Abstract
Background
Chronic kidney disease (CKD) affects 10% of the general population in Western countries. Currently, CKD cannot be cured and there are only few strategies to prevent the onset of CKD, to reverse early stages of CKD, and to prevent the progression of established CKD to end-stage kidney disease. Cigarette smoking is a preventable cause of CKD.
Methods
This narrative review analyses the cause–effect relationship between cigarette smoking and CKD and discusses the association of inhaled cadmium and smoking-induced kidney damage.
Results
Cigarette smoking places individuals at risk for incident CKD. It accelerates the progression (decline in glomerular filtration rate, aggravation of proteinuria) of CKD to end-stage kidney disease (ESKD), and is associated with shortened kidney transplant graft survival. These harmful effects on kidney function/structure are dependent on the dose and duration of cigarette smoking. Smoking abstinence decreases the higher risk for proteinuria and CKD progression. Inhaled cadmium may be the biologic link between cigarette smoking and kidney dysfunction. Recent studies indicate that accumulation of cadmium in the blood mediates progression of CKD and places smokers at higher risk for all-cause mortality.
Conclusions
Smoking cessation is an effective intervention to reduce the risk of onset and progression of CKD as well as to reduce smoking-attributable morbidity and mortality.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is defined by persistent (more than 3 months) urine abnormalities, changes in kidney structures, or reduction of the glomerular filtration rate. CKD encompasses a heterogeneous group of disorders with considerably variable incidence and prevalence. Clinical markers of functional and/or structural kidney damage, the rate of progression, and the late clinical presentation of patients depend on the underlying cause and stage of CKD. CKD affects more than 10% of the general adult populations with Western lifestyles worldwide, especially patients with diabetes mellitus, chronic arterial hypertension, and chronic glomerulonephritis. At present, there is no cure of progressive CKD. The early identification of modifiable risk factors is desirable because it may offer the opportunity to slow the progression rate and to reduce the high cardiovascular morbidity and mortality associated with CKD [1, 2].
Kidney histological findings and urine protein excretion patterns of active smokers
Observational studies found that active cigarette smoking is an independent risk factor for the onset of CKD, for accelerated progression of CKD to ESKD, or poorer graft survival after kidney transplantation [3,4,5,6,7,8,9,10,11,12]. There appeared to be a dose–response relationship of cigarette exposure and kidney functional decline. A higher number of pack years was associated with increased CKD progression [13,14,15].
Renal biopsy specimens taken from cigarette smokers showed a range of long-term changes with varying degrees of glomerulosclerosis, ischemic glomeruli, interstitial fibrosis and tubular atrophy, and arteriolar hyalinosis [16], This broad spectrum of histologic findings combined with initial renal hyperfiltration suggested that cigarette smoking induced renal damage was primarily the result of alterations of renal hemodynamics and its sequelae on glomerular and tubular function /structure [17,18,19]. However, the patterns of proteinuria in cigarette smoking healthy individuals pointed to tubular damage as an additional initiating mechanism of abnormal urinary albumin and enzyme excretion—the hallmark of incident smoking-associated CKD.
Hypothetical mechanisms of cigarette smoking-induced kidney damage
The precise nature of the biologic connection of cigarette smoking and renal damage is not well understood (and beyond the scope of this narrative review).
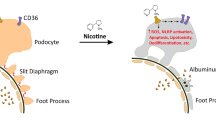
Cigarette smoking contains thousands of often toxic compounds, most of which have not been tested individually. Of importance, the susceptibility of individuals for smoking-induced kidney damage may vary. Undoubtedly, nicotine and cadmium (Cd) are well recognized nephrotoxins, but they are not the only ones. Nicotine alters renal hemodynamics by vasoconstriction and induces vascular endothelial cell dysfunction and small vessel or microvascular damage. It causes podocyte dysfunction and glomerular fibrosis and, as a result, albuminuria and progressive decline of glomerular filtration rate [20]. Furthermore, chronic low Cd exposure in cigarette smoke causes tubular toxicity. Metallothionein-bound Cd is freely filtered by the glomerular membrane and reabsorbed by the proximal tubular cells. Smoking-associated Cd exposure leads to accumulation of this nephrotoxin in the renal cortex, and places diabetic patients at risk for progression of CKD [21, 22]. Early evidence for Cd-induced tubular toxicity are low-molecular-weight proteinuria due to impaired endocytosis of filtered albumin and the detection of enzymes in the urine. Simultaneous exposure to low environmental cadmium and cigarette smoking was associated with tubular and glomerular dysfunctions (Fig. 1) [23].
