Abstract
Background
Sepsis 3 definitions have shifted the focus from nonspecific inflammation to sepsis as an organ dysfunction caused by a dysregulated host response to infection. Neutrophils have become therapeutic targets because of their intimate but complex involvement in sepsis. We conducted ex vivo and animal experiments to apply a granulocyte and monocyte adsorption column, which is clinically used for inflammatory bowel disease, in sepsis. In this study, the biocompatibility was evaluated in sepsis-like hypercytokinemia.
Methods
Six female outbred pigs were anesthetized. Extracorporeal direct hemoperfusion (DHP) with an Adacolumn or a sham column was initiated after lipopolysaccharide (LPS) administration. The DHP was performed for 2 h at a blood flow rate (QB) of 30 or 60 mL/min. Blood samples were collected before and during the DHP (30, 60, 90, and 120 min). The percentage change in white blood cell count, platelet count, and cytokine concentration was compared between the Adacolumn and sham columns.
Results
The percentage change in white blood cells were 96 (95–98)% and 106 (101–108)% in the Adacolumn and sham groups, respectively, at QB = 60 mL/min (p < 0.01). The percentage change in platelets were 95 (90–96)% and 97 (93–99)% in the in the Adacolumn and sham groups, respectively, at QB = 60 mL/min (not significant; n.s.). At QB = 60 mL/min, the percentage change in tumor necrosis factor-α, interleukin (IL)-6, and IL-10 were 92 (81–106)%, 95 (93–102)%, and 98 (95–100)%, respectively, for the Adacolumn and 100 (95–102)%, 98 (87–104)%, and 97 (93–99)%, respectively, for the sham column. The percentage change in white blood cell counts, platelet counts, and all cytokines at QB = 30 and 60 mL/min showed similar trends.
Conclusion
The biocompatibility of the Adacolumn was evaluated using a porcine LPS-induced inflammation model. No decrease in platelet counts or significant cytokine production was observed, suggesting that the Adacolumn could be safely used in patients with sepsis with QB = 30–60 mL/min for 2 h. However, production of mediators other than cytokines remains unknown and requires further investigation.
Similar content being viewed by others
Background
Sepsis-3, defined as life-threatening organ damage resulting from an uncontrolled host response to infection, has shifted the focus from non-specific inflammation to organ dysfunction caused by a dysregulated host response to infection [1]. Increasing evidence supports a central role of the immune system in sepsis; however, the current view of how sepsis affects immunity and vice versa is still rudimentary [2]. Neutrophils play essential roles in controlling infections under normal conditions. However, under hypercytokinemia, they cannot migrate to the infection site and their lifespan, which is normally short, is prolonged, resulting in persistent tissue damage and the development of organ dysfunction via release of several cytotoxic mediators, such as free radicals, proteolytic enzymes, and neutrophil extracellular traps (NETs) [3, 4]. The production of NETs causes microcirculatory disturbances that may lead to organ damage [5, 6]. The intimate but complex involvement of neutrophils in sepsis makes them promising targets for therapeutic intervention. Several approaches have been developed to therapeutically target neutrophils [7, 8].
In Japan, 40% of patients requiring blood purification have sepsis [9]. Blood purification for sepsis has long targeted molecules such as endotoxins and cytokines. Although polymyxin B-immobilized fiber columns (PMX) and cytokine-adsorbing hemofilters (CAH) are currently used in practice [10,11,12], blood purification methods targeting immune cells do not exist. However, in chronic inflammatory settings, such as inflammatory bowel disease (IBD), granulocyte and monocyte adsorption apheresis (GMA) with Adacolumn (JIMRO Co., Ltd., Takasaki, Japan) has been used safely in clinical practice.
We previously reported the basic performance of the Adacolumn in ex vivo experiments using lipopolysaccharide (LPS)-challenged porcine blood [13].
This study aimed to investigate whether leukocytes adsorbed on the Adacolumn produce cytokines or whether platelets are adsorbed on the column. We used a porcine LPS-induced inflammation model to mimic sepsis and determine whether Adacolumns could be safely used in patients with sepsis.
