Abstract
Background
Fat embolism syndrome (FES) is a rare syndrome that typically occurs 12–72 h after long bone or pelvic fractures with a classic triad of respiratory distress, neurologic changes, and petechial rash. Although Gurd’s criteria for FES include anuria or oliguria, the mechanism of acute kidney injury (AKI) remain unknown. Here, we present a case of FES complicated by AKI that required blood purification.
Case presentation
A 79-year-old woman was admitted to our hospital because of a right humerus and pelvic fracture caused by a traffic accident. On the second day of hospitalization, she developed impaired consciousness, respiratory failure, and disseminated intravascular coagulation (DIC). Chest radiography revealed bilateral diffuse alveolar infiltration. Brain magnetic resonance imaging revealed diffuse high signal intensity on diffusion-weighted imaging and diffuse low signal intensity on susceptibility-weighted imaging in the cerebral and cerebellar regions. The diagnosis of FES was confirmed and the patient was treated with methylprednisolone (40 mg/day) and ulinastatin. On the third day of hospitalization, she was admitted to our department because of AKI with oliguria. Although echocardiography showed an elevated right ventricular artery systolic pressure suggestive of pulmonary hypertension (PH), pulmonary congestion was initially considered on chest imaging, and hemodialysis and rapid ultrafiltration were initiated. However, she developed hypovolemic shock and treatment was switched to continuous hemodiafiltration and slow ultrafiltration. Thereafter, her consciousness, hypoxemia, DIC and PH completely improved. She was weaned from blood purification therapy on the 29th day of hospitalization. She had hemolytic anemia that might have been caused by thrombotic microangiopathy (TMA), but it resolved without plasmapheresis. On the 51st day of hospitalization, the patient was transferred to another hospital for rehabilitation.
Conclusions
FES can be complicated by AKI. In this case, DIC, which was difficult to differentiate from TMA, and/or renal congestion were considered to be a cause of AKI. Chest radiographs of FES may be indistinguishable from pulmonary congestion. In our case, chest radiography showed bilateral diffuse alveolar infiltrates which was not indicative of pulmonary congestion but pulmonary involvement of FES. FES is associated with PH, which may lead to right heart failure. Therefore, the patient could have developed hypovolemic shock due to hemodialysis and rapid ultrafiltration. Clinicians should pay attention to the hemodynamics when blood purification for FES is performed.
Similar content being viewed by others
Background
Fat embolism syndrome (FES) is a rare syndrome that typically occurs 24–72 h after long bone or pelvic fractures. The classic triad of symptoms of FES includes respiratory distress, neurologic changes, and petechial rash [1]. Although there is a lack of standardization of diagnostic criteria, Gurd and Wilson’s [2] and Schonfeld’s criteria [3] are useful.
Treatment is primarily supportive care, with the goals of maintaining oxygenation and ventilation, supporting hemodynamics, and resuscitating with fluids and blood products. In most cases, the symptoms are transient and fully reversible, often within a few days, although they may persist beyond one week when FES is severe [4]. The mortality rate is 5–15% [5, 6].
Although Gurd’s criteria for FES include anuria or oliguria, the incidence and mechanism of acute kidney injury (AKI) remain unknown, and there are few reports of FES complicated by AKI. Herein, we present a case of FES complicated by AKI that required blood purification and review related articles.
