Abstract
Background
IgA nephropathy (IgAN) is a common primary glomerulonephritis leading to end-stage renal disease. Living donor kidney transplantation (LDKT) is considered a favorable treatment option, but IgAN recurrence rates and the impact on outcome is unclear. In the present study, we retrospectively analyzed the rate of IgAN recurrence, risk factors and clinical outcomes in LDKT recipients.
Methods
We analyzed 193 adult patients who received a LDKT between 2011 and 2017 of which 32 (16.7%) had IgAN as underlying disease. Demographic data and clinical outcomes, especially regarding IgAN recurrence, were evaluated. Mean follow-up period was 102.13 months in the IgAN group vs. 97.27 months in the control group.
Results
Among the IgAN cohort, five (15.63%) experienced IgAN recurrence. Patients with IgAN underwent LDKT at a significantly younger age (p < 0.001). No significant differences were observed in demographic factors, HLA-mismatches, or AB0-compatibility. Patients receiving an immunosuppressive regimen including Cyclosporine A (60% vs. 7.4%) instead of Tacrolimus (40% vs. 92.6%) were significantly more likely to develop IgAN recurrences (p < 0.001). Moreover, early post-surgery serum creatinine levels were higher in the recurrence group at one week (4.25 vs. 1.65 mg/dl; p < 0.001), one month (2.13 vs. 1.53 mg/dl; p = 0.027) and two years (2.65 vs. 1.34 mg/dl; p = 0.008). Although graft survival displayed a tendency towards inferior outcomes after recurrence, significance was not reached (p = 0.14, log-rank test).
Conclusion
Living donor kidney transplantation continues to be recommended as the preferred treatment option for IgAN patients, despite the potential risk of recurrence and graft loss.
Similar content being viewed by others
Introduction
IgA nephropathy (IgAN) is a common primary glomerulonephritis (GN) characterized by the deposition of IgA immunoglobulins within the glomerular mesangium. With an Incidence of 2.5 per 100,000 population per year it is the most common GN worldwide and a significant contributor to end-stage renal disease (ESRD) cases with dialysis dependency [1]. Living kidney transplantation (LDKT) offers patients a chance at a better quality of life and long-term survival. However, the risk of IgAN recurrence, potentially resulting in graft dysfunction and ultimately in graft loss, remains a concern for both patients and transplant physicians. In addition, the risk of disease recurrence should be carefully considered with LDKT, as the positive effects for the recipient may be accompanied by surgical risks and long-term consequences for the donor, such as arterial hypertension, fatigue, or renal failure [2]. IgAN recurrence refers to the reappearance of IgA deposits and associated glomerular pathology in the transplanted kidney. Recurrence rates vary from 10 to 50% in studies with clinically-driven biopsies, as performed in our center [3, 4]. Nevertheless, there is conflicting evidence regarding the outcomes of LDKT in patients with IgAN. Certain studies suggest a higher incidence of IgAN recurrence in grafts from living related donors while other studies found no higher incidence [3, 4]. In this context, a review of Australia-New Zealand registry data revealed a significantly increased frequency of IgAN recurrence in grafts with zero HLA mismatches from living donors [5]. Furthermore, with the development of newer immunosuppressive agents such as mycophenolate mofetil or calcineurin inhibitors as Cyclosporine A or Tacrolimus, important improvements have been made over the last two decades, but the ideal immunosuppressive regimen for recipients with IgAN is still not established [4, 6].
Moreover, there exist divergent conclusions in the literature about the outcome after IgAN recurrence. McDonald et al. reported that IgAN recurrence was a major cause of graft loss, ranking third in prevalence behind chronic allograft nephropathy and death with functioning graft [5]. Intriguingly, other authors concluded that IgAN recurrence did not substantially increase the risk of allograft loss, as the rate of graft loss due to recurrence was similar to that seen in recipients without IgAN recurrence and appears to be also in the same range as other forms of GN [7,8,9]. Since most of the data is derived from registry studies, there is a need for real-world evidence of IgAN recurrence after LDKT.