Experiment 1: porcine model
Materials and methods
Study animals
This study was approved by the Institutional Animal Care and Use Committee of the Fujita Health University (approval number: M3762). Six female outbred pigs (age: 3–4 months; body weight: 35–40 kg) were used in the study. The pigs were housed under standard environmental conditions for at least 2 d before each experiment.
Animal preparation
Considerations were made to minimize the number of animals used in the experiments as much as possible and choose methods that caused as little pain to the animals as possible. The pigs were anesthetized. Intramuscular injections of ketamine (5 mg/kg), atropine (10 μg/kg), and midazolam (0.5 mg/kg) were administered for premedication. Anesthesia was initiated with intravenous injection of ketamine (5 mg/kg) and midazolam (0.5 mg/kg), followed by 2% sevoflurane in oxygen. Subsequently, LPS-induced inflammation was induced by intravenous infusion of a saline solution containing 10 μg/kg of LPS (Escherichia coli 0111) for 30 min.
Adacolumn
The device contained a 335-mL column filled with 220 g of specially processed cellulose diacetate beads approximately 2 mm in diameter [14]. Granulocytes and monocytes are known to highly express complement component C3 (CR3, also known as CD11b/CD18, Mac1, or integrin αMβ2) and Fc gamma receptors (FcγRs) [15]. The Adacolumn beads are coated with immunoglobulin G and inactivated complement-3b (iC3b) when the blood goes through the column, which function as ligands for the FcγRs and CR3 expressed in the activated granulocytes and monocytes, respectively, allowing selective adsorption of these cells [16].
Experimental protocol
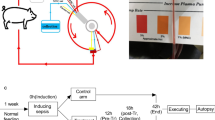
Vascular catheters for extracorporeal circulation (GamCath; Gambro Dialysatoren GmbH, Hechingen, Germany) were placed in the right external jugular and right femoral veins. The blood was debranched from the external jugular vein and returned to the femoral vein. An Adacolumn and sham column (control column not filled with Adacolumn beads) were connected using a two-way circuit (Fig. 1). This circuit is characterized by the simultaneous passage of blood from the same individual through two columns.
Schematic picture of the extracorporeal circuit. An Adacolumn and sham column were connected using a two-way circuit. The blood was debranched from the external jugular vein and returned to the femoral vein. Blood samples were collected from three sampling sites: the inlet side of both columns, outlet of the Adacolumn, and outlet of the sham column. The adsorption and production of blood cells and cytokines were compared using percentage change (ratio of outlet to inlet concentration multiplied by 100)
Extracorporeal direct hemoperfusion (DHP) was initiated 30 min after continuous LPS administration. The DHP was performed using a TR-525 (Toray Industries, Inc., Tokyo, Japan) for 2 h at a blood flow rate (QB) of 30 or 60 mL/min. Approximately 4000 IU of unfractionated heparin was injected intravenously at the start, and 4000 IU/h was administered continuously thereafter. Blood samples were collected before and at 30, 60, 90, and 120 min after DHP initiation. Blood samples were collected from three sampling sites: the inlet side of both columns, outlet of the Adacolumn, and outlet of the sham column. Blood cell counts and tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-8, and IL-10 were measured. The percentage change (ratio of outlet to inlet concentration multiplied by 100) in white blood cell counts, platelet counts, and cytokine concentrations was compared between both columns.
Measurements
Cytokine levels were measured by enzyme-linked immunosorbent assay (ELISA) using the following commercial kits: porcine IL-6 Quantikine ELISA (R&D Systems, Minneapolis, MN, USA), porcine IL-8/CXCL8 Quantikine ELISA (R&D Systems), porcine IL-10 Quantikine ELISA (R&D Systems), and porcine IL-10 Quantikine ELISA (R&D Systems) kits, according to the manufacturer’s instructions.
Statistical analysis
Percentage change data are expressed as medians (25–75% interquartile range). The nonparametric Mann–Whitney U test was used for statistical analysis to compare the percentage change between the Adacolumn and sham column. Statistical significance was set at p < 0.05. Data of white blood cell and platelets counts are expressed as the mean ± standard deviation (SD).
