Abstract
Background
Hepatitis C virus (HCV) infection is linked to a higher mortality rate in hemodialysis (HD) patients. We aimed to see if HCV eradication using interferon-free direct acting antivirals (DAAs) can affect bone-mineral and anemia biochemical parameters such as serum calcium (Ca++), phosphorus (PO4+), parathormone (PTH), fibroblast growth factor 23 (FGF23), hemoglobin (HB), and ferritin in HD patients and also peripheral insulin resistance by monitoring serum fasting insulin and HOMA insulin resistance (HOMA-IR).
Methods
Three hundred and thirty-four adults on regular HD with positive HCV genotype 4 (191 male and 143 female) were included; 157 of them had seroconversion during HD. All were hepatitis B virus (HBV) negative and received treatment with DAAs. All cases were examined for body mass index (BMI), HB, ferritin level, transferrin saturation (TSAT), Ca++, PO4+, PTH, FGF23, serum albumin, alanine transaminase (ALT), fasting insulin level, and HOMA-IR at the beginning and then were measured after 6 and 12 months from a sustained virological response (SVR).
Results
After 6 and 12 months from SVR, there was a significant increase in serum Ca despite no change in oral calcium dose requirement over that period (p = 0001), a significant increase in HB, serum iron (p = 0001), and a significant reduction in serum ferritin, PO4, PTH, and FGF23 (p = 0001). Both fasting insulin level and HOMA-IR were statistically significantly dropped.
Conclusion
HCV eradication with interferon-free DAAs showed a statistically significant impact on hemodialysis patients regarding hemoglobin, ferritin level, bone-mineral parameters, and improvement in peripheral insulin resistance.
Similar content being viewed by others
Introduction
Hepatitis C is a major public health issue that affects people all over the world. It causes chronic hepatitis, cirrhosis, and hepatocellular cancer. Individuals who have had blood transfusions or who have had invasive medical treatments such as hemodialysis have been thought to be at greater risk for hepatitis C [1, 2].
In 2015, Egypt has one of the highest HCV infection rates in the world, at 7% among adults, accounting for 7.6% of the country's mortality. Egypt began an aggressive screening and treatment campaign in 2014, which has since expanded into a nationwide effort to eradicate HCV as a public health issue by 2021 [3].
In HD patients receiving maintenance dialysis in affluent nations, the incidence of HCV infection ranges from 5 to 60%; however, the prevalence of HCV infection is substantially higher in patients receiving dialysis in developing countries [4]. According to the Dialysis Outcomes and Practice Patterns Study (DOPPS), the annual incidence of HCV seroconversion is 1.2 percent, signifying that at least 20,000 cases of HCV are acquired each year in HD units around the world [5].
HCV was shown to be prevalent in 34.8 percent of hemodialysis patients in a recent study conducted in Egypt. The rate of seroconversion was 13.2 percent. Positive blood transfusion history and frequency, as well as medical staff handling of equipment and blood products and the number of inserted temporary dialysis catheters, were discovered to be major risk factors for seroconversion [6].
DAAs are highly effective and safe. Chronic kidney disease (CKD) stages 4–5, including dialysis patients and kidney transplant recipients, can be cured of HCV. HCV genotype, viral load, estimated glomerular filtration rate (e-GFR), concomitant medications, comorbidities, and transplantation candidacy should all be taken into account when selecting a DAAs regimen [7]. The approval of an oral IFN-free, direct acting antiviral (DAA) regimen for hemodialysis patients in Egypt in 2014 heralded a paradigm shift in hepatitis C treatment.
The majority of hemodialysis patients are on subcutaneous (SC) erythropoietin and intravenous (IV) iron for anemia management guided by hemoglobin level, serum ferritin, and transferrin saturation (TSAT). Also, they are on oral calcium replacement and phosphate binders for control of bone-mineral disease (BMD).
The effect of HCV eradication in hemodialysis patients on BMD biochemical parameters such as serum Ca++, PO4+, PTH, and FGF23 had not been previously investigated.
Aim of the study
The study aimed to assess the impact of HCV eradication in a large cohort of hemodialysis patients using DAAs on BMD biochemical parameters such as Ca++, PO4+, PTH, FGF23, and anemia parameters such as HB, ferritin, iron, and TSAT. Additionally, if HCV eradication affects peripheral insulin resistance by evaluating blood fasting inulin and HOMA-IR, SC erythropoietin and IV iron requirements among hemodialysis patients.
