Abstract
Background
Angiotensin-converting enzyme inhibitors (ACE-I) and angiotensin receptor blockers (ARB) exert a renoprotective effect on patients with chronic kidney disease (CKD). Despite their benefit, one of their side effects is hyperkalemia, and it is one of the most common reasons for discontinuation of these drugs. Hyperchloremic metabolic acidosis is a known risk factor for hyperkalemia in patients with CKD. However, whether it is a risk factor for hyperkalemia after initiating ACE-I or ARB remains unclear.
Methods
In a previous study, serum sodium minus chloride level ([Na+) − (Cl−]) was identified as useful for diagnosing metabolic acidosis. To estimate the baseline acid–base status, we determined for the cutoff value of [Na+] − [Cl−] that correlates with [HCO3−] below 24 mEq/L in patients with CKD. We then investigated whether this cutoff value was associated with hyperkalemia (serum potassium level ≥ 5.0 mEq/L) after initiating ACE-I or ARB in patients with CKD.
Results
In the investigation of the cutoff value of [Na+] − [Cl−], 612 patients were examined, and [Na+] − [Cl−] showed a good correlation with [HCO3−] (r = 0.67, p < 0.001). Based on receiver operating curve analysis, we derived a cut-off value of [Na+] − [Cl−] below 33.5 mEq/L. Using this cut off value, the sensitivity and specificity of [Na+] − [Cl−] for metabolic acidosis were 75.2% and 75.0%, respectively. To explore whether metabolic acidosis is associated with hyperkalemia after initiating ACE-I or ARB, we examined 1143 patients with CKD. Among this cohort, 403 (35.3%) patients had [Na+] − [Cl−] < 33.5 mEq/L at baseline, and the incidence of hyperkalemia was significantly higher in univariate analysis (9.2% versus 4.2%, p = 0.03). However, in multivariate analysis, [Na+] − [Cl−] < 33.5 mEq/L was not associated with hyperkalemia (odds ratio 1.13; 95% confidence interval 0.65–1.95).
Conclusions
Hyperchloremic metabolic acidosis was not associated with hyperkalemia after initiation of ACE-I or ARB in patients with CKD.
Similar content being viewed by others
Background
Angiotensin-converting enzyme inhibitors (ACE-I) and angiotensin receptor blockers (ARB) are well known for their ability to reduce cardiovascular events and exert renoprotective effects in patients with chronic kidney disease (CKD) [1]. However, hyperkalemia defined as serum potassium level ([K+]) ≥ 5.0 mEq/L is one of the major side effects associated with these drugs. Since hyperkalemia adversely affects long term prognosis in patients with CKD [2], preventing the development of hyperkalemia after initiation of ACE-I or ARB is crucial in CKD management. Few risk factors for developing hyperkalemia following initiation of ACE-I or ARB include patient age, baseline estimated glomerular filtration rate (eGFR), doses of ACE-I or ARB, and diabetes [3, 4]. Although hyperchloremic metabolic acidosis is one of the risk factors for hyperkalemia in patients with CKD, it is unclear whether this is also a risk factor for hyperkalemia after initiation of ACE-I or ARB in patients with CKD.
Blood gas analysis is required for diagnosing hyperchloremic metabolic acidosis. Another method to assess serum bicarbonate level ([HCO3−]) is total CO2 (tCO2) measurement. However, because measuring tCO2 requires specialized testing equipment, it is thought that it is not as popular in Japan as it is in the USA [5]. Since a Kidney Disease: Improving Global Outcomes and Japanese CKD guideline recommend measuring [HCO3−] in the management of patients with CKD [6, 7], it is likely that nephrologists evaluate [HCO3−] in daily practice even in Japan. On the other hand, ACE-I and ARB are also used in patients with heart failure and hypertension, so these drugs are prescribed in medical departments other than nephrology. Therefore, such medical departments may not routinely assess [HCO3−]. Interestingly, serum sodium level ([Na+]) minus serum chloride level ([Cl−]) enables prediction of metabolic acidosis [8]. Therefore, if measurement of [Na+] minus [Cl−] ([Na+] − [Cl−]) could predict the development of hyperkalemia after initiation of ACE-I or ARB, it would subsequently be beneficial in CKD management.