Results
White blood cells and platelets before and after column
DHP was performed in six pigs, three for each group at 30 and 60 mL/min, and the data were summed for 12-point percentage changes at four timepoints: 30, 60, 90, and 120 min. The trends in white blood cell counts during 2 h of DHP and 12-point percentage changes are shown in Fig. 2. The percentage changes were 96 [95–98]% and 106 [101–108]% in the Adacolumn and sham groups, respectively, at QB = 30 mL/min (p < 0.01) and 92 [90–98]% and 104 [100–105]% in the Adacolumn and sham groups, respectively, at QB = 60 mL/min (p < 0.01). No differences were observed in white blood cell count between QB = 30 and 60 mL/min.
Trends in white blood cell counts and percentage change during passage through the columns. Because of the small sample size, the trends in white blood cells are expressed as mean ± standard deviation (SD) (n = 3) (left). The percentage change in the white blood cell count as blood was passed through the column is indicated by the percentage change. Boxplot demonstrating the upper quartile, median, and lower quartile along with the maximum and minimum values obtained for the percentage change in white blood cell counts in the Adacolumn and sham column groups (right). X shows the average value. n.s., not significant; QB, blood flow rate
The trends in platelet counts during 2 h of DHP and the 12-point percentage changes are shown in Fig. 3. The percentage changes were 95 [90–96]% and 97 [93–99]% in the Adacolumn and sham groups, respectively, at QB = 30 mL/min (n.s.), and 95 [93–100]% and 98 [96–100]% in the Adacolumn and sham groups, respectively, at QB = 60 mL/min (n.s.). The changes in platelet counts when blood passed through the columns were comparable between the two columns and no significant decrease was observed.
Trends in platelet counts and percentage change during passage through the columns. Because of the small sample size, trends in white blood cells are expressed as mean ± SD (n = 3) (left). The percentage change in platelet count as blood was passed through the column is indicated by the percentage change. Boxplot demonstrating the upper quartile, median, and lower quartile along with the maximum and minimum values obtained for the percentage change in platelet counts in the Adacolumn and sham column groups (right). X shows the average value. n.s., not significant; QB, blood flow rate
Cytokine concentrations at column inlet and outlet
Three of the six pigs, numbered one to three, were subjected to DHP at QB = 30 mL/min, and three pigs, numbered four to six, were subjected to DHP at QB = 60 mL/min. Plasma cytokine concentrations at each blood collection point for each individual are shown in Fig. 4. However, IL-8 could not be evaluated because many results were below the detection limit, even over time.
Plasma cytokine concentrations before and after passing through the columns. Three of the six pigs (numbered one to three) were subjected to extracorporeal circulation at blood flow rate (QB) = 30 mL/min, and three pigs (numbered four to six) were subjected to extracorporeal circulation at QB = 60 mL/min. Cytokine concentrations in the blood at each time point are shown for each individual. TNF: tumor necrosis factor, IL: interleukin,
Percentage change in cytokine concentration during passage through column
As shown in Fig. 4, the blood cytokine concentrations increased with time and were high at 90 and 120 min. Since there were no missing data at these two timepoints, the median values at 90 and 120 min for the three pigs were compared between the Adacolumn and sham column at QB = 30 and 60 mL/min. The percentage change in TNF-α, IL-6, and IL-10 at QB = 30 mL/min were 102 [95–116]%, 85 [82–91]%, and 100 [94–103]% for the Adacolumn, respectively, whereas, the percentage change in the sham columns were 99 [96–102]%, 91 [87–102]%, and 96 [91–101]%, respectively. The percentage change in TNF-α, IL-6, and IL-10 at QB = 60 mL/min were 92 [81–106]%, 95 [93–102]%, and 98 [95–100]% for the Adacolumn, respectively, whereas, those for the sham columns were 100 [95–102]%, 98 [87–104]%, and 97 [93–99]%, respectively. The cytokine levels at both QBs were not significantly different between the two groups (Fig. 5).