Methods and materials
This study was conducted between October 2019 and April 2021. Three hundred and thirty-four seropositive C patients genotype 4 were compliant with regular chronic hemodialysis for at least a year with adequate hemodialysis measured by KT/V to confirm the resolution of any deleterious effects of uremia on hematopoietic function. They were recruited from Cairo University Hospitals, New Giza community hospital, and other two large hospitals in Cairo. The patients were infected with HCV either before starting hemodialysis or became seroconversion after the start of dialysis and were diagnosed by the presence of HCV antibodies using enzyme-linked immunosorbent assay (ELISA) and confirmed by the presence of HCV-RNA tested by real-time reverse transcription-PCR (RT-PCR), and all of them were negative for hepatitis B virus ( HBV).
Exclusion criteria included hemodialysis patients who were not compliant with hemodialysis for 6 months with more than three missed dialysis sessions per month, patients with a history of recurrent hospitalization, major surgeries, decompensated liver cirrhosis, episodes of active GI bleeding, access clotting, bacteremia or chronic infection, pregnancy, bone marrow disorders (including multiple myeloma), and the presence of mass or multiple cysts on renal ultrasound.
All of the patients were subjected to full medical history and clinical examination. Blood samples were drawn, and the following biochemical parameters testing were measured before starting HCV treatment: serum albumin level, serum alanine aminotransferase (ALT) level, complete blood picture (CBC), serum ferritin, corrected serum calcium, serum phosphorus, intact PTH, and FGF23. Also, we measured fasting insulin level and calculated homeostatic model assessment (HOMA-IR) based on fasting insulin level to assess insulin resistance (IR) associated with HCV, which was calculated as fasting glucose (mmol/L) × fasting insulin (mU/mL)/22.5 [8]. All these biochemical parameters were measured using the AU system with reagents (Beckman Coulter, Brea, CA, USA). A two-site (NH2-terminal/C-terminal) enzyme-linked immunosorbent test (Immutopics, San Clemente, CA, USA) was used to evaluate FGF23 in the blood, and following the manufacturer's recommendations, samples were taken in the morning following a 12-h fast.
At the beginning of the study, our patients were on subcutaneous Epoetin alpha 4000 IU and IV iron sucrose either weekly, twice per week, or thrice weekly based on hemoglobin level. Also, they were on oral calcium carbonate replacement range between 800 and 1200 mg guided by serum calcium level and PTH. They were not on any phosphate binder as it was not available at the time of the study.
All the patients had received DAAs according to the National program in Egypt for treatment of HCV using Qurevo (Ritonavir 50 mg, Paritaprevir 75 mg, and Ombitasvir 12.5 mg) for 12 weeks, and they were followed up to 12 months after they had a sustained virological response (SVR). HCV by real-time reverse transcription-PCR (RT-PCR) was done 6 months and 12 months after HCV course treatment for SVR.
After the hemodialysis patients had a sustained virological response, biochemical parameters such as hemoglobin, serum ferritin, serum calcium, and serum phosphorus were monitored monthly for a total of 12 months. Intact PTH was assessed every three months. Fasting insulin level, calculated HOMA-IR, and FGF23 were measured again after 12 months. The requirements of SC erythropoietin (EPO-Alpha, 4000 IU) and intravenous (IV) iron sucrose doses were reported as average weekly doses during these 6 and 12 months of SVR.
Our primary outcome was to find the impact of treatment of HCV hemodialysis patients on levels of hemoglobin, serum Ca++, serum PO4+, transferrin saturation ratio (TSAT), and ferritin. Also, the effect of HCV eradication on the requirement of weekly doses of Epoetin alpha and IV iron sucrose. The secondary outcome was to find the effect of treatment of HCV hemodialysis patients on PTH, FGF23, fasting insulin level, and HOMA-IR.
Statistical analysis
SPSS (Statistical Package for Social Science) version 26 was used for all statistical analyses. The data were presented as a mean ± standard deviation. Student t test and Chi-squared tests were performed to compare the two groups for non-numerical data. The Pearson correlation test was used to determine the correlation between variables. Repeated measured ANOVA was conducted to assess changes in biochemical parameters over time. Significant was defined as a p value of less than 0.05. Multiple regression analyses were performed to describe if there is any direct effect of multiple explanatory variables on the outcome.
