Abstract
Background
Malnutrition is associated with discontinuing peritoneal dialysis (PD). The prognostic nutritional index (PNI), composed of serum albumin level and total lymphocyte count, has been suggested as a prognostic marker for mortality in patients undergoing PD. However, the relationship between PNI and PD discontinuation has not yet been well addressed. We evaluated the relationship between PNI and PD discontinuation in patients with end-stage kidney disease who initiated PD treatment.
Methods
This retrospective cohort study included patients who underwent PD at a single academic hospital between 2007 and 2022. We examined the association between PNI (< 40 vs. ≥ 40) and PD discontinuation using Cox proportional hazards regression models. We used restricted cubic spline analysis to examine the continuous associations between the PNI and outcomes.
Results
The mean age (and standard deviation) of the 91 patients was 57.1 ± 13.4 years; 72 (79.1%) discontinued PD during the median follow-up period of 25.0 months. Lower PNI was associated with an increased risk of PD discontinuation. The hazard ratios (95% confidence intervals) with three levels of adjustments were 1.74 (1.08, 2.79), 2.21 (1.32, 3.66), and 1.81 (1.01, 3.24) (reference: PNI ≥ 40). Restricted cubic spline analysis showed that a PNI < 40 was continuously associated with a higher risk of PD discontinuation.
Conclusion
A lower PNI (< 40) was associated with a higher risk of PD discontinuation. Our findings suggest that evaluating the PNI may help identify patients at high risk of PD discontinuation and lead to appropriate nutritional management for dialysis maintenance.
Similar content being viewed by others
Background
Peritoneal dialysis (PD) accounts for approximately 11% of the treatment modalities for end-stage kidney disease (ESKD) worldwide [1]. PD has several advantages over hemodialysis (HD), such as cost-effectiveness, fewer harmful hemodynamic effects, and preservation of residual kidney function [2, 3]. However, patients are often unwilling to discontinue PD and are forced to transition to HD after a shorter-than-expected period of PD treatment. Therefore, detecting patients at high risk of PD discontinuation is important for both dialysis modality selection and maintenance of PD treatment [2].
Malnutrition is an important factor in PD discontinuation and can be a modifiable factor [4]. Objective screening tools comprising laboratory markers and anthropometric parameters have been used to assess nutritional status [5]. The prognostic nutritional index (PNI) is a simple and useful nutritional marker calculated from the serum albumin level and total lymphocyte count. It has been widely used in the risk assessment of surgical intervention in patients with cancer [6]. In patients with ESKD, some studies have investigated the association between PNI and mortality, showing that a lower PNI is associated with higher mortality in both HD and PD patients [7,8,9,10]. However, there have been few reports on the relationship between PNI and PD discontinuation, and only one retrospective study has assessed the predictive values of nutritional indices for PD discontinuation [4].
In clinical settings, biomarkers and indices are often divided into categories based on optimal cutoff values. These objective criteria can help clinicians interpret data and select the optimal treatment [11]. To our knowledge, the association between well-recognized PNI cutoff values and PD discontinuation has not yet been examined. It may be important to investigate the association between the PNI and PD discontinuation using a well-recognized and reasonable cutoff value for the practical use of the PNI in clinical settings.
Consequently, this study aimed to examine the relationship between PNI and PD discontinuation in patients with ESKD who initiated PD treatment at an academic hospital by determining a certain cutoff value for PNI.
Methods
Study population and data source
This retrospective cohort study was conducted at a single academic hospital. The study cohort included patients who initiated PD treatment between April 1, 2007, and March 31, 2022. Patients aged < 20 years at baseline, those who did not receive PD treatment for at least a month, or those who switched from maintenance HD to PD were excluded from the study. All data were obtained from the electronic records of the Kumamoto University Hospital. Due to the anonymity of the patients included and the nonintrusive nature of the research, the requirement for written consent was waived. This study was approved by the Institutional Review Committee of the Kumamoto University Hospital (No. 2529).
Demographic, clinical, and laboratory measures
We obtained data on age, sex, height, weight, blood pressure, urine volume, information on the primary disease of ESKD, comorbidities, cardiovascular disease (CVD) events, CVD history or death, PD prescription, PD treatment period and peritonitis, HD treatment period, the use of renin–angiotensin–aldosterone system inhibitors, statins, diuretics, phosphate binders, vitamin D, and erythropoiesis-stimulating agents, and laboratory results from electronic medical records. Weight and height were used to calculate the body mass index (BMI).