In this study, to assess the association between baseline acid–base status and hyperkalemia after initiation of these drugs, we initially investigated the cutoff value of [Na+] − [Cl−] that correlates with [HCO3−] below 24 mEq/L in patients with CKD. Then, using this cutoff value, we examined whether it was useful for predicting hyperkalemia after the initiation of ACE-I or ARB in patients with CKD.
Methods
Study design and population
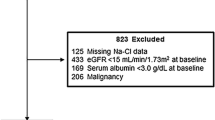
This was a single center retrospective case control study. Patients with CKD not on dialysis on an outpatient or inpatient basis, who were newly initiated on ACE-I or ARB from January 2011 to December 2021 at St. Luke’s International Hospital in Japan, were included in the study. Since we defined hyperkalemia as [K+] ≥ 5.0 mEq/L, we only included the patients with CKD whose [K+] were below 5.0 mEq/L at the initiation of ACE-I or ARB. CKD was defined as eGFR less than 60 mL/min/1.73 m2 at or before initiation of ACE-I or ARB. Serum creatinine, age, and sex were used for determining eGFR [9]. Exclusion criteria were: patients who had medical record of taking ACE-I or ARB before the initiation of ACE-I or ARB; patients who had medical record of dialysis before the initiation of ACE-I or ARB; patients who had baseline eGFR < 8 mL/min/1.73 m2; and patients whose baseline [Na+], [K+], [Cl−], and serum albumin level were not measured on the same day. We also excluded patients who were prescribed ACE-I or ARB within 7 days of hospitalization and patients ≥ 75 years old with an eGFR < 30 mL/min/1.73 m2, as the Japanese CKD guideline does not recommend initiating ACE-I or ARB to this population. For the investigation of whether [Na+] − [Cl−] correlates with [HCO3−] in patients with CKD, we only included the patients whose [HCO3−] were measured at the initiation of ACE-I or ARB.
Data sources
All the laboratory data were extracted from venous blood samples and were measured at the St.Luke’s International Hospital specimen laboratory. Serum albumin levels were measured by a bromocresol purple method. Serum creatine levels were measured by an enzyme method. [HCO3−] was measured by tCO2 detection using a dry chemistry method. [Na+], [Cl–], and [K+] levels were measured by electrode method.
Cutoff value for serum sodium minus chloride level
Linear regression analysis was performed to evaluate the relationship between [Na+] − [Cl−] and [HCO3−]. Furthermore, receiver operating curve (ROC) analysis and the Yoden index were established to evaluate diagnostic sensitivity, specificity, and the cutoff value for [Na+] − [Cl−] to predict metabolic acidosis ([HCO3−] < 24 mEq/L).
Association of serum sodium minus chloride level at baseline and hyperkalemia after initiation
Using the cutoff value of [Na+] − [Cl−] that correlates with [HCO3−] < 24 mEq/L, which was derived from our cohort, we divided all eligible patients into two groups: those with [Na+] − [Cl−] below the cutoff value and those with [Na+] − [Cl−] at or above the cutoff value. The outcome was [K+] ≥ 5.0 mEq/L at the first blood test after the initiation of ACE-I or ARB. All blood tests were performed within 1 year after the initiation of ACE-I or ARB.
Statistical analyses
Univariate analyses were conducted using the χ2 test and Fisher’s exact test. A multivariate analysis was performed by conducting a logistic regression analysis adjusted in accordance with the baseline data; age, sex, diabetes mellitus, eGFR, [K+], complication of hypoalbuminemia, and concomitant use of drugs that can increase [K+] or decrease [K+]. Hypoalbuminemia was defined as serum albumin level < 4 g/dL. These covariates were selected based on prior studies and clinical plausibility [3, 4]. Mineralocorticoid receptor antagonists and tolvaptan were defined as the drugs that increase [K+] and gastrointestinal cation exchangers, loop diuretics, thiazide diuretics, acetazolamide, and bicarbonate supplementation agents were defined as the drugs that decrease [K+]. A significant difference was defined as p-value < 0.05. All the analyses were conducted using RStudio version 4.2.0. (RStudio Team (2020). RStudio: Integrated Development for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com/), and the study was approved by the ethical review board of St. Luke’s International Hospital.
