Abstract
Background
Septic shock is a life-threatening condition and one of the most common causes of acute kidney injury (AKI). The acrylonitrile-co-methallyl sulfonate surface-treated (AN69ST) membrane used in severe sepsis was formally launched in Japan in 2014, as a non-renal indication. This membrane provides hemofiltration in dialysis and improves hemodynamics in patients with sepsis and hypercytokinemia. However, the clinical literature regarding continuous renal replacement therapy (CRRT) with the AN69ST membrane is very limited, especially in infants.
Case presentation
A 3-month-old female infant weighing 4.2 kg was hospitalized for septic shock and AKI secondary to necrotizing enterocolitis. Although she underwent palliative surgery, her vital signs did not recover from shock, and she developed reduced urine output. Her pediatric sequential organ failure assessment score was 10 points. Thus, we strongly suspected septic shock and sepsis-induced AKI, which were refractory to conservative treatment, and we decided to introduce CRRT with the AN69ST membrane for both renal replacement and anti-hypercytokinemic indications. After initiating CRRT for 72 h, her blood pressure increased sufficiently to maintain urine output, and improvements in the electrolyte abnormalities and metabolic acidosis were observed. Notably, her serum inflammatory cytokine levels decreased in parallel with improvement in her general condition. Despite successfully recovering from the AKI and being stable enough to allow discontinuing CRRT, she died of multiple organ dysfunction syndrome 3 weeks after CRRT was discontinued.
Conclusions
CRRT may complement standard treatment in patients with sepsis-induced AKI to control the amplitude of the systemic inflammatory response regarding acute tissue and organ damage. We expect that CRRT with the AN69ST membrane will be recognized as an option for the treatment of septic shock and sepsis-induced AKI, even in infants.
Similar content being viewed by others
Background
Sepsis is a leading cause of morbidity, mortality, and healthcare utilization for children worldwide. Most children who die of sepsis suffer from refractory shock and/or multiple organ dysfunction syndrome (MODS), with many deaths occurring within the initial 48–72 h of treatment [1]. Moreover, hypercytokinemia associated with sepsis further promotes MODS. Patients with sepsis and septic shock also often develop acute kidney injury (AKI), leading to electrolyte abnormalities, metabolic acidosis, and fluid overload. The reported mortality from AKI in children ranges from 1.2 to 50.7%, and AKI is associated with the need for renal replacement therapy [2]. Continuous renal replacement therapy (CRRT) may be effective for such septic conditions, in addition to conservative therapy. The rationale for CRRT in septic shock is to address impending or established fluid overload following initial resuscitation or for inflammatory cytokine removal, reversal of coagulopathy, to buffer lactic acidosis, address AKI, or a combination of these factors. In addition, certain continuous blood purification techniques may help regulate systemic inflammation and promote kidney recovery. The acrylonitrile-co-methallyl sulfonate surface-treated (AN69ST) membrane used in severe sepsis was formally approved by the National Health Insurance System in Japan in July 2014, as a non-renal indication. Because the AN69ST membrane has excellent cytokine direct-adsorption capacities, this membrane is expected to have effects in hemofiltration dialysis in addition to improving hemodynamics in patients with sepsis with hypercytokinemia [3]. However, the clinical literature evaluating CRRT with the AN69ST membrane is very limited, especially in infants.
Case presentation
A female infant of 35 weeks and 1 day gestational age and birth weight of 2269 g was delivered to a 26-year-old woman via cesarean section because of excess amniotic fluid-related congenital intestinal obstruction detected during fetal ultrasonography. After delivery, the baby’s Apgar scores were 5 and 8 at 1 min and 5 min, respectively. After admission to the neonatal intensive care unit, she had mild abdominal distension, and plain abdominal X-ray revealed bulging sides, elevated diaphragm, and the double bubble sign. Two days after birth, she underwent surgery. Surgeons diagnosed congenital ileal atresia 45 cm distal to Treitz’ ligament and performed primary end-to-end anastomosis. Her abdominal symptoms improved after operation, and she was extubated 1 day post-operatively. Feeding was started on post-operative day 11, and her condition improved gradually. However, 3 weeks after surgery, although her general condition was stable, she developed vomiting and abdominal distension with increased enteral nutrition volumes. Thus, we placed a feeding tube for elemental diet administration, hoping to decompress the intestinal tract and facilitate weight gain with good enteral nutrition.