Case presentation
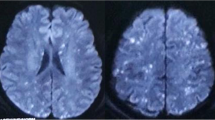
A 79-year-old woman was admitted to our hospital following a traffic accident. She had a history of hypertension and dyslipidemia and had undergone cardiac bypass surgery seven years earlier. The patient was treated with aspirin 100 mg/day, esomeprazole magnesium 20 mg/day, ezetimibe 10 mg/day, carvedilol 5 mg/day, furosemide 20 mg/day, and amlodipine besilate 5 mg/day. The baseline creatinine was 0.81 mg/dL, body temperature was 36.4℃, blood pressure was 118/65 mmHg, heart rate was 118 beats per minute, respiratory rate was 18 breaths per minute, SpO2 was 96% (room air), and consciousness was clear. Quick SOFA score was 0 points on admission. Radiographic images confirmed a right humerus and pelvic fracture. On the second day of hospitalization, she developed impaired consciousness and respiratory failure. Chest radiography revealed bilateral diffuse alveolar infiltrates which were absent on the first day of hospitalization (Fig. 1). Brain magnetic resonance imaging revealed diffuse high signal intensity on diffusion-weighted imaging (Fig. 2) and diffuse low signal intensity on susceptibility-weighted imaging in the cerebral and cerebellar regions (Fig. 3). FES was diagnosed according to Gurd and Wilson and Schonfeld’s criteria. Intravenous methylprednisolone (40 mg/day) and ulinastatin (300,000 units/day) were administered. Despite the administration of 3 L of fluid over the first 2 days of hospitalization, oliguria continued. On the third day of hospitalization, the patient was admitted to our department because of AKI with oliguria. Her body temperature was 36.6 °C, blood pressure was 113/60 mmHg, and heart rate was 86 beats per minute. The Glasgow Coma Scale score was 13/15(E3V4M6). Pulse oximetry showed 95% oxygen saturation when five liter per minutes oxygen was administered with mask. On physical examination, she had anemia in the eyelid conjunctiva and bilateral lower extremity edema. The auscultation of the lungs revealed no rales or murmurs, and no hemorrhagic spots were observed. She had cognitive dysfunction but no other neurological abnormalities, such as hemiplegia were evident. She did not have any symptoms of collagen disease, such as joint inflammation or skin rash. Laboratory tests showed a marked increase in D-dimer and a rapid decrease in platelets, together with leukocytosis, anemia, and elevation of liver enzymes and LDH (Table 1). Along with a clear cause of DIC, she was diagnosed with disseminated intravascular coagulation (DIC) according to the DIC diagnostic criteria of Japanese Ministry of Health, Labor and Welfare (scoring 7 points), and the International Society on Thrombosis and Haemostasis (scoring 5 points). However, she also had worsening anemia and a marked increase in LDH levels, and no haptoglobin was detected, raising suspicion of TMA. Blood sampling on the 5th day of hospitalization showed a PLASMIC score of 4 points, suggesting no decrease in ADAMTS-13 [7]. No enteritis symptoms were observed, so Shiga toxin-producing Escherichia coli associated hemolytic uremic syndrome (STEC-HUS) was ruled out. Neither collagen diseases, infections, malignant tumors, nor other causes were found, and secondary TMA was also ruled out. However, C3 was mildly decreased to 69 mg/dL in the blood test on the 6th day of hospitalization, and it was difficult to rule out atypical HUS (aHUS). On the 9th day of hospitalization, a small amount of fragmented red blood cells (2.4%) was observed, and the level of ADAMTS-13 activity was maintained at 62%, ruling out TTP. On the 12th day of hospitalization, the urine test showed 2 + proteinuria and 3 + hematuria, but the urine sediment had only 1–4 RBCs/HPF. This indicated hemoglobinuria rather than hematuria. Additionally, we did not find any fat droplets in the urine. Considering all these results, it was thought that she had a TMA condition comparable to aHUS. Electrocardiogram findings were normal. Chest radiography demonstrated bilateral diffuse alveolar infiltrates. Contrast-enhanced computed tomography (CT) revealed bilateral gravity-dependent distribution and ground-glass opacities (Fig. 4). There were no findings of pulmonary artery emboli or hydronephrosis, and the kidney size was normal. Based on chest imaging findings, pulmonary edema was considered, and hemodialysis (HD) and ultrafiltration were initiated at a fluid removal rate of 900 mL (22.5 mL/kg) per hour. However, she developed hypovolemic shock after 40 min, and the treatment was switched to continuous hemodiafiltration (CHDF) and ultrafiltration at a fluid removal rate of 100 mL per hour. Echocardiography revealed that the right ventricle compressed the left ventricle, and the tricuspid valve systolic pressure gradient was elevated at 44.5 mmHg. Through supportive therapy with oxygenation, fluids, and blood products, the patient’s consciousness and hypoxemia completely improved. Thereafter, the renal function was restored and DIC was resolved. The PH disappeared on the 16th day of hospitalization. She was weaned from blood purification therapy on the 29th day of hospitalization and transferred to another hospital for rehabilitation on the 51st day of hospitalization (Fig. 5).
Discussion and conclusions
We described a case of FES complicated by AKI requiring blood purification. Although Gurd’s criteria include anuria or oliguria, the incidence and mechanism of AKI secondary to FES remain unknown. In a single-case meta-analysis, renal dysfunction was reported in 1.5% (2/139) of patients [8]. Autopsy results indicative of fat emboli in the kidneys have been reported [9,10,11,12]; however, the association between renal fat embolism and renal failure was not been proven [9, 13].