Percentage change in the concentration of TNF-α, IL-6, and IL-10 during passage through the columns. The percentage change in cytokine concentration as blood was passed through column is indicated by the percentage change. Boxplot demonstrating the upper quartile, median, and lower quartile, along with the maximum and minimum values. X shows the average value. n.s., not significant; TNF, tumor necrosis factor; IL, interleukin; QB, blood flow rate
Experiment 2: batch-wise test
Materials and methods
The experiments were based on previously reported Adacolumn bead adsorption tests [17]. The test solution was prepared by dissolving cytokines [recombinant human (rh) TNF-α, rhIL-6, and rhIL-8 (all from FUJIFILM Wako Pure Chemical Co., Osaka, Japan)] in phosphate buffer (pH 7.2). The test solutions were filled into 5 mL sterile plastic syringes (Terumo Corporation, Tokyo, Japan) with the Adacolumn beads at a 1 mL:2 g ratio and without beads (control group), and incubated at 37 °C for 30 min. Cytokine concentrations were measured before and after incubation.
Measurements
Cytokines were measured by ELISA using the following commercial kits: Quantikine HS ELISA human TNF-α immunoassay (R&D Systems, Minneapolis, MN, USA), human IL-8 ELISA (Life Technologies, Tokyo, Japan), and human IL-6 (Fujirebio Inc., Tokyo, Japan) kits, according to the manufacturer’s instructions.
Statistical analysis
All results are expressed as the mean ± SD. Student’s t-test was used to compare data between two groups. Statistical significance was set at p < 0.05.
Results
Batch tests were performed to confirm the cytokine adsorption properties of Adacolumn beads. The initial concentration of TNF-α was 12,600 pg/mL; after 30 min of incubation, the concentration was 13,000 ± 497 pg/mL and 11,567 ± ,087 pg/mL in the control and Adacolumn beads groups (n = 3), respectively. Furthermore, IL-6 concentration went from 34,400 pg/mL to 34,867 ± 694 pg/mL in the control group and to 29,900 ± 29,909 pg/mL in the Adacolumn beads group. The IL-8 concentration went from 67,100 pg/mL to 65,200 ± 726 pg/mL in the control group and to 58,667 ± 3,984 pg/mL in the Adacolumn beads group. The ratio of TNF-α, IL-6, and IL-8 between the Adacolumn and control groups were 0.89 for TNF-α, 0.86 for IL-6, and 0.90 for IL-8. The concentrations of TNF-α, IL-6, and IL-8 after incubation were significantly lower in the Adacolumn group (Fig. 6).
Cytokine concentration after incubation with Adacolumn beads in the batch-wise test. a The test solutions were filled into 5-mL sterile plastic syringes with or without (control group) the Adacolumn beads, and incubated at 37 °C for 30 min. b Cytokine concentrations were measured before and after incubation. Because of the small sample size, data are expressed as mean ± SD (n = 3). TNF, tumor necrosis factor; IL, interleukin
Discussion
Adacolumn was first approved in Japan as a GMA for IBDs in 1999. The safety profile of the GMA is a major advantage. Post-market surveillance from 53 medical institutions in Japan provides the largest ever clinical safety data; adverse events were of low to mild severity [18]. It has since been approved for Crohn’s disease [19], pustular psoriasis [20], and psoriatic arthritis [21]. Furthermore, it has been used in general clinical practice in approximately 66,000 patients with few reports of serious side effects and is recognized as a safe treatment method.
However, the degree of blood cell stimulation may differ between patients with chronic inflammation and sepsis. In particular, the degree of cytokine production from granulocytes and monocyte adsorbed on the column, and the decrease in platelet count, should be evaluated for safe use in patients with sepsis. The Adacolumn has been used for outpatients with chronic inflammation with QB = 30 mL/min and 1 h of DHP; however, increased blood flow is usually required for its use in sepsis in the intensive care unit. In this experiment, the perfusion time was 2 h and the QB was set at 30 and 60 mL/min.