Current cigarette smoking and direct tubular injury
Chronic smoking-induced damage to the renal proximal tubular epithelium has been studied with urinary enzymes as potential biomarkers of tubular toxicity. With tubular injury, enzymes normally present in tubular cells may be released into the lumen and will appear in the urine. Clinical cross-sectional studies demonstrated that urinary excretion of proximal tubular enzymes (beta-hexaminidase, N-acetyl-beta-d-glucosaminidase, neutral endopeptidase), and free filtered beta-2 microglobulin and retinol binding protein was higher in smoking individuals than in their nonsmoking counterparts [24,25,26,27]. Mounting data on tubular biomarkers challenged the widespread notion that renal tubules are victims of secondary injury. This concept shifted toward direct renal tubular injury as a driving force for the progression of CKD. In response to injury, tubular epithelial cells undergo structural and functional changes due to inflammatory and fibrogenic cells, with the consequent production of bioactive molecules that drive interstitial inflammation and fibrosis.
Dose of cigarette smoking and clinical course of chronic kidney disease
Observational studies assessing clinical characteristics as potential accelerators of progression of autosomal dominant polycystic kidney disease (ADPKD) reported that (a) patients with established proteinuria (more than 300 mg/day) had more pack years and a more severe aggressive clinical course (larger renal volumes, lower creatinine clearances) than their nonproteinuric counterparts [28] and (b) patients with a history of severe smoking (high number of pack years) had higher protein excretion rates and a more rapid progression of ADPKD [29]. Investigations with experimental models of polycystic kidney disease corroborated these clinical observations. Exposure of Pkd1-deficient cystic and noncystic mice to cigarette smoke enhanced tubular cell proliferation and apoptosis and increased renal fibrosis. These effects were more prominent in cystic than in noncystic mice [30]. Reproducible, highly controlled experimental models of chronic kidney disease help to identify the cellular processes that may contribute to disease progression. However, there are differences in smoking-induced kidney damage between smoking polycystic mice and smoking patients with adult polycystic kidney disease. Nonnephrotic proteinuria is present in a significant proportion of smoking patients, but there are no data on urinary protein analysis in mice models of polycystic kidney disease. The exact reason of proteinuria remains unknown (methodological problems) but there are differences in the progression of renal insufficiency (months versus decades). ADPKD in humans causes damage primarily to the proximal tubule while polycystic models demonstrate damage primarily to the distal tubule. Finally, there may be differences in the presence and severity of arterial hypertension between humans and mice models.
Kanauchi evaluated the glomerular and tubulo-interstitial changes in renal biopsy specimens from patients with type 2 diabetes mellitus and assessed the association of cigarette smoking and renal lesions [31]. Stepwise multiple regression analyses identified smoking index, but not duration of diabetes as independent risk factor for the severity of tubulo-interstitial lesions in smoking diabetic patients.
In a prospective study in parallel group design with matched groups, we compared the monthly decline of creatinine clearance in 45 current cigarette smoking patients (≥ 1 pack/day) with early stages of various glomerular and tubulo-interstitial CKDs with 45 nonsmoking patients matched for age, gender, cause, and severity of CKD. Current cigarette smoking accelerated the decline in excretory renal function in both etiologic subgroups of CKD patients. The harmful effects were dose dependent, and more prominent in smoking patients with tubulo-interstitial CKDs. In the stepwise multiple regression analysis, only smoking and the baseline creatinine clearance were statistically significant factors for acceleration of progressive renal functional deterioration [32].
Cessation of cigarette smoking and evolution of chronic kidney disease
The concept of active cigarette smoking as an independent nephrotoxic factor is further fueled by the documentation that the risk of new-onset CKD and accelerated progression of various kidney disorders decreases with time after smoking cessation.
In a prospective cohort study involving Korean patients with various degrees of CKD, smoking was associated with a significantly higher risk of worsening kidney function, particularly in patients with estimated glomerular filtration rate (eGFR) values below 45 ml/min and proteinuria (more than 1 g/day). The risk of adverse kidney outcomes was incrementally increased in smoking CKD patients with a higher number of cigarette pack years. The progression of CKD was attenuated with increased duration of smoking cessation [14].