Bone marrow fat possess prothrombotic features, and in circulation, it is quickly covered with platelets and fibrin and initiates the coagulation cascade, leading to thrombocytopenia and DIC [1, 13]. Some reports suggested that fat embolism may precipitate DIC and cause AKI [12]. Therefore, in our case, DIC due to FES might be considered as the cause of AKI. On the other hand, in this case, hemolytic anemia was prominent, and TMA was considered as a differential diagnosis of thrombocytopenia and organ damage. There were no gastrointestinal symptoms suggestive of STEC-HUS, and TTP was negative based on ADAMTS13 activity and anti-ADAMTS13 antibody levels. We excluded other causes of secondary TMA, but we could not rule out aHUS with certainty. Based on the clinical course, we thought that she did not have aHUS with complement-related gene mutations, because her hemolytic anemia and renal impairment resolved without plasma exchange or complement inhibitor treatment as her FES improved. One way to distinguish DIC from TMA is that DIC usually has a significant rise in fibrin related markers (FRMs; such as soluble fibrin monomers, fibrinogen, fibrin degradation products [FDPs], D dimers, etc.) [14]. DIC patients often have schistocytes, but usually at a low percentage, within or near the normal range (< 0.5%). Measuring schistocytes is not a key test for the initial diagnosis of DIC, but it might be clinically useful to indicate a coexisting or underlying TMA if ≥ 1%. [15]. As our case had both DIC and TMA features, it is often difficult to differentiate DIC and TMA based on biomarkers alone. Actually, many TMA patients are diagnosed with DIC, and 15% of DIC patients are also diagnosed with TMA [14]. It is suggested that this difference of clinical findings between DIC and TMA is due to the fact that TMA causes thrombotic occlusion in the arterial side of the capillaries, while DIC causes it in the venous side [14], so lipid droplets caused by FES may induce thrombosis in both the arterial and venous sides of the capillaries, resulting in DIC with features of TMA, which may be a major factor leading to AKI. Actually, several reports have described cases of FES mimicking TMA in patients with hemoglobinopathies [16, 17]. Another report describes hemolytic anemia and thrombocytopenia in FES caused by a fracture [18].
In this case, hemodynamic changes might be an additional cause of AKI, along with DIC. The patient presented with tachycardia and possible pre-renal AKI at admission. However, without a urine test, we could not confirm this diagnosis. Her blood pressure was stable, which excluded acute tubular necrosis from low blood flow. Regarding the hemodynamic changes, another cause of AKI might be renal congestion due to pulmonary fat embolism. Dadfarmay et al. [19] reported a case of AKI with renal congestion due to acute PH triggered by pulmonary embolism. In this case, AKI improved following initiation of ultrafiltration. Similarly, in our case, PH due to pulmonary fat embolism might have led to renal congestion and improve following ultrafiltration.
To explore the link between AKI and renal congestion in FES, we reviewed the literature on FES cases with AKI that needed renal replacement therapy. We searched medical databases, such as PubMed and Google Scholar, and only found three previous case reports (Table 2) [20,21,22]. Jemima et al. reported a case of renal transplant recipient who developed AKI with oliguria after surgical stabilization of the acetabulum and replacement of the proximal femur. In this report, a large fat embolus in the inferior vena cava (IVC) and left iliac veins below the level of the IVC filter impaired the renal perfusion, which could cause renal congestion. The patient was managed with CHDF and systemic anticoagulation. The urine output started to increase, and CHDF was discontinued 3 days later. In other two reports, AKI occurred after FES due to fracture of long bone [21, 22]. Although the cause of AKI was not described, one of them complicated PH, which might lead to AKI [21]. Cleveland et al. [22] reported the successful use of peritoneal dialysis (PD) for a patient with AKI with anuria due to FES after a fracture of the right femur. As urinary output increased, PD was discontinued 9 days later. In all cases, the patient eventually had recovered renal function. Similar to these reports, our patient recovered from AKI and blood purification therapy was discontinued. Therefore, AKI requiring renal replacement therapy due to FES can improve after the acute phase as well as respiratory distress and neurological changes.
Our patient was initially suspected of having pulmonary congestion, and hemodialysis and rapid ultrafiltration were initiated accordingly. Thereafter, she developed hypovolemic shock. Chest radiography in severe FES shows diffuse bilateral patchy infiltrates [23] and may be indistinguishable from that in pulmonary congestion [24]. Echocardiographic confirmation of PH may be important in differentiating between RHF due to pulmonary involvement in FES and pulmonary congestion (left heart failure). The mechanism of PH is thought to be directly due to rapid occlusion of the pulmonary artery by the fat emboli themselves and indirectly due to reactive vasoconstriction caused by hypoxia [25]. Management of RHF in the setting of FES remains a challenge; Either increase of contractility through preload optimization and inotropes, or decrease of afterload is important [24]. When blood purification therapy for FES is performed, clinicians should differentiate between pulmonary involvement and pulmonary congestion due to fat embolism and determine dialysis conditions with attention to hemodynamics.