First, leukocyte adsorption by Adacolumn was observed at both QBs over a 2-h period. Notably, the percentages of granulocytes and monocytes targeted by the Adacolumn-DHP were lower in pigs (approximately 35%) than in humans. The mechanism of adsorption of porcine granulocytes and monocytes to Adacolumn is not yet clear. Recent studies have advanced the similarity between human and porcine surface markers [22], and the fact that monoclonal antibodies that inhibit CD11b or the α-chain of CR3 inhibit the phagocytosis of polymorphonuclear neutrophils (PMNs) to iC3b-opsonized particles and their ability to adhere to plastic suggests that porcine CD11b may function similarly to its human counterpart. CD11b is thought to have a similar function to that of human CD11b [23]. This suggests that the mechanism of neutrophil adsorption on Adacolumn is similar in humans and pigs. Platelet counts are often reportedly lower in patients with DHP [24]. However, no differences were observed in the percentage change in the platelet count between the Adacolumn and sham column. This suggests that fewer adverse events occur when this technique is applied to patients with sepsis having low platelet counts or complicated disseminated intravascular coagulation.
We reported the results of basic ex vivo experiments to develop a new blood purification system for regulating excessive immune reactions in sepsis using an Adacolumn and a CAH (AN69ST hemofilter). The phagocytic activity of granulocytes decreases and adhesiveness increases; however, the number of CD11b-positive cells markedly decreases [13]. This study showed that the second filter can adsorb cytokines, but the study did not examine pathogen associated molecular patterns or damage-associated molecular patterns, and the results were unknown. In this study, producing cytokines upstream of the Adacolumn was necessary to confirm the cytokine adsorption capacity of the CAHs downstream of the blood purification system. To this end, a large amount of LPS was injected into porcine blood, which was repeatedly circulated in a closed circulation system for 6 h. Naturally, this ex vivo experiment is distinct from clinical practice; therefore, we were interested in the extent of cytokine production under conditions similar to those in clinical practice. Therefore, we conducted the current study using a porcine LPS-induced inflammation model.
To the best of our knowledge, this is the first study to evaluate the biocompatibility of the Adacolumn in pigs with sepsis-like inflammation. The cytokine concentrations were measured over 2 h. Therefore, the cytokine concentrations were measured over time in all individuals at 90 and 120 min. Contrary to expectations, the cytokine concentrations in the blood passing through the Adacolumn tended to be lower than those passing through the sham column. Therefore, we evaluated the cytokine adsorption capacity of the beads using an in vitro phosphate buffer system (Methods 2). The results showed weak adsorption of TNF-α, IL-6, and IL-8 on the Adacolumn beads. This explains why cytokine concentrations may decrease when passing through the Adacolumn.
Interestingly, in reviewing research on chronic inflammation such as IBD, Adacolumn DHP not only reduces inflammation by adsorbing granulocytes and monocytes but also immunomodulates leukocyte anti-inflammatory activity when they pass through a column. The cytokine-producing capacity of neutrophils is reduced in patients with IBD after passing through the Adacolumn, and the number of anti-inflammatory CD10-negative premature granulocytes increases, indicating an increased turnover of these cells in circulation [17, 25]. Recently, Adacolumn beads have been suggested to modulate the immune system by inducing anti-inflammatory myeloid-derived suppressor cells via iC3b activation [26] and phagocytosis-induced cell death responses [27]. These immunomodulatory effects may contribute to the suppression of inflammation during sepsis. However, further studies are required to confirm these effects in patients with sepsis.
This study has several limitations. First, although LPS-induced models are important for elucidating the integrated pathophysiology of infection and inflammation, this approach has limitations because it does not completely mimic the physiological changes that would occur during bona fide sepsis. However, since the purpose of this study was not to evaluate sepsis pathogenesis but to confirm the biocompatibility of Adacolumn beads with granulocytes and monocytes under hypercytokinemia, we believe that this inflammation model will allow us to evaluate the results. In fact, the average concentration of IL-6 at 120 min in the six pigs was 2300 pg/mL, which is higher than that detected in sepsis (approximately 1000 pg/mL) [28] and may be used for evaluation. Second, the sample size used in this study was small. Although we considered using small animals such as rats to increase numbers, we chose a porcine model that is structurally and immunologically similar to humans. The porcine immune system resembles humans for > 80% of analyzed parameters in contrast to the mouse with only about 10% similarity [29]. In addition, by running the Adacolumn and sham column from the same pig, we theorized we could minimize the individual difference factor as much as possible and withstand the evaluation. Third, this study evaluated only the production of biocompatible cytokines. Therefore, the production of factors such as those resulting from hypotension, which were not measured, remains unknown. When an Adacolumn is used to treat sepsis, continuous hemo(dia)filtration is combined in many cases. When using the Adacolumn and hemofilter simultaneously, connecting the Adacolumn upstream and the hemofilter downstream in series and parallel is recommended [30]. Finally, in this study, the blood flow rate was set at 30 mL/min and 60 mL/min, but the optimal blood flow rate, and duration time for the granulocyte adsorption rate and the number of granulocytes adsorbed, needs further investigation.