Most laboratory parameters measured were obtained from the nearest day of PD initiation, which was within a week before or after initiation, including the levels of hemogram, albumin, urea nitrogen, creatinine, and electrolytes. Serum levels of ferritin, iron, total iron binding capacity, lipids, plasma brain natriuretic peptide (BNP), and intact parathyroid hormone (PTH) were measured within a month before or after PD initiation. Hemoglobin (Hb) A1c levels were measured within 3 months. The PNI was calculated using the following formula: (10 × serum albumin [g/dL]) + (0.005 × total lymphocyte count [/mm3)] [6].
Outcome ascertainment
The outcome of interest was the discontinuation of PD. Based on previous reports, the discontinuation of PD was defined as a switch to HD or hybrid therapy with HD, kidney transplantation, or death [12, 13]. Censoring was performed at another dialysis clinic or at the end of the study.
Statistical analysis
Data are described as proportion, mean (± standard deviation, SD), or median (interquartile range, IQR), as appropriate. We divided the PNI levels into two categories (< 40 and ≥ 40) according to previous reports [6, 8]. We used the t-test, Mann–Whitney U test, chi-square test, or Fisher’s exact test to assess patients’ characteristics between PNI categories. We conducted a Kaplan–Meier analysis to estimate the survival rates between the PNI groups, and the survival estimates were compared using the log-rank test. To assess the relationship between PNI and PD discontinuation, we used Cox proportional hazards regression models using PNI ≥ 40 as a reference. Plots of log [− log (survival rate)] against log (survival time) were generated to test the proportionality of this assumption.
In Cox proportional hazards regression models, three models were examined based on the level of multivariate adjustment: (a) model 1: a minimally adjusted model that included PNI (< 40 and ≥ 40), (b) model 2: PNI, age, and sex, (c) model 3: PNI, age, sex, presence of diabetes mellitus, CVD history, Hb, and urine volume. The selected variables were based on previous studies [14,15,16,17,18]. Associations between continuous PNI and PD discontinuation across the three adjustment levels were also modeled using restricted cubic splines with knots at 10, 50, and 90 percentiles of the PNI.
Most analyses were performed using JMP version 12 (SAS Institute Inc., Cary, NC, USA). Kaplan–Meier and spline analyses were performed using Stata version 17.1 (Stata Corporation, College Station, TX, USA). Statistical significance was set at P < 0.05.
Results
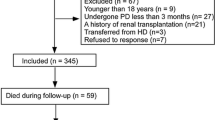
In total, 119 patients had initiated PD in Kumamoto University Hospital during the study period. After excluding patients who switched from HD, discontinued PD within a month, or had missing data in the main analysis, the final study population consisted of 91 patients (Additional file 1: Figure S1). The mean age of patients was 57.1 ± 13.4 years; 61.5% were males, and 44.0% had diabetes. Table 1 shows the baseline demographic, clinical, and laboratory characteristics of all patients and those in each PNI subset (< 40, and ≥ 40). Patients with PNI < 40 had a significantly higher prevalence of diabetes and exhibited significantly lower urine volume and Hb level than those with PNI ≥ 40 (Table 1).
The duration of PD therapy averaged 25.0 months (median) in the total cohort (Additional file 2: Table S1): 17.5 months in patients with PNI < 40 and 34.0 months in patients with PNI ≥ 40 (P < 0.05). A total of 72 patients (79.1%) discontinued PD therapy during the study period; 33 (78.6%) had PNI < 40, and 39 (79.6%) had PNI ≥ 40 (Additional file 3: Table S2). The incidences of peritonitis, CVD events, and death until PD discontinuation or censor were not significantly different between the two PNI categories (Additional file 2: Table S1). The causes of PD discontinuation are shown in Additional file 4: Figure S2 and Additional file 5: Table S3. When we compared the PNI < 40 group with the PNI ≥ 40 group, there was a significant difference in survival estimates by the log-rank test (P = 0.019) (Fig. 1).
In the Cox proportional hazards regression models before and after adjustment for age, sex, diabetes, CVD history, Hb level, and urine volume, a lower PNI was significantly associated with a higher risk of PD discontinuation (Table 2). When comparing the PNI < 40 group with the PNI ≥ 40 group, the hazard ratios (HRs) (95% confidence interval) of PD discontinuation in each model were 1,74 (1.08, 2.79), 2.21 (1.32, 3.66), and 1.81 (1.01, 3.24), respectively (Table 2). Additional file 6: Table S4 shows the HRs of the other adjusted variables in Models 2 and 3. Restricted cubic spline analysis showed that a lower PNI was continuously associated with a higher risk of PD discontinuation when the reference was set at PNI = 40 (Fig. 2). However, a higher PNI level showed no significant difference in the HR for PD discontinuation (Fig. 2).