The population-based Singapore Chinese healthy study analyzed the risk of cigarette smoking induced kidney failure [13]. A total of 674 cases of incident kidney failure occurred during the median follow up of 13.3 years. Active male smokers had a significant increase in the adjusted risk of kidney failure. There was a strong dose-dependent association between the number of years of smoking and kidney failure. The risk decreased with prolonged smoking cessation (more than 10 years) since baseline.
A cross-sectional study of Japanese men who had undergone general health screening suggested that active cigarette smoking might increase the prevalence of albuminuria and hyperfiltration. These early changes of CKD might be reversed by smoking cessation [33].
The prevention of renal and vascular end-stage disease(PREVEND) study included 7476 nondiabetic participants. Active smoking was associated with albuminuria and abnormal renal function. However, these associations were less pronounced or absent in former smokers [34].
There is mounting evidence that stopping active smoking slowed accelerated progression of renal failure in primary renal diseases or ameliorated renal injury in type 2 diabetes [14, 15, 35].
Limitations of the epidemiologic associations of active smoking and CKD
Currently, there is a lack of definitive proof for the association between cigarette smoking and kidney injury and the efficacy of smoking cessation from large randomized trials or well performed meta-analyses.
Most (but not all) studies assessing the association between cigarette smoking and kidney injury suggested a risk of smoking on the incidence and progression of CKD, irrespective of the original nature of the underlying kidney disease. Considerable heterogeneity across the epidemiologic research may represent the most plausible explanation of the controversial findings and make the results of meta-analyses less robust [11]. There are different sources of heterogeneity: (a) clinical heterogeneity (differences associated with participants or patients such as susceptibility to CKD, race, existence of other risk factors for CKD); (b) methodological differences such as prospective or retrospective study design, definition and classification of smoking habits (active smoker, never smokers, ex-smokers), quantification of exposure (pack years, duration of cigarette smoking) classification of CKD (proteinuria, serum creatinine levels, eGFR), duration of follow-up after cessation of smoking, presence of comorbidities, life style, health care system, and medications; and (c) statistical heterogeneity (selection of participants or patients, recall bias of exposure, control of confounding by other renal risk factors, sample size).
Cadmium exposure and renal function deterioration
Exposure to cadmium poses a health risk for humans and is a recognized risk factor for cancer, osteoporosis, lung and hepatic damage, and chronic kidney damage. The initial clinical sign of cadmium-induced renal lesions is tubular proteinuria, usually detected as increased urinary excretion of low-molecular-weight proteins (such as β-2-microglobulin, or alpha-1 macroglobulin) or tubular enzymes such as N-acetyl-/β-glucaminidase (NAG). Continuous exposure to cadmium leads to progressive tubular dysfunction and secondary glomerular damage with decreased glomerular filtration rate [36,37,38,39].
Barregard and coworkers determined kidney cadmium concentrations and histopathology in kidneys from 109 healthy kidney donors [40]. The results of these investigations suggested that even low environmental levels of cadmium can induce mild tubular atrophy. The amount of tubular atrophy and interstitial fibrosis was increased with active smoking.
Histopathologic evidence of tubular damage due to cadmium exposure has been also demonstrated at autopsy or in kidney biopsies in Japanese patients with itai-itai disease (combination of severe renal tubular damage and osteomalacia) [41]. These patients had experienced very high long-term environmental exposure to cadmium from contaminated rice.
In general, the diagnostic usefulness of urinary cadmium excretion (total urinary cadmium excretion/24 h or µg cadmium/g creatinine) is questionable. The recommended threshold levels of tolerable monthly cadmium intake (25 µg per body weight per month) or urinary cadmium excretion (5.2 µg/g creatinine) do not guarantee kidney protection [42, 43]. Clinical data from Japan and China indicated that cadmium-induced renal dysfunction varied between subjects. Almost 50% of subjects with high urinary cadmium excretion (> 20 µg/g creatinine) showed normal renal tubular function based on normal urinary NAG excretion and urinary β-2-microglobulin excretion rates. Renal tubular dysfunction was only observed in 20% of subjects living in cadmium polluted areas. Many factors such as patient demographics (age, gender), renal characteristics (urine flow rate, GFR), and comorbidities affect urinary cadmium excretion rates [44]. At present, only markers of tubular structure/function allow early prediction of cadmium-induced renal tubular dysfunction.