In the present study, we investigated the incidence of IgAN recurrence in the allograft after LDKT and the impact on the clinical outcome in a single high-volume transplant center performing clinically-driven graft biopsies as the diagnostic standard.
Methods
Patients
We retrospectively analyzed data from adult patients who underwent LDKT at Charité Hospital Berlin, Department for Urology, between 2011 and 2017. Our analysis focused on identifying patients with IgAN as the primary cause of their ESRD. We collected comprehensive patient data, including demographics, laboratory parameters and graft survival information, along with clinical and pathological data related to IgAN recurrence, from their medical records. However, no protocol biopsies were performed at our center, but biopsies were regularly taken if there was a suspicion of recurrence or rejection after LDKT. Accordingly, the diagnosis of IgAN recurrence in patients with clinical presentation of hematuria, proteinuria or renal failure was made by clincically-driven graft biopsy and subsequent histological examinations including electron microscopy to identify IgA deposits in the mesangium. Detailed descriptions of patient preparation and the surgical technique, including laparoscopic donor nephrectomy and LDKT, were reported in previous studies of our center [10]. The standard immunosupression regime of our LDKT cohort included corticosteroids, Mycophenolat mofetil, and Tacrolimus [11]. Cyclosporin A was used instead of Tacrolimus in cases of Tacrolimus intolerance or to avoid Tacrolimus-induced side effects such as diabetes mellitus or tremor [12]. Two hours before LDKT and on the fourth postoperative day, 20 mg basiliximab were administered intravenously. The following standard immunosuppression consisted of 2000 mg/day Mycophenolate mofetil, 500 mg/day Methylprednisolone, tapered to 4 mg after 4 weeks and Cyclosporine A with target trough levels of 150– 220 ng/mL in the first 3 months, tapered to 100–130 ng/mL thereafter, or Tacrolimus with target trough levels of 8–10 ng/mL for the first 3 months and 6–8 ng/mL thereafter [11]. Inosine Monophosphate Dehydrogenase (IMPDH) activity in erythrocytes was used as a surrogate for therapeutic drug monitoring for mycophenolate during follow-up [13].
Statistical analysis
Statistical analysis of the data was performed using Microsoft Office Excel 2022 and IBM SPSS Statistics 29 software. Student t-test was used for continuous variables and chi-square test for nominal variables. Kaplan–Meier analysis was done to compare graft survival data (log-rank test), p-values < 0.05 were considered statistically significant.
Results
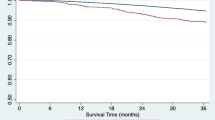
Our center conducted living donor kidney transplants (LDKT) on 193 adult patients between 2011 and 2017. In 32 cases (16.6%) IgAN was the underlying disease for ESRD. Patients with IgAN were significantly younger (average age 36.6 vs. 46.9, p < 0.001). However, other demographic factors, including BMI, waiting time for transplantation, preemptive transplantation rates, follow-up time, AB0-incompatible transplantation rates, and transplant outcomes such as serum creatinine levels, delayed graft function, rejection rates, graft loss and patient mortality, did not differ significantly between the two groups (Table 1). Kaplan–Meier analysis of death-censored graft survival showed a trend toward slightly decreased graft survival in the IgAN group compared to the rest of our cohort, but not in a statistically significant manner (p = 0.40), as shown in Fig. 1.