Hazard ratios of PD discontinuation according to PNI by cubic splines of Cox regression analyses. Cubic spline models of Cox proportional regression analyses reflecting hazard ratios for PD discontinuation according to the PNI in a cohort of 91 patients who underwent PD are shown (A: model 1; B: model 2; C: model 3). Histograms show the frequencies of the participants. Solid and dotted lines represent hazard ratios and 95% confidence intervals, respectively. The reference value was set at PNI = 40
Discussion
In this retrospective cohort study, we examined the association between PNI and PD discontinuation using the clinical data of PD patients in an academic hospital. A lower PNI level (< 40) was associated with a higher HR for PD discontinuation, and this association was consistent when we examined continuous associations between PNI and outcome with a reference level of PNI (PNI = 40).
The PNI consists of blood albumin level and lymphocyte count, and this index represents nutritional status [6]. PNI could be useful as a prognostic marker, including mortality, in patients with ESKD [7,8,9,10]. As malnutrition has been reported to be an important factor in PD discontinuation [18], the PNI may also serve as a useful marker for PD discontinuation. One observational study examined the association between several nutritional indices and PD discontinuation. The authors found that the PNI was a better prognostic marker than the geriatric nutritional risk index (GNRI) and controlling nutritional status (CONUT) scores, both of which are well-known prognostic markers for nutritional status [4]. Additionally, they set the PNI cutoff value from the receiver operating characteristic (ROC) curve. They also discussed the need for further studies to determine and validate the optimal PNI cutoff value. In the present study, we used a PNI value of 40, a well-used cutoff value in the risk assessment of several diseases [6, 8, 19,20,21,22,23]. We found that a PNI < 40 can be a useful cutoff level for identifying the risk of PD discontinuation.
This study found an association between the PNI during the initiation period of dialysis and withdrawal from PD. Notably, up to 75% of patients are reported to be malnourished at PD initiation [24]. Malnutrition in PD patients has been reported to be associated with increased mortality and infections such as peritonitis, which may be reflected in the high mortality and infection rates as causes of PD discontinuation in the low PNI group in this study (Additional file 5: Table S3) [8,9,10, 25]. In addition, patients undergoing PD tend to lose protein more easily via the PD fluid [26]. Therefore, nutritional management and adequate dialysis prescriptions based on the assessment of nutritional status at PD initiation are extremely important for maintaining PD. Nutritional management is one of the most important topics in patients with kidney disease from the non-dialysis-dependent phase to the dialysis-dependent phase [27]. Based on our findings that nutritional status during PD initiation was associated with the risk of PD discontinuation, it is also important to ensure adequate nutritional status during the initiation period of dialysis through preemptive nutritional management. As the current study highlighted the PNI upon PD initiation, further studies are needed to clarify whether continuous nutritional intervention with adequate assessment, including PNI over time, can influence prognosis, including PD discontinuation.
Although the proportion of inadequate dialysis, including ultrafiltration failure and insufficient dialysis, among the causes of PD discontinuation was similar to that in a previous report [12], ultrafiltration failure was the leading cause of PD discontinuation in the present study (Additional file 4: Figure S2). In previous studies, fluid overload was associated with PD technical failure, and hypoalbuminemia was associated with overhydration [28, 29]. From these points, it could be argued that an abnormal PNI may indicate fluid overload during the induction phase of dialysis because albumin is the main component of PNI. In this regard, Kang et al. showed an independent association between PNI and mortality by adjusting for fluid status in PD patients [8]. Therefore, in this study, we adjusted for residual urine volume, a predictor of overhydration, as a confounding factor and showed an association between PNI and PD discontinuation [30]. This association remained consistent when we adjusted for BNP levels instead of urine volume (Additional file 7: Table S5). These findings suggest that PNI is not only an indicator of fluid overload but may also be used as a marker of malnutrition and that PD patients with a low PNI level will require measures to improve nutritional status and fluid control.
This study had several limitations. First, because of the nature of the observational study, we could not clarify the causal relationship between PNI and PD discontinuation. Second, we could not exclude the possibility of residual confounding due to unmeasured confounders and the limitation of adjusting for variables due to the relatively small sample size. Third, because the participants’ data were from a single academic hospital, generalizability should be considered when adapting the evidence of our findings.