Three months after the first surgery, the patient’s bodyweight was 4.2 kg. However, 3 months and 9 days after the first surgery, she suddenly developed bile intestinal fluid in the feeding tube, and bloody stool. Abdominal radiography showed unilateral subdiaphragmatic free air and portal venous gas. Necrotizing enterocolitis of at least stage IIb according to Bell’s criteria [4] was suspected. She underwent emergency laparotomy, and extensive necrotizing enterocolitis with emphysematous changes was seen. Two intraperitoneal drains were placed because radical surgery was difficult, and a second-look surgery was scheduled after drainage decompression and general condition recovery. However, 12 h after emergency operation, she developed an unstable hemodynamic status (blood pressure and pulse were 35/20 mmHg and 160 beats/min, respectively) with oliguria, which led to refractory anuria. Her laboratory results indicated renal insufficiency (creatinine, 1.49 mg/dL [normal, < 0.3 mg/dL]; blood urea nitrogen, 35 mg/dL [normal, < 10 mg/dL]); mixed acidosis (pH, 7.068; HCO3−, 20.6 mEq/L; base excess, −5.3; lactic acid, 3.3 mmol/L [normal, < 2 mmol/L]); hyperkalemia (9.0 mEq/L; normal, < 5.5 mEq/L); increased C-reactive protein (2.4 mg/dL; normal, < 0.3 mg/dL); coagulation abnormalities (prothrombin time-INR, 4.00; activated partial thromboplastin time, 74.5 s; fibrin degradation products, 84.9 μg/mL [normal, < 5 μg/mL]); thrombocytopenia (103,000 platelets/μL); and low white blood cell count, 1600 cells/μL. Serum interleukin (IL)-6 measured using a semi-quantitative measurement kit (STICKELISA®; Toray, Tokyo, Japan) exceeded 5000 pg/mL (Fig. 1) [5]. Her pediatric sequential organ failure assessment (pSOFA) score was 10 points according to the criteria reported by Matics et al. [6]. Refractory hyperkalemia persisted despite insulin and glucose therapy and calcium infusion. Although peaked T waves, prolonged PR interval, decreased P wave, and widening QRS complex were observed on a bedside-monitored 3-lead electrocardiogram, no fatal arrhythmia occurred. Thus, we strongly suspected septic shock and sepsis-induced AKI, which were refractory to conservative treatment, and we decided to introduce CRRT with the AN69ST membrane for both renal replacement and anti-hypercytokinemic indications. She was also given respiratory support, vasoactive drugs (dopamine, 10 μg/kg/min), hydrocortisone (5 mg/kg/dose), appropriate volume replacement, and empirical antibiotic therapy (vancomycin, 10 mg/kg/day with a target trough value of 10–20 μg/mL, and meropenem, 60 mg/kg/day).
Interleukin-6 measurement. Interleukin (IL)-6 semi-quantitative analysis using the STICKELISA® (Toray, Tokyo, Japan) quantitative measurement kit before continuous renal replacement therapy with the acrylonitrile-co-methallyl sulfonate surface-treated (AN69ST) membrane. IL-6 produced an intensely dark color at > 5000 pg/mL (arrow), which was obtained within 45 min using 0.2 mL of serum at bedside
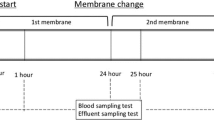
We initiated CRRT using continuous hemodialysis with the AN69ST membrane (sepXiris 60; Baxter International, Chicago, IL) (Table 1) and placed a percutaneous double-lumen central venous catheter (diameter: 4.5 F, length: 6 cm) in the left external jugular vein for blood return and a 24-G catheter in the brachial artery for blood drainage. Nafamostat mesilate, which is an anticoagulant agent, was administered at a dose of 0.5–1 mg/kg/h to maintain activated coagulation time between 150 s and 200 s.