Results
Results are summarized in Tables 1, 2, 3, 4, and 5. Three hundred and thirty-four patients had a confirmed diagnosis of HCV on regular HD for more than 12 months with a mean of 34.07 ± 17.41. Overall, mean age was 41.69 ± 9.67, and 57.2% were male. The underlying causes of the end-stage renal disease in our study were diabetic nephropathy (19.2%), hypertension (11.4%), chronic GN (4.2%), gouty nephropathy (12.6%), chronic interstitial disease from analgesic and herbs (7.2%), obstructed uropathy (22.8%), and hereditary (3.6%), and the remaining (19.2%) had an unknown cause. 53% of patients had HCV before initiation of hemodialysis, and the rest had developed seroconversion during dialysis. Table 1 shows patients' demographic and laboratory data at the beginning of the study.
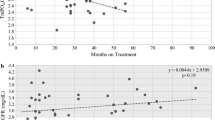
Repeated measured ANOVA tests were conducted and revealed that there was a statistically significant increase in HB, serum Ca, and TSAT over six and twelve months from HCV eradication with SVR (p value < 0.001) despite no change in oral calcium carbonate doses from starting of HCV treatment and during 12-month follow-up from SVR as shown in Table 2. There was a significant reduction in weekly IV iron sucrose and subcutaneous erythropoietin-alpha requirements for hemodialysis patients over 6 months and 12 months from HCV eradication with SVR as shown in Table 3.
There was a statistically significant decrease in both serum PO4 and PTH after twelve months from SVR (p value < 0.001) as shown in Table 2. A paired T-test was conducted and revealed that there is a statistically significant decrease in fasting insulin level, HOMA-IR, and FGF23 after twelve months from SVR (p value < 0.001) as shown in Table 2.
To detect percent changes, a mean difference change between biochemical markers serum HB concentration, ferritin, corrected calcium, phosphorus, PTH, FGF23, fasting insulin level, and HOMA-IR was calculated at baseline before starting HCV treatment and after 12 months from SVR, as shown in Fig. 1.
Multiple regression analyses were used to determine if serum iron, ferritin, and TSAT would positively predict HB level at 6 months and 12 months, and the result showed a non-significant effect (p value = 0.599, F = 0. 691 and R2 = 0.008) and HOMA-IR percent change were not predicted by percent changes in serum iron, ferritin, PTH, calcium, and PO4 (p value = 0.329, F = 1.160 and R2 = 0.017) as shown in Table 4.
Also, multiple regression analyses showed that serum calcium and PO4 at 6 and 12 months had a non-significant effect on change in serum PTH at 6 and 12 months (p value = 0.184, F = 1.564, and R2 = 0.019) as shown in Table 5.
Multiple regression analyses were used to determine if percent change in serum calcium, PO4, and PTH will positively predict change in FGF23 after 12 months from SVR, and the result showed a non-significant effect (p value = 0.852, F = 0.236, and R2 = 0.002) (Table 5).
Discussion
HCV infection is quite common in maintenance of hemodialysis patients, and DAAs have shown efficacy and safety in these patients. The effect of HCV on hemoglobin levels was previously investigated, and it was linked to increased endogenous erythropoietin synthesis due to chronic hepatic inflammation [9,10,11]. However, the exact location of EPO production in the liver is unknown. In dialysis patients with HCV, Sahin et al. discovered a decreased need for IV iron [12]. Chronic inflammation caused by HCV infection or enhanced production from regenerated liver cells results in a rise in circulating EPO, which leads to an improvement in hematocrit in these patients [10].
Our cohort analysis found that treating seropositive HCV hemodialysis patients with a sustained virological response resulted in a significant increase in hemoglobin, serum iron, and TSAT levels throughout the trial and were linked to a considerable reduction in the weekly EPO-alpha and IV iron sucrose dosage requirements.
Ferritin is an acute-phase reactant generated from the liver in response to hepatic inflammation; hence, individuals with HCV have higher ferritin levels than non-HCV patients [13]. During the follow-up period after the patients had achieved SVR, our study found that serum ferritin had decreased statistically significantly.