Numerous experimental studies have focused on the pathophysiology of cadmium-induced nephrotoxicity and have identified inflammatory processes, oxidative stress, mitochondrial dysfunction, disturbed endoplasmic reticulum homeostasis, and genotoxicity as key factors that lead to cell cycle arrest and eventual apoptosis of glomerular or tubular epithelial cells and endothelial cells. However, the data are still fragmentary, and the complex pathogenesis of cadmium induced nephrotoxicity remains to be established [45, 46].
Cadmium, smoking, and progressive diabetic nephropathy
Cigarette smoke contains more than 7000 often toxic compounds, most of which have not been tested individually. Amongst them, cadmium is one of the key players of the negative effects of cigarette smoke. Active heavy smokers have significantly higher blood cadmium concentrations compared with nonsmokers. The prospective long-term cohort study (median follow up 6 years) performed in the Dutch Ziekenhuis Group Twente hospitals investigated the hazards of blood cadmium concentration and smoking status on renal function in patients with mildly impaired renal function (mean eGFR at baseline 69 ± 27 ml/min/1.73 m2) in patients with type 2 diabetes [47]. The major findings of this observational study were that (a) active smokers had higher blood cadmium levels than former or never smokers. Measured blood cadmium levels were within the range that is currently considered “normal” by international guidelines. Cigarette smoking was the main but not the sole source of blood cadmium. (b) Both blood cadmium concentration and active smoking were associated with an increased hazard for deterioration of renal function. The unfavorable nephrotoxic effects of cadmium concentrations were in large parts the result of smoking status. (c) Comparison of renal functional decline in smoking and never-smoking diabetic patients suggested that smoking cessation lowered blood cadmium concentrations within 3 months and reduced the hazard for renal functional deterioration. The authors claimed the need for rigorous assessment of smoking status in diabetic patients with high risk of irreversible kidney damage.
E-cigarette use and chronic kidney disease
There has been a dramatic increase in the use of e-cigarettes (e-cig) or other types of electronic nicotine delivery systems (ENDS) as alternatives and/or cessation tools for conventional cigarettes, especially among young adolescents and young adults [48]. The heating of e-cig fluid generates an aerosol that contains humectants (vegetable glycerin, propyl glycol) and their toxic byproducts when heated (reactive aldehydes, volatile organic compounds), flavorings, heavy metals, and nicotine. There is no doubt, that nicotine and cadmium are components of e-cigarettes, depending on the type of vaping used [49]. E-cigarette use is associated with elevated blood cadmium levels [50].
Currently, data are scarce regarding the long-term impact of vaping on the onset or progression of CKD [51, 52]. Using data from the Chronic Kidney Disease in Children study, Molino et al. [53] found in a cohort of adolescents and young adults with pediatric-onset CKD, that chronic e-cig use (at least 1 year) was significantly associated with progression of CKD severity (rise in proteinuria, decline in eGFR). The multiple regression analysis of the results of a Russian cross-sectional study revealed that vaping was associated with onset of CKD in healthy subjects. E-cig users had higher urinary albumin excretion than combustible cigarette smokers and compared with nonsmokers or non-e-cig users [54].
Limited animal studies using histologic examinations of renal tissue or measurements of renal function indicate that e-cig use can increase susceptibility to CKD and progression of CKD in experimental models [55,56,57,58,59,60,61].
These preliminary results reinforce the uncertainty surrounding long-term health consequences of vaping. However, further studies are needed to comprehensively investigate the renal toxicity of e-cig in healthy subjects and patients with CKD. There is an urgent need to protect public health from the life threatening effects (lung, cardiovascular system) of vaping.
Conclusions
Smoking prevention at the population level, and particularly in patients at risk for CKD or with established CKD, must be part of public policies for the prevention of kidney disease progression. Efforts should continue to focus on all modifiable risk factors of CKD, and nephrologists should promote lifestyle modifications as effective means to prevent new-onset CKD or to slow progression of primary and secondary kidney disorders to ESKD. Research is uncertain on whether replacement of cigarettes by e-cigarettes helps smoking cessation, and data are yet incipient to achieve truthful conclusions on their effects on the kidneys.
Data availability
Data sharing is not applicable to this review as new data sets were not generated or analyzed.
References
Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022;12:7–11.
Schrauben SJ, Apple BJ, Chang AR. Modifiable Lifestyle Behaviors and CKD Progression: A Narrative Review. Kidney. 2022;3:752–78.
Fu YC, Xu ZL, Zhao MY, Xu K. The association between smoking and renal function in people over 20 years old. Front Med. 2022;9:870278.