In summary, Adacolumn-DHP was performed for 2 h at QBs of 30 and 60 mL/min using a porcine model with an intravenous endotoxin. White blood cell counts decreased significantly before and after passing through the Adacolumn, whereas platelet counts did not. No significant production of TNF-α, IL-6, and IL-10 was observed when passing through the column.
Conclusion
The biocompatibility of the Adacolumn was evaluated using a porcine LPS-induced inflammation model. No decrease in platelet counts or significant production of cytokines was observed, suggesting that the Adacolumn could be safely used in patients with sepsis with QB = 30–60 mL/min for 2 h. However, production of mediators other than cytokines remains unknown and requires further investigation.
Availability of data and materials
The data used in this article are available from the corresponding author upon reasonable request.
Abbreviations
- NETs:
-
Neutrophil extracellular traps
- PMX:
-
Polymyxin B-immobilized fiber column
- CAH:
-
Cytokine-adsorbing hemofilter
- IBD:
-
Inflammatory bowel disease
- GMA:
-
A granulocyte and monocyte adsorption apheresis
- LPS:
-
Lipopolysaccharide
- CR3:
-
Complement component C3 receptor
- FcγR:
-
Fc gamma receptors
- iC3b:
-
Inactivated complement-3b
- DHP:
-
Direct hemoperfusion
- ELISA:
-
Enzyme-linked immunosorbent assay
- QB:
-
Blood flow rate
References
Singer M, Deutschman CCS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10.
Rubio I, Osuchowski MF, Shankar-Hari M, et al. Current gaps in sepsis immunology: new opportunities for translational research. Lancet Infect Dis. 2019;19:e422–36.
Brown KA, Brain SD, Pearson JD, et al. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368:157–69.
Stiel L, Meziani F, Helms J. Neutrophil activation during septic shock. Shock. 2018;49:371–84.
Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2018;16:231–41.
Shen XF, Cao K, Jiang JP, et al. Neutrophil dysregulation during sepsis: an overview and update. J Cell Mol Med. 2017;21:1687–97.
Németh T, Sperandio M, Mócsai A. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov. 2020;19:253–75.
Shen X, Cao K, Zhao Y, et al. Targeting neutrophils in sepsis: from mechanism to translation. Front Pharmacol. 2021;12:644270.
Abe M, Shiga H, Tatsumi H, et al. Results of the 2018 Japan Society for Blood Purifcation in Critical Care survey: current status and outcomes. Ren Replace Ther. 2022;8:58.
Moriyama K, Nishida O. Targeting cytokines, pathogen-associated molecular patterns, and damage-associated molecular patterns in sepsis via blood purification. Int J Mol Sci. 2021;22:8882.
Kawaji T, Okamoto A, Moriyama K, et al. Sustained high-efficiency daily diafiltration using a mediator-adsorbing membrane in Burkitt lymphoma with a very high risk of tumor lysis syndrome: a case series with literature review. Ren Replace Ther. 2023;9:53.
Hattori N, Oda S. Cytokine-adsorbing hemofilter: old but new modality for septic acute kidney injury. Ren Replace Ther. 2016;2:41.
Hara Y, Shimomura Y, Nakamura T, et al. Novel blood purification system for regulating excessive immune reactions in severe sepsis and septic shock: an ex vivo pilot study. Ther Apher Dial. 2015;19:308–15.
Kashiwagi N, Hirata I, Kasukawa R. A role for granulocyte and monocyte apheresis in the treatment of rheumatoid arthritis. Ther Apher. 1998;2:134–41.
Uribe-Querol E, Rosales C. Phagocytosis: our current understanding of a universal biological process. Front Immunol. 2020;11:531655.