HCV-positive patients are more likely to develop severe arterial calcification, according to Fayed et al. This could be due to an increase in intact PTH, Ca X P product [14]. In this study, we discovered that eradicating HCV with DAA significantly impacted serum PO4, intact PTH, and FGF23, all of which were dramatically reduced. Also, despite no change in oral calcium over the research period, serum calcium increased significantly, implying that HCV eradication had a major influence on CKD-MBD. Chronic HCV infection can lead to a heightened immune reaction and activation leading to a chronic inflammatory state that can affect several systems [15], and HCV treatment with DAAs showed normalization of regulatory T-cell and other specific T- and B-cell populations [16]. Activated T cells and B cells secrete RANKL and other inflammatory cytokines such as TNFα and IL-17A that promote RANK expression on monocytes increasing the number of osteoclast precursors and thus of RANKL responsive cells capable of differentiating into osteoclasts [17], which enhance bone resorption and release excess phosphorus into the blood. As known, hyperphosphatemia complexes serum calcium, decreasing the levels of ionized calcium and triggering the release of PTH, resulting in secondary hyperparathyroidism in CKD and ESRD. Also, FGF23 serum levels are reported to increase in CLD patients [18]. This may explain why increased PTH levels are negatively associated with secondary osteoporosis in these patients [19]. All the above-mentioned can speculate why HCV eradication using DAAs in hemodialysis patients improved bone-mineral parameters, including a dramatic decrease in serum phosphorus, intact PTH, and FGF23 with a significant increase in serum calcium.
Insulin resistance is linked to a variety of HCV-related problems. Hepatic fibrosis, steatosis, hepatocellular carcinoma, and antiviral therapy resistance can all be caused by HCV-related insulin resistance. Insulin resistance caused by HCV infection is thus a therapeutic target at any stage of infection. HCV interacts with the insulin signaling system by modulating normal cellular gene expression [20]. HCV infection is linked to a high prevalence of insulin resistance, as well as increased insulin and glucose levels in chronic hemodialysis patients [21]. The eradication of HCV in hemodialysis patients reduced peripheral insulin resistance as measured by a considerable drop in fasting insulin levels and HOMA-IR, according to our findings. Using a combination of sofosbuvir/ledipasvir and ribavirin for 12 weeks, Giacomo et al. investigated the effect of treating HCV patients with DAA on insulin resistance in non-diabetic patients who were not on dialysis and found an improvement in peripheral insulin sensitivity, but no difference in endogenous glucose production, lipolysis suppression, or substrate oxidation (22).
Conclusion
Eradication of HCV in hemodialysis patients with interferon-free direct acting antivirals showed a statistically significant impact on hemoglobin, ferritin level, bone-mineral parameters, FGF23, and improvement on peripheral insulin resistance and also reduction in subcutaneous Erythropoietin and IV iron requirements for correction of anemia in hemodialysis patients which may have an impact on national income.
Data availability
All relevant data are within the manuscript and the supporting information files.
Abbreviations
- ALT:
-
Alanine transaminase
- BMD:
-
Bone-mineral disease
- CBC:
-
Complete blood cell count
- Ca++ :
-
Serum calcium
- CLD:
-
Chronic liver disease
- DAA:
-
Direct acting antiviral
- EPO:
-
Erythropoietin
- FGF23:
-
Fibroblast growth factor 23
- HB:
-
Hemoglobin
- HCV:
-
Hepatitis C virus
- HD:
-
Hemodialysis
- HOMA-IR:
-
Homeostatic model assessment-Insulin resistance
- IV:
-
Intravenous
- PTH:
-
Parathormone hormone
- PO4+ :
-
Serum phosphorus
- RANKL:
-
Receptor activator of nuclear factor kappaB ligand
- SVR:
-
Sustained virological response
- TSAT:
-
Transferrin saturation ratio
References
Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47(19):1–39.
Lunel F, Musset L. Hepatitis C virus infection and cryoglobulinemia. J Hepatol. 1998;29(5):848–55.
Hassanin A, Kamel S, Waked I, Fort M. Egypt’s ambitious strategy to eliminate hepatitis C virus: a case study. Glob Health Sci Pract. 2021;9(1):187–200.
Ozer Etik D, Ocal S, Boyacioglu AS. Hepatitis C infection in hemodialysis patients: a review. World J Hepatol. 2015;7(6):885–95.
Jadoul M. The prevention of hepatitis C virus transmission to hemodialysis patients and staff members. Hemodial Int. 2018;22(Suppl 1):S104–9.
Kerollos KM, El-Ameen HA, El Wahed LA, Azoz NM. Prevalence and seroconversion of hepatitis C among hemodialysis patients in Assiut governorate, Egypt. Egypt J Intern Med. 2020;32(2):1–6.
Saadi G, Kalantar-Zadeh K, Almasio P, Ashuntantang G, Barsoum R, Bruchfeld A, et al. Hepatitis C virus infection and global kidney health: the consensus proceedings of the International Federation of Kidney Foundations. Afr J Nephrol. 2020;23(1):159–68.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Boubaker K, Mahfoudhi M, Battikh A, Bounemra A, Maktouf C, Kheder A. Higher endogenous erythropoietin levels in hemodialysis patients with hepatitis C virus infection and effect on anemia. Open J Nephrol. 2015;5(2):29–34.