Conclusions
We showed that a lower PNI was associated with a higher risk of PD discontinuation in a single academic hospital cohort. We propose that upon initiation of dialysis treatment, patients must be evaluated for their nutritional status and potential risks of PD treatment. To this end, our findings suggest that evaluating the PNI may help identify patients at high risk of PD discontinuation and lead to appropriate nutritional management for dialysis maintenance.
Availability of data and materials
As the participants in this study did not agree that their data would be shared publicly, supporting data were not available.
Abbreviations
- PD:
-
Peritoneal dialysis
- ESKD:
-
End-stage kidney disease
- HD:
-
Hemodialysis
- PNI:
-
Prognostic nutritional index
- CVD:
-
Cardiovascular disease
- BMI:
-
Body mass index
- BNP:
-
Brain natriuretic peptide
- PTH:
-
Parathyroid hormone
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- Hb:
-
Hemoglobin
- HR:
-
Hazard ratio
- GNRI:
-
Geriatric nutritional risk index
- CONUT:
-
Controlling nutritional status
- ROC:
-
Receiver operating characteristic
References
Cho Y, Bello AK, Levin A, Lunney M, Osman MA, Ye F, et al. Peritoneal dialysis use and practice patterns: an international survey study. Am J Kidney Dis. 2021;77:315–25.
Teitelbaum I. Peritoneal Dialysis. N Engl J Med. 2021;385:1786–95.
Karopadi AN, Mason G, Rettore E, Ronco C. Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dial Transpl. 2013;28:2553–69.
Yang Y, Xu Y, Zhang P, Zhou H, Yang M, Xiang L. Predictive value of objective nutritional indexes in technique failure in peritoneal dialysis patients. J Ren Nutr. 2022;32:605–12.
Keller U. Nutritional laboratory markers in malnutrition. J Clin Med. 2019;8:775.
Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85:1001–5.
Miyasato Y, Hanna RM, Morinaga J, Mukoyama M, Kalantar-Zadeh K. Prognostic nutritional index as a predictor of mortality in 101,616 patients undergoing hemodialysis. Nutrients. 2023;15:311.
Kang SH, Cho KH, Park JW, Yoon KW, Do JY. Onodera’s prognostic nutritional index as a risk factor for mortality in peritoneal dialysis patients. J Korean Med Sci. 2012;27:1354–8.
Peng F, Chen W, Zhou W, Li P, Niu H, Chen Y, et al. Low prognostic nutritional index associated with cardiovascular disease mortality in incident peritoneal dialysis patients. Int Urol Nephrol. 2017;49:1095–101.
Cai L, Yu J, Yu J, Peng Y, Ullah H, Yi C, et al. Prognostic value of inflammation-based prognostic scores on outcome in patients undergoing continuous ambulatory peritoneal dialysis. BMC Nephrol. 2018;19:297.
Woo SY, Kim S. Determination of cutoff values for biomarkers in clinical studies. Precis Future Med. 2020;4:2–8.
Taki Y, Sakurada T, Koitabashi K, Imai N, Shibagaki Y. Predictive factors for withdrawal from peritoneal dialysis: a retrospective cohort study at two centers in Japan. Adv Perit Dial. 2017;33:68–73.
Luo Q, Xia X, Lin Z, Lin J, Yang X, Huang F, et al. Very early withdrawal from treatment in patients starting peritoneal dialysis. Ren Fail. 2018;40:8–14.
Gamba G, Mejía JL, Saldívar S, Peña JC, Correa-Rotter R. Death risk in CAPD patients. The predictive value of the initial clinical and laboratory variables. Nephron. 1993;65:23–7.
Struijk DG, Krediet RT, Koomen GC, Boeschoten EW, Arisz L. The effect of serum albumin at the start of continuous ambulatory peritoneal dialysis treatment on patient survival. Perit Dial Int. 1994;14:121–6.
Holland DC, Meers C, Lawlor ME, Lam M. Serial prealbumin levels as predictors of outcomes in a retrospective cohort of peritoneal and hemodialysis patients. J Ren Nutr. 2001;11:129–38.
Zhang F, Liu H, Gong X, Liu F, Peng Y, Cheng M, et al. Risk factors for mortality in Chinese patients on continuous ambulatory peritoneal dialysis. Perit Dial Int. 2015;35:199–205.
Da Luz LG, Ankawi G, Digvijay K, Rosner MH, Ronco C. Technique failure in peritoneal dialysis: etiologies and risk assessment. Blood Purif. 2021;50:42–9.
Nozoe T, Kohno M, Iguchi T, Mori E, Maeda T, Matsukuma A, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42:532–5.