Hiraishi K, Takeda Y, Shiobara N, et al. Studies on the mechanisms of leukocyte adhesion to cellulose acetate beads: an in vitro model to assess the efficacy of cellulose acetate carrier-based granulocyte and monocyte adsorptive apheresis. Ther Apher Dial. 2003;7:334–40.
Kashiwagi N, Sugimura K, Koiwai H, et al. Immunomodulatory effects of granulocyte and monocyte adsorption apheresis as a treatment for patients with ulcerative colitis. Dig Dis Sci. 2002;47:1334–41.
Hibi T, Sameshima Y, Sekiguchi Y, et al. Treating ulcerative colitis by Adacolumn therapeutic leucocytapheresis: clinical efficacy and safety based on surveillance of 656 patients in 53 centres in Japan. Dig Liver Dis. 2009;41:570–7.
Fukuda Y, Matsui T, Suzuki Y, et al. Adsorptive granulocyte and monocyte apheresis for refractory Crohn’s disease: an open multicenter prospective study. J Ggastroenterol. 2004;39:1158–64.
Ikeda S, Takahashi H, Suga Y, et al. Therapeutic depletion of myeloid lineage leukocytes in patients with generalized pustular psoriasis indicates a major role for neutrophils in the immunopathogenesis of psoriasis. J Am Acad Dermatol. 2013;68:609–17.
Kanekura T, Seishima M, Honma M, et al. Therapeutic depletion of myeloid lineage leukocytes by adsorptive apheresis for psoriatic arthritis: Efficacy of a nondrug intervention for patients refractory to pharmacologics. J Dermatol. 2017;44:1353–9.
Dawson HD, Lunney JK. Porcine cluster of differentiation (CD) markers 2018 update. Res Vet Sci. 2018;118:199–246.
Guzylack-Piriou L, Salmon H. Membrane markers of the immune cells in swine: an update. Vet Res. 2008;39:54.
Ikeda T. Hemoadsorption in critical care. Ther Apher. 2002;6:189–92.
Hanai H, Takeda Y, Eberhardson M, et al. The mode of actions of the Adacolumn therapeutic leucocytapheresis in patients with inflammatory bowel disease: a concise review. Clin Exp Immunol. 2011;163:50–8.
Sakanoue M, Higashi Y, Kanekura T. Inhibition of inflammatory cytokines and induction of myeloid-derived suppressor cells by the effects of granulocyte and monocyte adsorption apheresis. Ther Apher Dial. 2017;21:628–34.
Kashiwagi N, Saito F, Maegawa H, et al. Granulocyte and monocyte adsorptive apheresis induces apoptosis of neutrophils and release of a novel chemoattractant for desensitization of interleukin-8 response–In vitro and in vivo results. Cytokine. 2021;139:155410.
Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8:1233–44.
Pabst R. The pig as a model for immunology research. Cell Tissue Res. 2020;380:287–304.
Yonekawa C, Nakae H, Tajimi K, et al. Combining continuous endotoxin apheresis and continuous hemodiafiltration in the treatment of patients with septic multiple organ dysfunction syndrome. Ther Apher Dial. 2006;10:19–24.
Acknowledgements
Not applicable.
Funding
This study did not receive any funding.
Author information
Authors and Affiliations
Contributions
T.N. and K.M. wrote the manuscript and analyzed the data. K.M. and O.N. designed the study and revised the manuscript. T.N., T.S., Y.K., and K.M. contributed to the data collection. K.M., T.N., T.S., Y.K., and O.N. discussed the results and contributed to the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Animal Care and Use Committee of the Fujita Health University (approval number: M3762).
Consent for publication
Not applicable.
Competing interests
Kazuhiro Moriyama is a member of the Endowed Chair of JIMRO Co., Ltd., and has consulting contracts with Baxter Ltd., Toray Industries Inc., and Asahi Kasei Medical Co., Ltd.. The remaining authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nakamura, T., Moriyama, K., Sakai, T. et al. Changes in cytokine concentrations during passage through a granulocyte and monocyte adsorption column in a porcine lipopolysaccharide-induced inflammation model. Ren Replace Ther 10, 51 (2024). https://doi.org/10.1186/s41100-024-00565-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-024-00565-9