Jones-Burton C, Seliger SL, Scherer RW, Mishra SI, Vessal G, et al. Cigarette smoking and incident chronic kidney disease: a systematic review. Am J Nephrol. 2007;27:342–51.
Eid HA, Moazen EM, Elhussini M, Shoman H, Hassan A, et al. The influence of smoking on renal functions among apparently healthy smokers. J Multidiscip Healthc. 2022;15:2969–78.
Leonberg-Yoo AK, Rudnick MR. Tobacco use: a chronic kidney disease accelerant. Am J Nephrol. 2017;46:257–9.
Liao D, Ma L, Liu J, Fu P. Cigarette smoking as a risk factor for diabetic nephropathy: A systematic review and meta-analysis of prospective cohort studies. PLoS One. 2019;14:e0210213.
Oliveira Coelho F, Andrade L. Smoking and kidney disease: risk factors, challenges, and preventive strategies. Contrib Nephrol. 2021;199:179–87.
Wang L, Smith-Salzberg B, Meyers KE, Glenn DA, Tuttle KR, et al. Tobacco exposure in adults and children with proteinuric glomerulopathies: a NEPTUNE cohort study. BMC Nephrol. 2023;24:30.
Wang S, Qin A, Pei G, Jiang Z, Dong L, et al. Cigarette smoking may accelerate the progression of IgA nephropathy. BMC Nephrol. 2021;22:239.
Xia J, Wang L, Ma Z, Zhong L, Wang Y, et al. Cigarette smoking and chronic kidney disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Nephrol Dial Transplant. 2017;32:475–87.
Sung RS, Althoen M, Howell TA, Ojo AO, Merion RM. Excess risk of renal allograft loss associated with cigarette smoking. Transplantation. 2001;71:1752–7.
Jin A, Koh WP, Chow KY, Yuan JM, Jafar TH. Smoking and risk of kidney failure in the Singapore Chinese health study. PLoS One. 2013;8:e62962.
Lee S, Kang S, Joo YS, Lee C, Nam KH, et al. Smoking, smoking cessation, and progression of chronic kidney disease: results from KNOW-CKD study. Nicotine Tob Res. 2021;23:92–8.
Schiffl H, Lang SM, Fischer R. Stopping smoking slows accelerated progression of renal failure in primary renal disease. J Nephrol. 2002;15:270–4.
Liang KV, Greene EL, Oei LS, Lewin M, Lager D, et al. Nodular glomerulosclerosis: renal lesions in chronic smokers mimic chronic thrombotic microangiopathy and hypertensive lesions. Am J Kidney Dis. 2007;49:552–9.
Hammer Y, Cohen E, Levi A, Krause I. The relationship between cigarette smoking and renal function: a large cohort study. Isr Med Assoc J. 2016;18:553–6.
Maeda I, Hayashi T, Sato KK, Koh H, Harita N, et al. Cigarette smoking and the association with glomerular hyperfiltration and proteinuria in healthy middle-aged men. Clin J Am Soc Nephrol. 2011;6:2462–9.
Mickelsson M, Söderström E, Stefansson K, Andersson J, Söderberg S, et al. Smoking tobacco is associated with renal hyperfiltration. Scand J Clin Lab Invest. 2021;81:622–8.
Van Laecke S, Van Biesen W. Smoking and chronic kidney disease: seeing the signs through the smoke? Nephrol Dial Transplant. 2017;32:403–5.
Yan LJ, Allen DC. Cadmium-induced kidney injury: oxidative damage as a unifying mechanism. Biomolecules 2021; 11.
Yimthiang S, Vesey DA, Pouyfung P, Khamphaya T, Gobe GC et al. Chronic kidney disease induced by cadmium and diabetes: a quantitative case-control study. Int J Mol Sci 2023; 24.
Satarug S, Ujjin P, Vanavanitkun Y, Nishijo M, Baker JR, et al. Effects of cigarette smoking and exposure to cadmium and lead on phenotypic variability of hepatic CYP2A6 and renal function biomarkers in men. Toxicology. 2004;204:161–73.
IA EL S, Afifi AM, Shouman AE, Akel S. Effects of smoking and lead exposure on proximal tubular integrity among Egyptian industrial workers. Arch Med Res. 2004;35:59–65.
Roszczenko A, Gałazyn-Sidorczuk M, Brzóska MM, Moniuszko-Jakoniuk J, Zwierz K. Select parameters of renal function in smokers in correlation with the exposure to cadmium. Przegl Lek. 2004;61:348–50.