Results
During the observation period, 4385 patients with CKD were initiated on either ACE-I or ARB. After applying the exclusion criteria, 612 patients had their [HCO3−] measured at baseline and were included in the investigation of whether [Na+] − [Cl−] correlates with [HCO3−]. A significant relationship was detected between [Na+] − [Cl−] and [HCO3−] (r = 0.67, p < 0.001) through regression analysis (Fig. 1A). ROC analysis indicated that [Na+] − [Cl−] of 33.5 mEq/L had the highest Yoden indices (sensitivity + specificity − 1) to predict metabolic acidosis ([HCO3−] < 24 mEq/L) (Fig. 1B). Using this cutoff value, the sensitivity and specificity of [Na+] − [Cl−] for metabolic acidosis were 75.2% and 75.0%, respectively, while the area under the ROC curve was 0.81 (95% confidential interval 0.78–0.85).
Relationship between [HCO3−] and [Na+] − [Cl−] at the initiation of ACE-I or ARB. A. Scatter plot of [HCO3−] and [Na+] − [Cl−]. From the linear regression analysis, [Na+] − [Cl−] was significantly related with [HCO3−] (r = 0.67 and p < 0.001). B. Receiver operating curve analysis for [Na+] − [Cl−] for predicting metabolic acidosis ([HCO3−] < 24 mEq/L). From this analysis, 33.5 mEq/L had the highest Yoden indices (sensitivity + specificity − 1). Using this cutoff value, the sensitivity and specificity of [Na+] − [Cl−] for metabolic acidosis was 75.2% and 75.0%, respectively, while the area under the ROC curve was 0.81 (95% confidential interval 0.78–0.85). [HCO3−], serum bicarbonate level; [Na+], serum sodium level; [Cl−], serum chloride level; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker
By using the cutoff value of [Na+] − [Cl−], we then investigated whether this cutoff value was associated with hyperkalemia after initiation of ACE-I or ARB. In this cohort, 1143 patients were enrolled, among which there were 960 outpatients and 183 inpatients. The distribution of [Na+] − [Cl−] at the initiation of ACE-I or ARB in 1143 patients is depicted in Fig. 2. At the initiation of ACE-I or ARB, [Na+] − [Cl−] was almost normally distributed. Using the cutoff value of [Na+] − [Cl−] at 33.5 mEq/L, the 1143 patients were divided into two groups: those with [Na+] − [Cl−] < 33.5 mEq/L (Na − Cl < 33.5 group) and those with [Na+] − [Cl−] ≥ 33.5 mEq/L (Na − Cl ≥ 33.5 group). The Na − Cl < 33.5 group consisted of 403 patients, while the Na − Cl ≥ 33.5 group consisted of 740 patients.
Distribution of serum sodium minus chloride level at the initiation of ACE-I or ARB. At the initiation of ACE-I or ARB, [Na+] − [Cl−] was almost normally distributed. ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; [Na+], serum sodium level; [Cl−], serum chloride level
Table 1 demonstrates the clinical characteristics of the patients at initiation of ACE-I or ARB for each group. Baseline eGFR was observed to be significantly lower, baseline [K+] was significantly higher, hypoalbuminemia was significantly more prevalent, and the incidence of hyperkalemia after the initiation of ACE-I or ARB was significantly higher in the Na − Cl < 33.5 group as compared with the Na − Cl ≥ 33.5 group.
Table 2 demonstrates the results of the univariate analysis for association of hyperkalemia after the initiation of ACE-I or ARB with baseline clinical characteristics. According to the univariate analysis, baseline [Na+] − [Cl−] < 33.5 mEq/L, baseline eGFR, baseline [K+], and concomitant use of drugs which decrease [K+] were observed to be significantly associated with hyperkalemia. However, multivariate analysis indicated that only baseline eGFR and baseline [K+] were significantly associated with hyperkalemia after the initiation of ACE-I or ARB (Table 3).
Discussion
In this study, we investigated the association between [Na+] − [Cl−] and [HCO3−] and derived the cut-off value of [Na+] − [Cl−] < 33.5 mEq/L as a surrogate for [HCO3−] < 24 mEq/L. Then, we explored whether [Na+] − [Cl−] < 33.5 mEq/L at the initiation of ACE-I or ARB was associated with the development of hyperkalemia in patients with CKD. A significant association between [Na+] − [Cl−] at initiation of ACE-I or ARB and hyperkalemia was observed in univariate analysis. However, no such significance was observed in multivariate analysis adjusted by age, sex, diabetes mellitus, eGFR, [K+], complication of hypoalbuminemia, and concomitant use of drugs that increase [K+] or decrease [K+].