Kaplan–Meier analysis of death-censored graft survival in the IgA Nephropathy group (IgAN) (red) and the rest of the living donor kidney transplant cohort with divergent underlying disease for end stage renal failure (blue) showed a tendency for an inferior graft survival in the IgAN group, but not in a significant manner in the performed log-rank test (p = 0.40)
Furthermore, 5 out of the 32 patients (15.6%) in the IgAN group developed a IgAN recurrence. With 60%, a immunosuppression containing Cyclosporine A was significantly more common in the IgAN recurrence group (p < 0.001). Accordingly, with 92.6%, Tacrolimus was significantly more frequent in the immunosuppression regime of non-recurrent cases (p < 0.001). Additionally, the IgAN recurrence group exhibited higher early post-surgery serum creatinine levels at one week, one month and two years (p < 0.001, p = 0.027, and p = 0.008, respectively). However, there were no significant differences observed in terms of patient characteristics, including Age, HLA-mismatches, AB0-incompatibility and degree of kinship, dialysis type, time interval to the first biopsy, or transplant outcomes, such as rejection rates, death-censored graft loss, or death, as shown in Table 2. With a mean time interval days from LDKT to the first graft biopsy of 792.80 versus 1067.43 days, a tendency towards earlier biopses in the IgAN recurrence group was shown. Moreover, the number of biopsies performed was significantly higher in the IgAN recurrence group, with a mean of 2.8 per recepient compared to 0.62 in the non-recurrence group (p = 0.001) (Table 2). Symptoms for which a graft biopsy was performed and the diagnosis of a IgAN recurrence was made, ranged from microhematuria, proteinuria, kidney failure to hypertension.
As shown in Table 3, IgAN recurrences occurred at various time intervals, ranging from 13 to 123 months with a mean of 64 months (± 39.52) post-transplantation. In two cases de-novo donor specific antibodies were detected. In addition, the IgAN recurrence cases were evaluated for tonsillectomy before or after recurrence. However, none of the patients underwent tonsillectomy. Treatment strategies included the application of high-dose corticosteroids; Cyclophosphamide, an immunosuppressing alkylating agent; Budesonide, a glucocorticoide targeting intestinal immunity in the gut mucosa; and a therapy switch to Belatacept, a selective T-cell inhibitor which may reduce a humoral graft immunization [14, 15]. Additionally, in one case plasmapheresis was performed and in another case, which showed fulminant functional deterioration of the graft without clinical prospect of recovery, no further therapy for IgAN recurrence was executed. Moreover, we observed two graft losses, one at 16 and one at 123 months after transplantation, due to IgAN recurrence, accounting for 40% in that group and 6.25% of all recipients with IgAN. Kaplan–Meier analysis revealed no significant difference in graft survival when IgAN recurrence occurred, compared with patients with IgAN as a primary disease without recurrence, but a trend toward less favorable outcome for the recurrence group was shown (p = 0.14 (log-rank test); Fig. 2). Blood levels of Cyclosporin A, trough levels of Tacrolimus and the IMPDH activity during the clinical course are depicted in Fig. 3.
Kaplan–Meier analysis of death-censored graft survival in the IgA Nephropathy group (IgAN) with recurrence (red) versus IgAN as a primary disease without recurrence (blue) showed a tendency for an inferior graft survival in the IgAN group, but not in a significant manner in the performed log-rank test (p = 0.141)
Discussion
Kidney transplantation, particularly LDKT, is a valuable treatment option for ESRD patients, providing improved quality of life and long-term survival without need for dialysis. However, the risk of IgAN recurrence in the graft raises important considerations, as the favorable impact for the recipient may also be associated with risks for the donor. In the present real-world analysis, we observed an IgAN recurrence rate of 15.6% among patients with IgAN as the primary cause of their ESRD. This rate is within the lower range reported in previous studies, which varies from 10 to 50% in clinically-driven biopsies as performed in our center [3, 4]. In this regard, it is a controversial issue which characteristics of the recipient may represent the risk factors for an increased IgAN recurrence. In the present study, in which patients with IgAN as primary disease were significantly younger at the time of transplantation, also a tendency towards a younger age in the IgAN recurrence group compared to the non-recurrent group was shown, but not to a significant extent. This is in line with several studies identified transplantation at a younger age as a risk factor for IgAN recurrence [9, 