After 12 h of treatment, as her blood pressure gradually recovered (90/55 mmHg), and her heart rate decreased (130 beats/min), we gradually decreased the dopamine administration. Her pSOFA score decreased from 10 points to 6 points. In addition, her serum potassium levels normalized, and the indicators of metabolic acidosis improved. CRRT was performed in the same manner for 3 days (total: 72 h), and CRRT was discontinued because her urine volume recovered (> 2 mL/kg/h) with loop diuretics (6 mg/kg/day). The serial changes in inflammatory and immunological data before and after CRRT are shown in Table 2. No significant microorganisms were detected in the results of the blood and ascites cultures. Despite improved vital signs and diuresis, disseminated intravascular coagulation persisted. Attempted second-look surgery was outside the indications at this time because of intracranial hemorrhage, which occurred 2 days after discontinuing CRRT. The patient died secondary to MODS associated with septic shock 3 weeks after discontinuing CRRT.
Discussion and conclusions
To the best of our knowledge, this is the first report describing the effective use of CRRT with the AN69ST membrane for septic shock in an infant. Sepsis and septic shock are secondary to a systemic inflammatory response (hypercytokinemia) and represent a clinical spectrum of dysregulated host responses to an infection that can result in MODS. Thus, strategies aimed at controlling the host response are important. Since the 2000s, polymyxin-B immobilized column-direct hemoperfusion (PMX-DHP) has been reported for neonatal and pediatric septic shock following its use in adults [7,8,9,10,11,12,13,14,15]. However, because there are no randomized controlled trials in neonatal and pediatric patients, unlike in adults, the efficacy of PMX-DHP is controversial. We previously reported the effects of PMX-DHP in neonatal septic shock and found that the efficacy for septic shock secondary to late-onset sepsis (LOS) (> 72 h after birth) was very low (in-hospital mortality: 80%) compared with early-onset sepsis (≤ 72 h after birth) (hospital mortality: 0%) [16]. One of the reasons for this difference is that it is difficult to recognize the non-specific signs of LOS. In addition, if LOS-induced AKI has already developed, electrolyte abnormalities, metabolic acidosis, and fluid overload, which are associated with prognosis, may have occurred. Indeed, although PMX-DHP directly removes endotoxins and indirectly decreases the numbers of inflammatory mediators, PMX-DHP has no renal replacement effect such as fluid removal or electrolyte correction. Although an experienced tertiary care hospital can obtain “hybrid CRRT” by connecting a PMX-DHP column and a hemodialysis column in series [9], this method has drawbacks in that the circuit design becomes complicated, circuit coagulation occurs easily, and the total priming volume increases according to patient physique.
In comparison, AT69ST membranes address both renal and non-renal indications, and may be a favorable treatment strategy for septic shock with AKI. Our patient experienced improvement in both electrolyte abnormalities and metabolic acidosis as well as in the abnormal serum cytokine levels. Notably, 72 h after beginning CRRT, the serum IL-6 levels decreased by 99% from 315,000 pg/mL to 1080 pg/mL (Table 2). Furthermore, tumor necrosis factor-α levels also decreased before and after CRRT (Table 2). Typically, sepsis and septic shock involve the production and release of a variety of cytokines, such as IL-6, IL-10, and tumor necrosis factor-α. IL-6 is produced by B and T lymphocytes, monocytes, endothelial cells, and fibroblasts in the acute phase of infection [16]. IL-6 induces hepatocytes to produce acute phase reactants, such as C-reactive protein. In our patient, the effects of antibiotics and steroids overlapped, but short-term decreases in inflammatory cytokines secondary to CRRT may be associated with improved general condition. Two filter types, namely polymethyl methacrylate (PMMA) and AN69ST membranes, have been used for CRRT to remove cytokines in Japan. Interestingly, Kobashi et al. recently reported a comparison of PMMA and AN69ST membranes in adult cases of sepsis and AKI. The authors concluded that the 28-day survival rates were higher in the AN69ST group than in the PMMA group in patients with or without sepsis [17]. Although the cause and pathophysiology of sepsis and AKI may differ between adults and infants, considering the patients’ physique and therapeutic effects, CRRT using the AN69ST membrane may be an option for infants.