Ramadan A, Abouellail H, Shawky S, Abdelraouf S. Does hepatitis C affect hematological parameters, CO morbidity, and functional status in hemodialysis patients? J Med Sci Clin Res. 2016;4(6):11042–51.
Simon P, Meyrier A, Tanquerel T, Ang KS. Improvement of anemia in haemodialysed patients after viral or toxic hepatic cytolysis. Br Med J. 1980;280(6218):892–4.
Sahin I, Arabaci F, Sahin HA, Ilhan M, Ustun Y, Mercan R, Eminov L. Does hepatitis C virus infection increase hematocrit and hemoglobin levels in hemodialyzed patients? Clin Nephrol. 2003;60(6):401–4.
Shan Y, Lambrecht RW, Bonkovsky HL. Association of hepatitis C virus infection with serum iron status: analysis of data from the third National Health and Nutrition Examination Survey. Clin Infect Dis. 2005;40(6):834–41.
Fayed A, Soliman A, El Mahdy H, Hamza W, Abdulazim DO, Salem MM, Sharaf El Din UA, Vascular Calcification Group (VCG). Impact of hepatitis virus infection on arterial calcification among incident hemodialysis patients. Nefrologia (Engl Ed). 2020;40(3):336–44.
Kuller LH, Tracy R, Belloso W, Wit SD, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203.
Emery JS, Kuczynski M, La D, Almarzooqi S, Kowgier M, Shah H, et al. Efficacy and safety of direct-acting antivirals for the treatment of mixed cryoglobulinemia. Am Gastroenterol. 2017;112:1298–308.
Adamopoulos IE, Chao CC, Geissler R, Laface D, Blumenschein W, Iwakura Y, et al. Interleukin-17A upregulates receptor activator of NF-kappaB on osteoclast precursors. Arthritis Res Ther. 2010;12(1):R29.
He X, Shen Y, Ma X, Ying L, Peng J, Pan X, Bao Y, Zhou J. The association of serum FGF23 and non-alcoholic fatty liver disease is independent of vitamin D in type 2 diabetes patients. Clin Exp Pharmacol Physiol. 2018;45(7):668–74.
Bihari C, Lal D, Thakur M, Sukriti S, Mathur D, Patil AG, et al. Suboptimal level of bone-forming cells in advanced cirrhosis are associated with hepatic osteodystrophy. Hepatol Commun. 2018;2(9):1095–110.
Bose SK, Ray R. Hepatitis C virus infection and insulin resistance. World J Diabetes. 2014;5(1):52–8.
Ozdemir A, Yalinbas B, Selamet U, Eres M, Turkmen F, Kumbasar F, Murat B, Keskin AT, Barut Y. The effect of hepatitis C virus infection on insulin resistance in chronic hemodialysis patients. Yonsei Med J. 2007;48(2):274–80.
Gastaldi G, Gomes D, Schneiter P, Montet X, Tappy L, Clément S, Negro F. Treatment with direct-acting antivirals improves peripheral insulin sensitivity in non-diabetic, lean chronic hepatitis C patients. PLoS ONE. 2019;14(6):e0217751.
Acknowledgements
The authors thank all the patients who enrolled in this study. The authors thank the nursing staff for their assistance in the sample collection.
Funding
The authors declare that there are no sources of funding to be acknowledged.
Author information
Authors and Affiliations
Contributions
MME involved in study design, writing the manuscript, interpretation of the data of the work, and drafting and revising of the work and corresponding author; RAD involved in the collection of samples and preparation of data; MTH involved in the collection of samples and preparation of data; SA involved in the collection of samples and preparation of data; AF involved in study design, writing the manuscript, interpretation of the data of the work, and drafting and revising of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research was conducted in accordance with the ethical standards of the Internal Medicine department's ethical committee number N-11-2023 (New Giza (NGU) University). The paper adheres to the ethical forms of the 1975 Declaration of Helsinki, as revised in 2008. Informed consent was obtained from all patients to be included in the study.
Consent for publication
We confirm that each author has seen and approved the contents of the submitted manuscript.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Elnokeety, M.M., Darwish, R.A., Hegazy, M.T. et al. The impact of HCV eradication using interferon-free direct acting antivirals on bone-mineral, anemia parameters and peripheral insulin resistance in hepatitis c-infected Egyptian hemodialysis cohort. Ren Replace Ther 9, 49 (2023). https://doi.org/10.1186/s41100-023-00500-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-023-00500-4