16,17,18]. Furthermore, the question of whether living-related donors, such as family members, pose a higher risk of IgAN recurrence compared to unrelated donors has garnered considerable attention [3, 19, 20]. McDonald et al. demonstrated a significantly increased rate of IgAN recurrence in grafts with zero HLA-mismatches from living donors [5]. However, no significant differences in HLA-mismatches, first- and second-degree kinship or AB0-compatibility were found between the two groups. Although the small number of cases in this study limits the validity of a risk factor analysis, our results did not confirm an increased risk of recurrence for living related donors or an increased risk of AB0-incompatibility in the IgAN cases of our cohort. Moreover, the ideal immunosuppressive regimen for recipients with IgAN is still not established [4, 6]. Maintenance therapy typically consists of mycophenolate mofetil, low-dose methylprednisolone, and a calcineurin inhibitor such as Cyclosporine A or Tacrolimus. The results of the present study are consistent with those of Lionakis et al., who showed significantly lower rates of IgAN recurrence and lower serum creatinine levels under immunosuppression with a regimen containing Tacrolimus compared to one with Cyclosporine A [6]. In contrast, Ortiz et al. reported a significantly lower recurrence rate under immunosuppression containing Cyclosporine A in recipients who underwent a protocol biopsy within the first two years. However, it should be emphasized that in our study clinically-driven biopsies were performed and the majority of patients in the study by Ortiz et al. showed no clinical manifestations of a IgAN recurrence at the time of biopsy [21]. Together with the fact that recurrent IgAN rarely manifests clinically before three years post transplantation, it seems uncertain whether recurrence diagnosed by protocol biopsy as performed by Ortiz et al. would become clinically apparent [3]. Accordingly, the question of the most adequate immunosuppression regimen with regard to IgAN recurrence has not been conclusively clarified and prospective studies are required. However, in the present study significantly less biopsies were performed in the clinically non-recurrent group compared to the IgAN recurrent group, which also means a reduced risk of peri-interventional complications for grafts with a good function and an uncertain benefit from a possible protocol biopsy [2, 3].
Furthermore, our findings revealed higher early post-surgery serum creatinine levels in patients with IgAN recurrence at one week, one month and two years. In this regard, it is questionable whether early graft function could possibly be an indicator for recurrent IgAN in the subsequent long-term course. Due to the relatively small sample size of the present study, this question cannot be answered conclusively and should be evaluated in further studies. However, it is important to note that these differences in serum creatinine levels did not persist in the long term, as demonstrated by the comparable levels at subsequent time points. The underlying reason may be a higher incidence of delayed graft function, acute tubulus necrosis, toxicity of the used calcineurin inhibitors or a history of rejection, which occurred in the IgAN recurrence group at a relatively early stage after LDKT as described in Table 3.
While IgAN recurrence presents a clinical challenge, the question of graft survival after recurrence is a further highly debated. Some studies have reported an increased risk of graft loss associated with recurrence, while others have not observed such an association [5, 8, 22]. The findings of the present study did not observe statistically significant differences in death-censored graft loss rates, but a tendency toward inferior graft survival in the IgAN and IgAN recurrence group could be shown. Our results are thus in contrast to the previously mentioned findings of Briganti et al. indicating no higher graft loss rates after IgAN recurrence [8]. In a previous study with the LDKT patient cohort from our center, Mang et al. compared the incidence and graft loss rates of patients with focal segmental glomerulosclerosis (FSGS) as another form of GN, and were able to show recurrence rates of FSGS of 14% and graft losses of 9% [23]. The incidence rate of recurrent disease (15.6%) and graft loss rate in recipients with IgAN (15.8%) were slightly higher in the present study, but this could also be due to a longer follow-up period of 102 months compared to only 46.7 months in the study conducted by Mang et al. In addition, it should be mentioned that in the present study, graft loss due to IgAN recurrence occurred in only two of 32 grafts (6.25%) with IgAN, and therefore, specific graft survival after IgAN recurrence appeared to be superior to FSGS recurrence [9, 23].