This case and literature review had some limitations. First, we did not perform a renal biopsy, which would have clarified the pathophysiology. This was because the patient needed bed rest after pelvic fracture and was taking aspirin for angina pectoris. Second, we did not evaluate renal congestion adequately. We inferred renal congestion from pulmonary hypertension, but we should have used clinical methods to detect it. Recently, ultrasound using intrarenal venous flow (IRVF) [26] or VeXUS (venous excess ultrasound) grading system [27] has been reported to be effective for evaluating renal congestion.
In conclusions, FES can be complicated by AKI requiring blood purification. DIC which was difficult to differentiate from TMA and/or renal congestion due to RHF caused by FES might be the cause of AKI. AKI due to FES can improve after the acute phase, as well as respiratory distress and neurological changes. Clinicians should pay attention to the hemodynamics during blood purification for patients with AKI due to FES.
Abbreviations
- FES:
-
Fat embolism syndrome
- AKI:
-
Acute kidney injury
- DIC:
-
Disseminated intravascular coagulation
- TMA:
-
Thrombotic microangiopathy
- aHUS:
-
Atypical hemolytic uremic syndrome
- PH:
-
Pulmonary hypertension
- RHF:
-
Right heart failure
- CRP:
-
C-reactive protein
- CT:
-
Computed tomography
- HD:
-
Hemodialysis
- CHDF:
-
Continuous hemodiafiltration
- CRRT:
-
Continuous renal replacement therapy
- PD:
-
Peritoneal dialysis
References
Rothberg DL, Makarewich CA. Fat embolism and fat embolism syndrome. J Am Acad Orthop Surg. 2019;27:e346–55. https://doi.org/10.5435/JAAOS-D-17-00571.
Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Jt Surg Br. 1974;56B:408–16. https://doi.org/10.1302/0301-620X.56B3.408.
Schonfeld SA, Ploysongsang Y, DiLisio R, Crissman JD, Miller E, Hammerschmidt DE, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99:438–43. https://doi.org/10.7326/0003-4819-99-4-438.
Sethi D, Kajal S, Saxena A. Neuroimaging findings in a case of cerebral fat embolism syndrome with delayed recovery. Indian J Crit Care Med. 2015;19:674–7. https://doi.org/10.4103/0972-5229.169350.
Kwiatt ME, Seamon MJ. Fat embolism syndrome. Int J Crit Illn Inj Sci. 2013;3:64–8. https://doi.org/10.4103/2229-5151.109426.
Shaikh N. Emergency management of fat embolism syndrome. J Emerg Trauma Shock. 2009;2:29–33. https://doi.org/10.4103/0974-2700.44680.
Bendapudi PK, Hurwitz S, Fry A, Marques MB, Waldo SW, Li A, et al. Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. Lancet Haematol. 2017;4:e157–64. https://doi.org/10.1016/S2352-3026(17)30026-1.
He Z, Shi Z, Li C, Ni L, Sun Y, Arioli F, et al. Single-case metanalysis of fat embolism syndrome. Int J Cardiol. 2021;345:111–7. https://doi.org/10.1016/j.ijcard.2021.10.151.
Emson HE. Fat embolism studied in 100 patients dying after injury. J Clin Pathol. 1958;11:28–35. https://doi.org/10.1136/jcp.11.1.28.
Eriksson EA, Rickey J, Leon SM, Minshall CT, Fakhry SM, Schandl CA. Fat embolism in pediatric patients: an autopsy evaluation of incidence and etiology. J Crit Care. 2015;30(221):e1-5. https://doi.org/10.1016/j.jcrc.2014.09.008.
Milroy CM, Parai JL. Fat embolism, fat embolism syndrome and the autopsy. Acad Forensic Pathol. 2019;9:136–54. https://doi.org/10.1177/1925362119896351.
Uldall PR, Kerr DN. Post-traumatic acute renal failure. Br J Anaesth. 1972;44:283–90. https://doi.org/10.1093/bja/44.3.283.
Husebye EE, Lyberg T, Røise O. Bone marrow fat in the circulation: clinical entities and pathophysiological mechanisms. Injury. 2006;37(Suppl 4):S8-18. https://doi.org/10.1016/j.injury.2006.08.036.
Wada H, Matsumoto T, Suzuki K, Imai H, Katayama N, Iba T, et al. Differences and similarities between disseminated intravascular coagulation and thrombotic microangiopathy. Thromb J. 2018;16:14. https://doi.org/10.1186/s12959-018-0168-2.
Lesesve JF, Martin M, Banasiak C, André-Kerneïs E, Bardet V, Lusina D, et al. Schistocytes in disseminated intravascular coagulation. Int J Lab Hematol. 2014;36:439–43. https://doi.org/10.1111/ijlh.12168.