In this study, we identified a significant relationship between [Na+] − [Cl−] and [HCO3−]. A study that examined whether [Na+] − [Cl−] could predict metabolic acidosis included 341 patients in the intensive care unit (ICU) and defined metabolic acidosis based on the strong ion difference (SID) [8]. According to this study, metabolic acidosis was defined as SID < 42.7 mEq/L using data from healthy volunteers. Our study revealed that [Na+] − [Cl−] is useful not only for SID-defined metabolic acidosis,but also for [HCO3−]-defined metabolic acidosis. Furthermore, to the best of our knowledge, this is the first study to demonstrate that [Na+] − [Cl−] is useful for assessing metabolic acidosis in patients with CKD.
We adopted serum [Na+] − [Cl−] as a possible predictive value for developing hyperkalemia because we anticipated it would correlate with hyperchloremic metabolic acidosis. We specifically focused on hyperchloremic metabolic acidosis, as opposed to high anion gap metabolic acidosis, because we expected that the effect of serum [HCO3−] on [K+] was primarily related to the degree of hyperchloremic metabolic acidosis. From a physiological standpoint, the serum anion gap can be calculated using the equation:
We then rearranged this equation as follows:
Since hyperchloremic metabolic acidosis does not alter the serum anion gap, [Na+] − [Cl−] is lower in patients with hyperchloremic metabolic acidosis. In contrast, in patients diagnosed with high anion gap metabolic acidosis, an increase in serum anion gap would exactly match the decrease in [HCO3−] caused by buffering of hydrogen ions, resulting in [Na+] − [Cl−] remaining constant in these patients. Therefore, a decrease in [Na+] − [Cl−] suggests hyperchloremic metabolic acidosis but not high anion gap acidosis. Additionally, although hyperchloremic metabolic acidosis is known as a risk factor for hyperkalemia, high anion gap metabolic acidosis is not considered a risk factor for hyperkalemia according to previous studies [10,11,12]. The explanation for this difference is that organic acids, unlike mineral acids, can freely penetrate cell membranes and cause less efflux of potassium from the intracellular compartment [10]. Furthermore, organic acids are actively absorbed into the intracellular compartment via transporters on the cell membrane, causing greater decrease in cell pH [11]. This leads to the influx of Na+ and efflux of H+ via the Na+–H+ exchanger to mitigate the decrease in cell pH, subsequently enhancing the Na+-K+-ATPase, which causes an influx of potassium from the extracellular compartment. From this perspective, the association between acid–base disorders and hyperkalemia in patients with CKD may be assessed solely by the degree of hyperchloremic metabolic acidosis, which can be predicted by [Na+] − [Cl−]. Although high anion gap metabolic acidosis may occur in conjunction with hyperchloremic metabolic acidosis as the CKD stages progress, the accumulation of organic acid would theoretically not have an effect on [K+].
Considering the normal value of 24 mEq/L for [HCO3−] and 12 mEq/L for serum anion gap, [Na+] − [Cl−] < 36 mEq/L would theoretically be correlated with [HCO3−] < 24 mEq/L. However, from the ROC analysis of our cohort, [Na+] − [Cl−] of 33.5 mEq/L had the highest Yoden indices (sensitivity + specificity − 1) for predicting metabolic acidosis. Interestingly, the best cutoff value according to the previous study of [Na+] − [Cl−] for diagnosing SID-defined metabolic acidosis was 34 mEq/L [8]. As mentioned above, we transformed the equation of serum anion gap as follows:
[Na+] (mEq/L) − [Cl−] (mEq/L) = serum anion gap (mEq/L) + [HCO3−] (mEq/L).
Additionally, serum anion gap is defined as follows:
Serum anion gap (mEq/L) = all unmeasured anions (mEq/L) − all unmeasured cations (mEq/L).
Since the major unmeasured anion is albumin, patients with hypoalbuminemia have a lower anion gap. In general, for each 1 g/dL decrease in serum albumin concentration below 4 g/dL, the serum anion gap decreases by approximately 2.5 mEq/L [13, 14]. Therefore, hypoalbuminemia was suggested as the reason why the ROC cutoff value for [Na+] − [Cl−] was lower than the physiologically determined value. Almost half of patients with CKD enrolled in our study had hypoalbuminemia (511 of 1143 patients). Furthermore, in the previous study according to which the best cutoff value of [Na+] − [Cl−] to diagnose SID defined metabolic acidosis was 34 mEq/L, the median serum albumin of patients in the ICU was 2.4 g/dL [8].