Our findings highlight the need for examination of graft outcomes in patients with IgAN recurrence, as this may have long-term implications for graft survival. However, in the absence of significant differences, the results of this study do not contradict the evaluation of patients with IgAN as LDKT. Kidney transplantation may still be the best treatment option for patients with ESRD with IgAN as primary disease.
However, it is important to acknowledge the limitations of our retrospective analysis, including the absence of protocol biopsies. Furthermore, as mentioned above, the small number of cases must be taken into account, which limits the informative value of the risk factor analysis.
Conclusion
Our study identified the use of an immunosuppression regimen containing Cyclosporine A instead of Tacrolimus as a potential risk factor for IgAN recurrence, but despite a younger age at transplantation in the IgAN group compared to the rest of the cohort and a trend toward slightly inferior graft survival in the IgAN and IgAN recurrence group, no further significant differences were observed. Therefore, living donor kidney transplantation continues to be recommended as the preferred treatment option for patients with IgAN. Nevertheless, both the donor and the recipient should be carefully medically advised about the risks of a possible IgAN recurrence.
Availability of data and materials
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
Abbreviations
- BMI:
-
Body-mass-index
- ESRD:
-
End-stage renal disease
- FSGS:
-
Focal segmental glomerulosclerosis
- GN:
-
Glomerulonephritis
- HLA:
-
Humane leucocyte antigen
- IgAN:
-
IgA nephropathy
- LDKT:
-
Living kidney transplantation
References
Storrar J, Chinnadurai R, Sinha S, Kalra PA. The epidemiology and evolution of IgA nephropathy over two decades: a single centre experience. PLoS ONE. 2022;17:1–13.
Rush D, Arlen D, Boucher A, Busque S, Cockfield SM, Girardin C, et al. Lack of benefit of early protocol biopsies in renal transplant patients receiving TAC and MMF: a randomized study. Am J Transplant. 2007;7:2538–45.
Moroni G, Belingheri M, Frontini G, Tamborini F, Messa P. Immunoglobulin A nephropathy. Recurrence after renal transplantation. Front Immunol. 2019;10:1–8.
Lionaki S, Panagiotellis K, Melexopoulou C, Boletis JN. The clinical course of IgA nephropathy after kidney transplantation and its management. Transpl Rev. 2017;31:106–14.
McDonald SP, Russ GR. Recurrence of IgA nephropathy among renal allograft recipients from living donors is greater among those with zero HLA mismatches. Transplantation. 2006;82:759–62.
Lionaki S, Makropoulos I, Panagiotellis K, Vlachopanos G, Gavalas I, Marinaki S, et al. Kidney transplantation outcomes in patients with IgA nephropathy and other glomerular and non-glomerular primary diseases in the new era of immunosuppression. PLoS ONE. 2021;16:1–15.
Kawabe M, Yamamoto I. Current status and perspectives on recurrent IgA nephropathy after kidney transplantation. Nephron. 2023;147:9–13.
Briganti EM, Russ GR, McNeil JJ, Atkins RC, Chadban SJ. Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med. 2002;347:103–9.
Allen PJ, Chadban SJ, Craig JC, Lim WH, Allen RDM, Clayton PA, et al. Recurrent glomerulonephritis after kidney transplantation: risk factors and allograft outcomes. Kidney Int. 2017;92:461–9.
Mang J, Hennig L, Biernath N, Liefeldt L, Bichmann A, Ralla B, et al. Is a retroaortic vein a risk factor in laparoscopic living donor nephrectomy. Urol Int. 2020;104:641–5.
Liefeldt L, Brakemeier S, Glander P, Waiser J, Lachmann N, Schönemann C, et al. Donor-specific HLA antibodies in a cohort comparing everolimus with cyclosporine after kidney transplantation. Am J Transpl. 2012;12:1192–8.
Penninga L, Penninga EI, Møller CH, Iversen M, Steinbrüchel DA, Gluud C. Tacrolimus versus cyclosporin as primary immunosuppression for lung transplant recipients. Cochrane Database Syst Rev. 2013;2013.