Kammeyer R, Devnani R, Mehta R. Cerebral fat embolism syndrome mimicking thrombotic thrombocytopenic purpura in a patient with hemoglobin SC disease. Am J Hematol. 2016;91:539–42. https://doi.org/10.1002/ajh.24286.
Gangaraju R, May JE, Williams LA 3rd, Reddy VB, MacLennan P, Marques MB. Fat embolism syndrome due to bone marrow necrosis in patients with hemoglobinopathies: a life-threatening complication mimicking thrombotic thrombocytopenic purpura. Am J Hematol. 2019;94:E64–6. https://doi.org/10.1002/ajh.25363.
Salimi Z, Ami Ali M, Tazi R, Mimouni Y, Hazim A, Aasfara J. A rare case of cerebral fat embolism with no respiratory or dermatologic involvement. Cureus. 2022;14:e22192. https://doi.org/10.7759/cureus.22192.
Dadfarmay S, Wahba IM. Acute kidney injury due to pulmonary embolism: the case for ‘congestive renal failure.’ NDT Plus. 2011;4:295–8. https://doi.org/10.1093/ndtplus/sfr093.
Scott J, Collin N, Baker R, Ravanan R. Fat embolism: a rare cause of perioperative renal transplant dysfunction. BMJ Case Rep. 2017;2017:bcr2017221829. https://doi.org/10.1136/bcr-2017-221829.
Rassam S, Mathieson E, Gemmell L. Atrial septal defect: an important risk factor after trauma. Am J Emerg Med. 2005;23:223–4. https://doi.org/10.1016/j.ajem.2004.04.033.
Cleveland JC, Spitzer S. Renal shutdown secondary to fat embolism. Am J Surg. 1967;114:454–6. https://doi.org/10.1016/0002-9610(67)90172-9.
Schwalbach KT, Wade RC, Mkorombindo T, McElwee SK, Wells JM, Wille KM. Supportive care of right ventricular failure due to fat embolism syndrome. Respir Med Case Rep. 2021;34: 101499. https://doi.org/10.1016/j.rmcr.2021.101499.
Newbigin K, Souza CA, Torres C, Marchiori E, Gupta A, Inacio J, et al. Fat embolism syndrome: state-of-the-art review focused on pulmonary imaging findings. Respr Med. 2016;113:93–100. https://doi.org/10.1016/j.rmed.2016.01.018.
Fracasso T, Karger B, Pfeiffer H, Sauerland C, Schmeling A. Immunohistochemical identification of prevalent right ventricular ischemia causing right heart failure in cases of pulmonary fat embolism. Int J Legal Med. 2010;124:537–42. https://doi.org/10.1007/s00414-009-0382-3.
Iida N, Seo Y, Sai S, Machino-Ohtsuka T, Yamamoto M, Ishizu T, et al. Clinical implications of intrarenal hemodynamic evaluation by Doppler ultrasonography in heart failure. JACC Heart Fail. 2016;4:674–82. https://doi.org/10.1016/j.jchf.2016.03.016.
Beaubien-Souligny W, Rola P, Haycock K, Bouchard J, Lamarche Y, Spiegel R, et al. Quantifying systemic congestion with point-of-care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J. 2020;12:16. https://doi.org/10.1186/s13089-020-00163-w.
Acknowledgements
We thank the dialysis staff at Ishikawa Central Prefectural Hospital for providing invaluable technical assistance and dedicated patient care.
Funding
There was no funding source for this report.
Author information
Authors and Affiliations
Contributions
TS acquired and analyzed the data, and drafted the manuscript. HF interpreted the data and revised the manuscript. KA and MH revised the manuscript critically for important intellectual content. HF, KA, MH, RN, TK, YM, AN, and MK contributed to writing the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data supporting the findings of this report are available from the corresponding author, H.F., upon reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Suda, T., Fujii, H., Asakura, K. et al. Fat embolism syndrome after humerus and pelvis fracture complicated by acute kidney injury requiring blood purification: a case report and literature review. Ren Replace Ther 9, 51 (2023). https://doi.org/10.1186/s41100-023-00504-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-023-00504-0
Keywords
- Fat embolism syndrome
- Acute kidney injury
- Hemodialysis
- Continuous hemodiafiltration
- Continuous renal replacement therapy
- Pulmonary hypertension
- Right heart failure
- Susceptibility-weighted imaging
- Steroid
- Methylprednisolone
- Hemolytic anemia
- Thrombotic microangiopathy
- Disseminated intravascular coagulation
- Hemolytic uremic syndrome