In this study, at the initiation of ACE-I or ARB, [Na+] − [Cl−] was not associated with the development of hyperkalemia. We consider that the patients who were not hypoaldosteronism at the initiation of ACE-I or ARB may be more susceptible to these drugs. As presented in Table 1, patients belonging to the Na − Cl < 33.5 group had significantly impaired renal function and higher [K+] compared with the Na − Cl ≥ 33.5 group. Previous study indicated that hypoaldosteronism was observed in most patients with hyperkalemia and impaired renal function [15]. Therefore, the Na − Cl < 33.5 group could be hypoaldosteronism. ACE-I and ARB are known to inhibit the excretion of hydrogen and potassium at the collecting duct via the suppression of renin–angiotensin–aldosterone system, thereby inducing hyperchloremic acidosis [16]. In our view, patients without acidosis at the time of initiation of ACE-I or ARB were those who had higher eGFR and lower [K+] and were suspected to have normal activity level of renin–angiotensin–aldosterone system. Thus, they were more susceptible to developing metabolic acidosis after the initiation of ACE-I or ARB. Therefore, the acid–base status at the initiation of ACE-I or ARB may not be associated with the development of hyperkalemia.
There is a normal range for [HCO3−], which has been reported to be between 22 and 28 mEq/L [17, 18]. From this perspective, we conducted another analysis in which we adopted 22 mEq/L as the normal value for [HCO3−] instead of 24 mEq/L. Through this analysis, [Na+] − [Cl−] of 32.5 mEq/L had the highest Yoden indices for predicting metabolic acidosis (Additional file 1: Figure S1). Additional file 1: Table S1 presents a comparison of patient characteristics between the two groups with [Na+] − [Cl−] < 32.5 mEq/L and ≥ 32.5 mEq/L at the initiation of ACE-I or ARB. Additional file 1: Table S2 presents the results of the multivariate analysis for association of hyperkalemia after the initiation of ACE-I or ARB with baseline clinical characteristics. This multivariate analysis was performed by changing the cutoff value for [Na+] − [Cl−] to 32.5 mEq/L and leaving other covariates unchanged. Interestingly, similar to the results of multivariate analysis using a cutoff value predicting [HCO3−] < 24 mEq/L (Table 3), baseline eGFR and baseline serum potassium level were associated with hyperkalemia after the initiation of ACE-I or ARB.
There are several limitations in this study. First, our research was a single-center retrospective study conducted at a regional core hospital. This may introduce selection bias of the patient population. Second, although the doses of ACE-I or ARB were identified as a risk factor for hyperkalemia in a previous study [3], we could not assess the dosages of these drugs. Third, the acid–base status was not evaluated through blood gas analysis. However, to the best of our knowledge, this is the first study to demonstrate that [Na+] − [Cl−] is useful for assessing metabolic acidosis in patients with CKD and to investigate the relationship between [Na+] − [Cl−] at the initiation of ACE-I or ARB and the new onset of hyperkalemia in patients with CKD.
Conclusions
[Na+] − [Cl−] showed a strong correlation with [HCO3−] and may serve as a reliable surrogate for [HCO3−] in patients with CKD. However, the baseline acid–base status was not found to be associated with hyperkalemia after the initiation of ACE-I or ARB.
Availability of data and materials
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
Abbreviations
- ACE-I:
-
Angiotensin-converting enzyme inhibitor
- ARB:
-
Angiotensin receptor blocker
- CKD:
-
Chronic kidney disease
- eGFR:
-
Estimated glomerular filtration rate
- ICU:
-
Intensive care unit
- ROC:
-
Receiver operating curve
- SID:
-
Strong ion difference
- RAAS:
-
Renin–angiotensin–aldosterone system
- [Na+]:
-
Serum sodium level
- [Cl−]:
-
Serum chloride level
- [HCO3 −]:
-
Serum bicarbonate level
- [K+]:
-
Serum potassium level
- tCO2 :
-
total CO2
- Na − Cl ≥ 33.5 group:
-
[Na+] − [Cl−] ≥ 33.5 mEq/L
- Na − Cl < 33.5 group:
-
[Na+] − [Cl−] < 33.5 mEq/L
References
Xie X, Liu Y, Perkovic V, Li X, Ninomiya T, Hou W, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67:728–41.