Mino Y, Naito T, Otsuka A, Ozono S, Kagawa Y, Kawakami J. Inosine monophosphate dehydrogenase activity depends on plasma concentrations of mycophenolic acid and its glucuronides in kidney transplant recipients. Clin Chim Acta. 2009;409:56–61.
Liao J, Zhou Y, Xu X, Huang K, Chen P, Wu Y, et al. Current knowledge of targeted-release budesonide in immunoglobulin a nephropathy: a comprehensive review. Front Immunol. 2023;13:1–11.
Lombardi Y, François H. Belatacept in kidney transplantation: What are the true benefits? A systematic review. Front Med. 2022;9:942665.
Jäger C, Stampf S, Molyneux K, Barratt J, Golshayan D, Hadaya K, et al. Recurrence of IgA nephropathy after kidney transplantation: experience from the Swiss transplant cohort study. BMC Nephrol. 2022;23:1–11.
Han SS, Huh W, Park SK, Ahn C, Han JS, Kim S, et al. Impact of recurrent disease and chronic allograft nephropathy on the long-term allograft outcome in patients with IgA nephropathy. Transpl Int. 2010;23:169–75.
Cazorla-López JM, Wu J, Villanego-Fernández F, Naranjo-Muñoz J, Vigara-Sánchez LA, García-García-Doncel A, et al. IgA nephropathy after renal transplant: recurrences and de novo cases. Transpl Proc. 2020;52:515–8.
Andresdottir MB, Haasnoot GW, Doxiadis IIN, Persijn GG, Claas FHJ. Exclusive characteristics of graft survival and risk factors in recipients with immunoglobulin A nephropathy: a retrospective analysis of registry data. Transplantation. 2005;80:1012–8.
Okumi M, Okada D, Unagami K, Kakuta Y, Iizuka J, Takagi T, et al. Higher immunoglobulin A nephropathy recurrence in related-donor kidney transplants: the Japan Academic Consortium of Kidney Transplantation study. Int J Urol. 2019;26:903–9.
Ortiz F, Gelpi R, Koskinen P, Manonelles A, Räisänen-Sokolowski A, Carrera M, et al. IgA nephropathy recurs early in the graft when assessed by protocol biopsy. Nephrol Dial Transpl. 2012;27:2553–8.
Infante B, Rossini M, Di Lorenzo A, Coviello N, Giuseppe C, Gesualdo L, et al. Recurrence of immunoglobulin A nephropathy after kidney transplantation: a narrative review of the incidence, risk factors, pathophysiology and management of immunosuppressive therapy. Clin Kidney J. 2020;13:758–67.
Mang J, Hennig L, Liefeldt L, Duerr M, Lehner LJ, Bichmann A, et al. Focal segmental glomerulosclerosis and recurrence in living donor recipients. Res Rep Urol. 2021;13:495–9.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
JS contributed to conceptualization, data curation, formal analysis, writing the original draft, methodology and project administration. LL was involved in conceptualization, and editing the manuscript. EB, JDH, ML, and BR participated in editing the manuscript. ST contributed in data curation and editing the manuscript. FF contributed in conceptualization, methodology and supervision. RP contributed to methodology, conceptualization, project administration, supervision, and editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been conducted according to the Declaration of Helsinki; all organs were donated voluntarily with written informed consent, and this was conducted in accordance with the Declaration of Istanbul. The article is exempt from the local Ethical Committee approval (Institutional Review Board of Charité Hospital Berlin) because of this study’s retrospective, noninterventional design and because patient data confidentiality and privacy were always maintained.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Schmidt, J., Liefeldt, L., Baysal, E. et al. IgA nephropathy recurrence after living donor kidney transplantation: a retrospective analysis of postoperative outcomes at a single high-volume transplant center. Ren Replace Ther 10, 39 (2024). https://doi.org/10.1186/s41100-024-00558-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-024-00558-8