Hultberg B, Isaksson A, Brattström L, Israelsson B. Elevated urinary excretion of beta-hexosaminidase in smokers. Eur J Clin Chem Clin Biochem. 1992;30:131–3.
Nortier J, Bernard A, Roels H, Deschodt-Lanckman M, Gueuning C, et al. Urinary neutral endopeptidase in workers exposed to cadmium: interaction with cigarette smoking. Occup Environ Med. 1997;54:432–6.
Chapman AB, Johnson AM, Gabow PA, Schrier RW. Overt proteinuria and microalbuminuria in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1994;5:1349–54.
Ozkok A, Akpinar TS, Tufan F, Kanitez NA, Uysal M, et al. Clinical characteristics and predictors of progression of chronic kidney disease in autosomal dominant polycystic kidney disease: a single center experience. Clin Exp Nephrol. 2013;17:345–51.
Sousa MV, Amaral AG, Freitas JA, Murata GM, Watanabe EH, et al. Smoking accelerates renal cystic disease and worsens cardiac phenotype in Pkd1-deficient mice. Sci Rep. 2021;11:14443.
Kanauchi M. Cigarette smoking affects tubulointerstitial lesions in type 2 diabetes. Diabetes Care. 2002;25:1486–7.
Schiffl H, Lang SM, Fischer R, Bergner A. Cigarette smoking accelerates progression of renal failure in primary renal disease. A prospective study in parallel group design with matched groups. Nephrology. 2000;5:151–4.
Noborisaka Y, Ishizaki M, Yamada Y, Honda R, Yokoyama H, et al. The effects of continuing and discontinuing smoking on the development of chronic kidney disease (CKD) in the healthy middle-aged working population in Japan. Environ Health Prev Med. 2013;18:24–32.
Pinto-Sietsma SJ, Mulder J, Janssen WM, Hillege HL, de Zeeuw D, et al. Smoking is related to albuminuria and abnormal renal function in nondiabetic persons. Ann Intern Med. 2000;133:585–91.
Phisitkul K, Hegazy K, Chuahirun T, Hudson C, Simoni J, et al. Continued smoking exacerbates but cessation ameliorates progression of early type 2 diabetic nephropathy. Am J Med Sci. 2008;335:284–91.
Buchet JP, Lauwerys R, Roels H, Bernard A, Bruaux P, et al. Renal effects of cadmium body burden of the general population. Lancet. 1990;336:699–702.
Järup L, Hellström L, Alfvén T, Carlsson MD, Grubb A, et al. Low level exposure to cadmium and early kidney damage: the OSCAR study. Occup Environ Med. 2000;57:668–72.
Prozialeck WC, Edwards JR. Mechanisms of cadmium-induced proximal tubule injury: new insights with implications for biomonitoring and therapeutic interventions. J Pharmacol Exp Ther. 2012;343:2–12.
Thomas LD, Hodgson S, Nieuwenhuijsen M, Jarup L. Early kidney damage in a population exposed to cadmium and other heavy metals. Environ Health Perspect. 2009;117:181–4.
Barregard L, Sallsten G, Lundh T, Mölne J. Low-level exposure to lead, cadmium and mercury, and histopathological findings in kidney biopsies. Environ Res. 2022;211:113119.
Baba H, Tsuneyama K, Kumada T, Aoshima K, Imura J. Histopathological analysis for osteomalacia and tubulopathy in itai-itai disease. J Toxicol Sci. 2014;39:91–6.
Satarug S, Vesey DA, Gobe GC. Cadmium-induced proteinuria: mechanistic insights from dose-effect analyses. Int J Mol Sci 2023; 24.
Satarug S, Yimthiang S, Pouyfung P, Khamphaya T, Vesey DA Cadmium-induced tubular dysfunction in type 2 diabetes: a population-based cross-sectional study. Toxics 2023; 11.
Wang X, Chen X, He W, Zhu G, Jin T, et al. A nomogram to predict cadmium-induced renal tubular dysfunction. Sci Rep. 2020;10:10121.
Mishra M, Nichols L, Dave AA, Pittman EH, Cheek JP et al. Molecular mechanisms of cellular injury and role of toxic heavy metals in chronic kidney disease. Int J Mol Sci 2022; 23.