Hiraoka A, Kumada T, Tada T, Fukunishi S, Atsukawa M, Hirooka M, et al. Nutritional index as prognostic indicator in patients receiving Lenvatinib treatment for unresectable hepatocellular carcinoma. Oncology. 2020;98:295–302.
Akgül Ö, Bagante F, Olsen G, Cloyd JM, Weiss M, Merath K, et al. Preoperative prognostic nutritional index predicts survival of patients with intrahepatic cholangiocarcinoma after curative resection. J Surg Oncol. 2018;118:422–30.
Yamamoto S, Adachi S, Wada T, Narui K, Kimura A, Oshi M, et al. The modified Glasgow prognostic score and prognostic nutritional index as prognostic markers in patients with metastatic breast cancer treated with Eribulin. In Vivo. 2022;36:1854–9.
Zhou W, Cao Q, Qi W, Xu Y, Liu W, Xiang J, et al. Prognostic nutritional index predicts short-term postoperative outcomes after bowel resection for Crohn’s disease. Nutr Clin Pract. 2017;32:92–7.
Prasad N, Gupta A, Sinha A, Sharma RK, Kumar A, Kumar R. Changes in nutritional status on follow-up of an incident cohort of continuous ambulatory peritoneal dialysis patients. J Ren Nutr. 2008;18:195–201.
Prasad N, Gupta A, Sharma RK, Sinha A, Kumar R. Impact of nutritional status on peritonitis in CAPD patients. Perit Dial Int. 2007;27:42–7.
Kim SM, Kang BC, Kim HJ, Kyung MS, Oh HJ, Kim JH, et al. Comparison of hemodialysis and peritoneal dialysis patients’ dietary behaviors. BMC Nephrol. 2020;10:90.
Kalantar-Zadeh K, Fouque D. Nutritional management of chronic kidney disease. N Eng J Med. 2017;377:1765–76.
Ng JK, Kwan BC, Chan GC, Chow KM, Pang WF, Cheng PM, et al. Predictors and prognostic significance of persistent fluid overload: a longitudinal study in Chinese peritoneal dialysis patients. Perit Dial Int 2022; 8968608221110491.
John B, Tan BK, Dainty S, Spanel P, Smith D, Davies SJ. Plasma volume, albumin, and fluid status in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2010;5:1463–70.
Jung ES, Sung JY, Han SY, Kim AJ, Ro H, Jung JY, et al. Residual urinary volume is a predictor of overhydration in patients on peritoneal dialysis. Tohoku J Exp Med. 2014;233:295–300.
Acknowledgements
The authors thank N. Nakagawa for her support of this study.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
YM, JM, and MM designed the study. YM collected the data. YM and JM analyzed the data. YM and MM drafted the manuscript. HI, YN, MA, YI, YK, TM, TN, DF, AO, and TK supervised the study. All authors contributed substantially to the conception and interpretation of the work and critically revised the manuscript. The final manuscript was approved by all listed authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. It was approved by the Institutional Review Committee of Kumamoto University Hospital (No. 2529). Because of the anonymity of the patients studied and the nonintrusive nature of the research, the requirement for written consent was waived via the opt-out method on the hospital’s information website.
Consent to publication
Because of the anonymity of the patients studied and the nonintrusive nature of the research, the requirement for written consent was waived via the opt-out method on the hospital’s information website.
Competing interests
The authors declare no competing interests relevant to the contents of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1. Flowchart of patient selection in the study cohort.
Additional file 2: Table S1.
Time on peritoneal dialysis therapy and incidence of peritonitis, cardiovascular event, and death until peritoneal dialysis discontinuation or censor in 91 PD patients and patients in 2 groups of PNI.
Additional file 3:
Table S2. Case numbers of each outcome in all 72 cases and cases in 2 groups of PNI.
Additional file 4:
Figure S2. The causes of PD discontinuation.
Additional file 5:
Table S3. The causes of PD discontinuation in all 72 cases and cases in 2 groups of PNI.
Additional file 6:
Table S4. The hazard ratios of other adjusted variables for PD discontinuation using Cox proportional hazards models in 91 PD patients.
Additional file 7:
Table S5. Association of PNI with PD discontinuation in Cox proportional hazards models adjusting BNP.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Miyasato, Y., Morinaga, J., Inoue, H. et al. Association between prognostic nutritional index and peritoneal dialysis discontinuation: a retrospective cohort study. Ren Replace Ther 9, 58 (2023). https://doi.org/10.1186/s41100-023-00511-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-023-00511-1