Collins AJ, Pitt B, Reaven N, Funk S, McGaughey K, Wilson D, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46:213–21.
Johnson ES, Weinstein JR, Thorp ML, Platt RW, Petrik AF, Yang X, et al. Predicting the risk of hyperkalemia in patients with chronic kidney disease starting lisinopril. Pharmacoepidemiol Drug Saf. 2010;19:266–72.
Riccio E, Capuano I, Buonanno P, Andreucci M, Provenzano M, Amicone M, et al. RAAS inhibitor prescription and hyperkalemia event in patients with chronic kidney disease: a single-center retrospective study. Front Cardiovasc Med. 2022;9:824095.
Hideaki S. [Electrolyte/acid-base imbalance encountered in daily clinical practice—common pathological conditions and pathological conditions that should not be overlooked—. Topics:VIII. The usefulness of Na-CI for the differentiation of acid base disorders in general practice and precau- tions for its use]. Nihon Naika Gakkai Zasshi. 2022; 111: 957–64.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO. clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2012;2013(3):1–150.
Japanese Society of Nephrology. Essential points from evidence-based clinical practice guidelines for chronic kidney disease 2018. Clin Exp Nephrol. 2019;23:1–15.
Mallat J, Barrailler S, Lemyze M, Pepy F, Gasan G, Tronchon L, et al. Use of sodium-chloride difference and corrected anion gap as surrogates of Stewart variables in critically ill patients. PLoS ONE. 2013;8:e56635.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Perez GO, Oster JR, Vaamonde CA. Serum potassium concentration in acidemic states. Nephron. 1981;27:233–43.
Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol. 2015;10:1050–60.
Dépret F, Peacock WF, Liu KD, Rafique Z, Rossignol P, Legrand M. Management of hyperkalemia in the acutely ill patient. Ann Intensive Care. 2019;9:32.
Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol. 2007;2:162–74.
Feldman M, Soni N, Dickson B. Influence of hypoalbuminemia or hyperalbuminemia on the serum anion gap. J Lab Clin Med. 2005;146:317–20.
Schambelan M, Sebastian A, Biglieri EG. Prevalence, pathogenesis, and functional significance of aldosterone deficiency in hyperkalemic patients with chronic renal insufficiency. Kidney Int. 1980;17:89–101.
Karet FE. Mechanisms in hyperkalemic renal tubular acidosis. J Am Soc Nephrol. 2009;20:251–4.
Raphael KL. Metabolic acidosis in CKD: core curriculum 2019. Am J Kidney Dis. 2019;74:263–75.
Tucker AM, Johnson TN. Acid-base disorders: a primer for clinicians. Nutr Clin Pract. 2022;37:980–9.
Acknowledgements
Not applicable.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Research idea and study design: H.M. and T.F.; data acquisition: H.M. and T.F.; data analysis/interpretation: H.M. and T.F.; statistical analysis: H.M. and T.F.; supervision or mentorship: T.F., K.S., N.K., K.K., Y.I., M.N., F.T., and M.N. Each author contributed important intellectual content during manuscript drafting and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study protocol was reviewed and approved by the ethical review board of St. Luke’s International Hospital. Because of the anonymity of the patients studied and the nonintrusive nature of the research, the requirement for written consent was waived via the opt-out method on the hospital’s information website.
Consent for publication
Because of the anonymity of the patients studied and the nonintrusive nature of the research, the requirement for written consent was waived via the opt-out method on the hospital’s information website.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Table S1. Clinical characteristics of the cohort adopting 22 mEq/L as the normal value for serum bicarbonate level. Supplementary Table S2. Multivariate analysis for association of hyperkalemia after the initiation of ACE-I or ARB with baseline clinical characteristics. Supplementary Figure S1. Receiver operating curve analysis for [Na+] − [Cl−] for predicting [HCO−] < 22 mEq/L.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mae, H., Fujimaru, T., Shimoyama, K. et al. Association of serum sodium minus chloride level at initiation of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and hyperkalemia in patients with CKD: a case control study. Ren Replace Ther 10, 24 (2024). https://doi.org/10.1186/s41100-024-00541-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-024-00541-3