A review of the data in previous studies of neonatal and pediatric patients with septic shock treated with PMX-DHP since 2010, and our patient’s data describing treatment with AN69ST, are summarized in Table 3 [7,8,9,10,11,12,13,14,15]. We searched the PubMed and Google Scholar databases from inception to 20 April 2020 (citations containing abstracts only were excluded), and found that neonatal sepsis was most common, and 10/11 reports were from Japan. In most patients, serum IL-6 levels improved and survival prognosis was good.
The Surviving Sepsis Campaign Guidelines (SSCG) for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children were published in February 2020 [18]. According to the SSCG, CRRT for septic shock is indicated only as follows: “using renal replacement therapy to prevent or treat fluid overload in children with septic shock or other sepsis-associated organ dysfunction who are unresponsive to fluid restriction and diuretic therapy (weak recommendation, very low quality of evidence).” Although the SSCG includes a weak recommendation for CRRT for septic shock associated with refractory fluid overload, the guidelines do not discuss removing cytokines or mediators as part of CRRT. However, some in vitro and in vivo studies suggested that a PMX-DHP column and the AN69ST membrane may be particularly useful in the treatment of septic shock and sepsis-induced AKI [19, 20]. Therefore, we emphasize that it is important to understand the characteristics of individual hemofilters (PMX-DHP and AN69ST) according to the patient’s condition. Compared with the PMX-DHP column, which has a priming volume of 8 mL for the smallest size (PMX-01R®; Toray Medical), the smallest priming volume for the AT69ST membrane is currently 47 mL. Much smaller devices are required for neonates and infants, to perform research and obtain experience using these devices.
We conclude that CRRT for septic shock and sepsis-induced AKI using the AT69ST membrane may be an option, even in infants. This method has great advantages in infants with sepsis for direct cytokine adsorption and renal replacement therapy. In addition, in our case, we showed that CRRT with the AT69ST membrane was performed safely and effectively even in an infant. Future studies should focus on the eligibility criteria for CRRT with the AT69ST membrane in infants with sepsis and AKI, and prospective trials with larger sample sizes are needed.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MODS:
-
Multiple organ dysfunction syndrome
- AK:
-
Acute kidney injury
- CRRT:
-
Continuous renal replacement therapy
- AN69ST:
-
Acrylonitrile-co-methallyl sulfonate surface-treated
- IL:
-
Interleukin
- pSOFA:
-
Pediatric sequential organ failure assessment
- PMX-DHP:
-
Polymyxin-B immobilized column-direct hemoperfusion
- LOS:
-
Late-onset sepsis
- PMMA:
-
Polymethyl methacrylate
- SSCG:
-
The Surviving Sepsis Campaign Guidelines
References
Weiss SL, Balamuth F, Hensley J, Fitzgerald JC, Bush J, Nadkarni VM, et al. The epidemiology of hospital death following pediatric severe sepsis: when, why, and how children with sepsis die. Pediatr Crit Care Med. 2017;18:823–30.
Lawal TA, Raji YR, Ajayi SO, Ademola AD, Ademola AF, Ayandipo OO, et al. Predictors and outcome of acute kidney injury after non-cardiac paediatric surgery. Ren Replace Ther. 2019;5:15. https://doi.org/10.1186/s41100-019-0214-y.
Thomas M, Moriyama K, Ledebo I. AN69: evolution of the world’s first high permeability membrane. Contrib Nephrol. 2011;173:119–29.
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7.
Miwa K, Shibayama N, Moriguchi T, Goto J, Yanagisawa M, Yamazaki Y, et al. A rapid enzyme-linked immunosorbent assay with two modes of detection for measuring cytokine concentration. J Clin Lab Anal. 2009;23:40–4.
Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically ill children. JAMA Pediatr. 2017;171:e172352.