Zhang Y, Liu Z, He Q, Wu F, Xiao Y, et al. Construction of mode of action for cadmium-induced renal tubular dysfunction based on a toxicity pathway-oriented approach. Front Genet. 2021;12: 696892.
Oosterwijk MM, Hagedoorn IJM, Maatman R, Bakker SJL, Navis G, et al. Cadmium, active smoking and renal function deterioration in patients with type 2 diabetes. Nephrol Dial Transplant. 2023;38:876–83.
Bozier J, Chivers EK, Chapman DG, Larcombe AN, Bastian NA, et al. The evolving landscape of e-cigarettes: a systematic review of recent evidence. Chest. 2020;157:1362–90.
Smith DM, Christensen C, van Bemmel D, Borek N, Ambrose B, et al. Exposure to nicotine and toxicants among dual users of tobacco cigarettes and e-cigarettes: population assessment of tobacco and health (PATH) study, 2013–2014. Nicotine Tob Res. 2021;23:790–7.
Lee JW, Kim Y, Kim Y, Yoo H, Kang HT. Cigarette smoking in men and women and electronic cigarette smoking in men are associated with higher risk of elevated cadmium level in the blood. J Korean Med Sci. 2020;35:e15.
Bandara NA, Zhou XR, Alhamam A, Black PC, St-Laurent MP. The genitourinary impacts of electronic cigarette use: a systematic review of the literature. World J Urol. 2023.
Abraham J, Stonhill MA, Gregory MC, Callahan SJ. Renal abnormalities in patients with e-cigarette, or vaping, product use-associated lung injury (EVALI). JASN. 2020;31:98.
Molino AR, Jerry-Fluker J, Atkinson MA, Furth SL, Warady BA, et al. The association of alcohol, cigarette, e-cigarette, and marijuana use with disease severity in adolescents and young adults with pediatric chronic kidney disease. Pediatr Nephrol. 2021;36:2493–7.
Podzolkov VI, Bragina AE, Druzhinina NA, Vasil’eva LV, Osadchiy KK, et al. Relation between Tobacco smoking/electronic smoking and albuminuria/vascular stiffness in young people without cardiovascular diseases. Kidney Blood Press Res. 2020;45:467–76.
Crotty Alexander LE, Drummond CA, Hepokoski M, Mathew D, Moshensky A, et al. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am J Physiol Regul Integr Comp Physiol. 2018;314:R834-r847.
Drummond C, Alexander LEC, Tian J. Chronic Electronic cigarette vapor inhalation induces renal injury and functional decline in female mice. FASEB J. 2017;31:698.691-698.691.
Drummond CA, Crotty Alexander LE, Haller ST, Fan X, Xie JX, et al. Cigarette smoking causes epigenetic changes associated with cardiorenal fibrosis. Physiol Genomics. 2016;48:950–60.
Feng M, Bai X, Thorpe AE, Nguyen LT, Wang M et al. Effect of E-vaping on kidney health in mice consuming a high-fat diet. Nutrients 2023; 15.
Golli NE, Jrad-Lamine A, Neffati H, Dkhili H, Rahali D, et al. Impact of e-cigarette refill liquid exposure on rat kidney. Regul Toxicol Pharmacol. 2016;77:109–16.
Doğan K The effects of electronic cigarettes on oxidative stress markers in the kidney tissues of Wistar Albino Rats. Int J Med Biochem. 2022.
Salman RJ, Jebur HB, Idbeis HI. Study the adverse effect of E, cigarette and conventional cigarette in kidneys, stomach and testes in rats in comparison with control group. World Sci J Mod Res Methodol. 2023;2:56–70.
Acknowledgment
Not applicable
Funding
There was no funding for design of the study sand collection, analysis and interpretation of data and for writing the manuscript.
Author information
Authors and Affiliations
Contributions
Both authors ( S.L., H.S ) meet the International Committee of Medical Journal Editor’s criteria for authorship for this article. They take responsibility for the integrity of the work and have given their approval for the final version of the manuscript to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This narrative review is based on previously conducted studies and does not contain any unpublished study with participants or animals. It uses publicly accessible data. Institutional approval and patient consent were not necessary. The approval of an ethical committee is not necessary.
Consent for publication
Not applicable to this article.
Competing interests
The authors declare that they have no non-financial or financial competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lang, S.M., Schiffl, H. Smoking status, cadmium, and chronic kidney disease. Ren Replace Ther 10, 17 (2024). https://doi.org/10.1186/s41100-024-00533-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-024-00533-3