Newsholme P, Curi R, PithonCuri TC, et al. Glutamine metabolism by lymphocytes, macrophages, and neutrophils: its importance in health and disease. J Nutr Biochem. 1999;10:316–24. https://doi.org/10.1016/S0955-2863(99)00022-4.
Santos LA, dos Silvério A, SD, ORFÃO LH,. Perfil leucocitário de uma população do sul de minas gerais. Rev da Univ Val do Rio Verde. 2015;13:506–13.
Rosenfeld LG, Malta DC, Szwarcwald CL, et al. Reference values for blood count laboratory tests in the Brazilian adult population, national health survey. Rev Bras Epidemiol. 2019;22:1–13. https://doi.org/10.1590/1980-549720190003.supl.2.
Hou YC, Wu JM, Wang MY, et al. Glutamine supplementation attenuates expressions of adhesion molecules and chemokine receptors on t cells in a murine model of acute colitis. Mediators Inflamm. 2014. https://doi.org/10.1155/2014/837107.
Hu YM, Hsiung YC, Pai MH, Yeh SL. Glutamine administration in early or late septic phase downregulates lymphocyte PD-1/PD-L1 expression and the inflammatory response in mice with polymicrobial sepsis. J Parenter Enter Nutr. 2018;42:538–49. https://doi.org/10.1177/0148607117695245.
Cetinbas F, Yelken B, Gulbas Z. Role of glutamine administration on cellular immunity after total parenteral nutrition enriched with glutamine in patients with systemic inflammatory response syndrome. J Crit Care. 2010. https://doi.org/10.1016/j.jcrc.2010.03.011.
Wells SM, Kew S, Yaqoob P, et al. Dietary glutamine enhances cytokine production by murine macrophages. Nutrition. 1999;15:881–4. https://doi.org/10.1016/S0899-9007(99)00184-7.
Yaqoob P, Calder PC. Cytokine production by human peripheral blood mononuclear cells: differential senstivity to glutamine availability. Cytokine. 1998;10:790–4. https://doi.org/10.1006/cyto.1998.0358.
Raizel R, Leite JSM, Hypólito TM, et al. Determination of the anti-inflammatory and cytoprotective effects of l-glutamine and l-alanine, or dipeptide, supplementation in rats submitted to resistance exercise. Br J Nutr. 2016;116:470–9. https://doi.org/10.1017/S0007114516001999.
Porsani MYH, Paludetti M, Orlando DR, et al. Protective effect of β-glucan and glutamine on intestinal and immunological damage in mice induced by cytarabine (Ara-C). Pesqui Vet Bras. 2017;37:977–83. https://doi.org/10.1590/s0100-736x2017000900013.
Safi S, Batumalaie K, Mansor M, et al. Glutamine treatment attenuates hyperglycemia-induced mitochondrial stress and apoptosis in umbilical vein endothelial cells. Clinics. 2015;70:569–76. https://doi.org/10.6061/clinics/2015(08)07.
Chang WK, Yang KD, Shaio MF. Lymphocyte proliferation modulated by glutamine: involved in the endogenous redox reaction. Clin Exp Immunol. 1999. https://doi.org/10.1046/j.1365-2249.1999.01009.x.
Cruzat V, Rogero MM, Keane KN, et al. Glutamine: metabolism and immune function, supplementation and clinical translation. 2018;1–31. https://doi.org/10.3390/nu10111564
Janský L, Reymanová P, Kopecký J. Dynamics of cytokine production in human peripheral blood mononuclear cells stimulated by LPS or infected by Borrelia. 2003;593–598
Nunes A, Koterba E, Alves V, et al. Terapia Nutricional no Paciente Grave. Proj Diretrizes. 2011;1–13.
Funding
This study was funded by the Laboratory of Immunology and Molecular Biology-Federal University of Bahia and enteral and Parenteral Nutrition Service of Hospital Santa Izabel-BA. NEVES-AMORIM, EG; SANTOS, S. Q and ARAÚJO-PEREIRA, M received Research grants the Federal University of Bahia.
Author information
Authors and Affiliations
Contributions
Neves-Amorim, E. G.: formal analysis, investigation, writing-original draft; Santos, S. Q.: investigation; Araújo-Pereira, M.: investigation; Santana, Z. V. B.: investigation; Bomfim, E. K. S: investigation; Chagas, N. M. B. L.: investigation; Conceição, R. R.: investigation; Freire, M. D. M.: general analyses, review and editing; Torres, A. J. L.: formal analysis, conceptualization, writing-review and editing; Fortuna, V.: formal analysis, conceptualization, writing-review and editing; Carvalho, G. C.: formal analysis, conceptualization, writing-review and editing; Meyer, J. R.: conceptualization, resources; Freire, S. M.: conceptualization, methodology, supervision, project administration, resources, funding acquisition, writing-review and editing; Freire, A. N. M.: conceptualization, methodology, project administration, funding acquisition, resources.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. The institutional review board of the Bahiana School of Medicine and Public Health (CAAE:57274316.3.0000.5544) approved this study.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Neves-Amorim, E.G., Santos, S.Q., Araújo-Pereira, M. et al. Effect of l-Glutamine treatment on the expression of T and B cell surface molecules and secreted cytokines by cultured peripheral blood of healthy subjects. Nutrire 47, 19 (2022). https://doi.org/10.1186/s41110-022-00169-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-022-00169-5