Hirabayashi K, Shiohara M, Saito S, Tanaka M, Yanagisawa R, Tsuruta G, et al. Polymyxin-direct hemoperfusion for sepsis-induced multiple organ failure. Pediatr Blood Cancer. 2010;55:202–5.
Koga Y, Oba U, Takimoto T, Suminoe A, Takada H, Hara T. Polymyxin B-immobilized fiber column hemoperfusion therapy for septic shock. Shock. 2013;40:233.
Tokumasu H, Watabe S, Tokumasu S. Effect of hemodiafiltration therapy in a low-birthweight infant with congenital sepsis. Pediatr Int. 2015;58:237–40.
Nishizaki N, Nakagawa M, Hara S, Oda H, Kantake M, Obinata K, et al. Effect of PMX-DHP for sepsis due to ESBL-producing E. coli in an extremely low-birthweight infant. Pediatr Int. 2016;58:411–4.
Nanishi E, Hirata Y, Lee S, Kaku N, Momii K, Kubota K, et al. Polymyxin-B immobilized column-direct hemoperfusion for adolescent toxic shock syndrome. Pediatr Int. 2016;58:1051–4.
Nishizaki N, Hirano D, Miyasho T, Obinata K, Shoji H, Shimizu T. Evaluation of urinary IL-6 in neonates with septic shock treated with polymyxin B-immobilized fiber column. Pediatr Int. 2017;59:1032–3.
Kaneda H, Shimizu M, Yachie A. Successful treatment of enterohemorrhagic escherichia coli-induced acute encephalopathy and hemolytic-uremic syndrome with polymyxin-B direct hemoperfusion. Ther Apher Dial. 2017;21:419–21.
Kim YA, Kim H, Kim YM, Park SE. A successful application of adult polymyxin B-immobilized fiber column hemoperfusion to a neonate with septic shock. Acute Crit Care. 2019;34:28-8.
Hara K, Nagano N, Hijikata M, Urakami T, Morioka I. Successful treatment of enterohemorrhagic escherichia coli-induced acute encephalopathy and hemolytic-uremic syndrome with polymyxin-B direct hemoperfusion. J Nihon Univ Med Ass. 2020;79:41–5.
Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39:517–28.
Kobashi S, Maruhashi T, Nakamura T, et al. The 28-day survival rates of two cytokine-adsorbing hemofilters for continuous renal replacement therapy: a single-center retrospective comparative study. Acute Med Surg. 2018;13:60–7.
Weiss SL, Peters MJ, Alhazzani W, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. 2020;21:e52–e106.
Arimura T, Abe M, Shiga H, Katayama H, Kaizu K, Oda S. Clinical study of blood purification therapy in critical care in Japan: results from the survey research of the Japan Society for Blood Purification in Critical Care in 2013. J Artif Organs. 2017;20:244–51.
Hattori N, Oda S. Cytokine-adsorbing hemofilter: old but new modality for septic acute kidney injury. Ren Replace Ther. 2016;2:41. https://doi.org/10.1186/s41100-016-0051-1.
Acknowledgements
We acknowledge the Juntendo University Urayasu Hospital Clinical Engineering Team for their expertise regarding CRRT. The authors thank Jane Charbonneau, DVM, from Edanz Group for editing a draft of this manuscript.
Funding
The authors confirm that they received no funding for this study.
Author information
Authors and Affiliations
Contributions
NN, RU, YN, HA, AM, AM, and TO engaged in patient care; NN drafted the manuscript; KO and TS reviewed the manuscript and supervised the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was waived for this study by our institutional review board because the study is a case report. The patient’s parents gave voluntary written informed consent before each treatment.
Consent for publication
Consent for publication was obtained to use the patient’s medical information in this manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nishizaki, N., Ueno, R., Nagayama, Y. et al. Effects of continuous renal replacement therapy with the AN69ST membrane for septic shock and sepsis-induced AKI in an infant: a case report with literature review of cytokine/mediator removal therapy in children. Ren Replace Ther 6, 34 (2020). https://doi.org/10.1186/s41100-020-00284-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-020-00284-x