Abstract
Background
High-dose methotrexate (HD-MTX) therapy has been used to treat a wide range of oncological malignancies. While the therapy can be tolerated with hydration, urine pH control, and leucovorin rescue therapy, HD-MTX is cytotoxic and can cause renal failure. There have been several case reports of HD-MTX toxicity in patients with solid tumors; however, few case series of hematological malignancies have been published. Patients with hematological malignancies tend to be administered many concomitant drugs, which can affect the elimination of MTX and result in acute kidney injury.
Case presentation
Here, we present three cases of HD-MTX-induced acute kidney injury in patients with hematological malignancies. Several blood purification methods were used to attempt to eliminate MTX.
Conclusions
Rapid elimination of MTX is needed for patients with higher serum MTX concentrations to avoid additional cytotoxic effects, especially in patients who have experienced many complications. Although the most effective method for MTX elimination remains unknown, the results of our retrospective survey suggest that combined modalities, such as hemodialysis and plasma exchange, may be suitable for treatment of patients with hematological malignancies.
Similar content being viewed by others
Background
High-dose methotrexate (HD-MTX), defined as a dose higher than 500 mg/m2 [1] or 1000 mg/m2 [2], is used for adult and pediatric patients with a wide range of malignancies, including lymphoma and sarcoma [1, 3]. Alkalization of urine and high-dose leucovorin rescue therapy aid in the safe delivery of HD-MTX therapy, and the adverse effects of HD-MTX in established regimens were thought to be tolerated [4].
There have been many cases reports or case series of MTX toxicity which needed blood purifications in solid tumors, such as osteosarcoma [5,6,7,8,9,10,11], and investigated the incidence of nephrotoxicity by a large-scale retrospective survey [2]. In contrast, similar cases of toxicity in hematologic malignancies are relatively rare [6, 10,11,12,13,14]. The doses of MTX in patients with hematological malignancies are generally lower than those administered to patients with solid cancers [12], and cell toxicity is induced by MTX in a dose-dependent manner [15]; accordingly, the occurrence of MTX toxicity in hematological malignancies appears to be lower than that in solid tumors.
We report here three cases of acute kidney injury (AKI) resulting from MTX toxicity in patients with hematological malignancies. In these cases, we attempted to decrease the serum MTX concentration by using several blood purification methods.
Case presentation
Case 1
A 48-year-old man (body surface area 1.77 m2) was diagnosed with acute lymphoblastic leukemia 3 months before the administration of HD-MTX. He received a chemotherapy regimen consisting of cyclophosphamide at 1200 mg/m2, vincristine (VCR) at 1.5 mg/m2, daunorubicin at 45 mg/m2, prednisone at 60 mg/m2, and cytarabine (Ara-C) at 40 mg. The patient achieved and maintained complete remission (CR). He was prescribed no trimethoprim-sulfamethoxazole combinations or non-steroidal anti-inflammatory drugs (NSAIDs). Lansoprazole, a proton pump inhibitor (PPI), was prescribed at the time of HD-MTX therapy, but the PPI was soon replaced by a histamine receptor blocker (H2 blocker). The first course of intensification chemotherapy, which did not include HD-MTX, consisted of Ara-C at 2 g/m2, etoposide at 100 mg/m2, dexamethasone at 40 mg/whole body, and L-asparaginase (ASP) at 10,000 KU/m2 and commenced 1 month prior to the HD-MTX therapy. During the first course of therapy, grade 2 leukopenia (< 1500/mm3) and sepsis occurred. Cefepime was administered as a 1 g intravenous injection four times daily for 10 days to treat the sepsis. The patient was discharged from our hospital without renal dysfunction. At that point, no urinary abnormalities, such as proteinuria or hematuria, had been observed. One week later, the patient was readmitted to our hospital to receive the second course of intensification therapy. This regimen consisted of MTX at 3 g/m2 (over 24 h), VCR at 1.3 mg/m2, 6-mercaptopurine at 25 mg/m2, L-ASP at 10,000 KU/m2, and Ara-C at 40 mg. The patient’s urine pH remained above 7.0 throughout the course of therapy. On day 2, he developed a fever, and treatment with an injection of 1 g cefepime four times daily was initiated. The patient’s serum MTX concentration increased to 33.2 nmol/mL, and serum creatinine rose to 7.63 mg/dL on day 2, although the serum creatinine level was 0.77 mg/dL on day 0 and urinary volume was 2070 mL on day 1. He also suffered from diarrhea, another complication of HD-MTX, for several days. On day 2, the patient’s blood test results were as follows: creatine phosphokinase (CPK), 100 IU/L; blood urea nitrogen (BUN), 62 mg/dL (BUN/Cr ratio was 8.13); uric acid, 6.4 mg/dL; aspartate aminotransferase (AST), 19 IU/L; and alanine aminotransferase (ALT), 7 IU/L. These test results suggested that other etiologies for AKI, such as rhabdomyolysis, hyperuricemia, and liver failure could be excluded and MTX toxicity was the main etiology of AKI despite the patient’s dehydration. In addition to augmentation of the dose of leucovorin, we initiated plasma exchange therapy, which involved replacing the patient’s plasma with fresh frozen plasma (FFP), along with hemodialysis. Plasma exchange was performed eight times, and hemodialysis was performed four times. On day 10, the patient’s plasma MTX concentration was below 1.0 nmol/mL; leucovorin rescue was continued to day 20, and the patient was discharged from our hospital on day 34. His final serum MTX was 0.05 nmol/mL (day 22) and his creatinine was 1.39 mg/dL (day 28). The clinical course is illustrated in Fig. 1.
Case 2
A 19-year-old man (body surface area 1.36 m2) was diagnosed with mixed-phenotype acute leukemia and received chemotherapy 2 years prior to HD-MTX therapy. He attained CR but relapsed 1 year later and received chemotherapy again at that time. The induction and intensification therapy were performed with the same regimen administered to the patient in case 1. During the first course of intensification therapy, which did not include HD-MTX, he developed a fever and was diagnosed with febrile neutropenia. The patient was treated with an injection of 1 g cefepime four times daily for 2 days and an injection of 1 g doripenem three times daily for 5 days. The second course of intensification chemotherapy included HD-MTX therapy, as in case 1. The patient’s creatinine was 0.82 mg/dL on day 0, his urinary volume on day 1 was 3100 mL, and he had no history of urinary abnormalities. No PPI, trimethoprim-sulfamethoxazole combinations, or NSAIDs were prescribed to this patient, and his urine pH was maintained above 7.0. Despite careful attention to MTX toxicity, AKI occurred and resulted in a serum MTX concentration of 9.51 nmol/mL and a serum creatinine concentration of 5.95 mg/dL on day 2. Blood tests revealed CPK 29 IU/L, BUN 56 mg/dL (BUN/Cr ratio was 9.41), uric acid 3.0 mg/dL, AST 16 IU/L, and ALT 18 IU/L, suggesting that the main etiology for AKI was MTX toxicity, as in case 1. We performed plasma exchange twice with 5% albumin as the replacement fluid. Hemodialysis was performed twice, and charcoal hemoperfusion once. On day 15, the patient’s plasma MTX concentration was below 1.0 nmol/mL, and leucovorin rescue was continued until day 22. Grade 2 leukopenia, another complication caused by HD-MTX, occurred on day 11 during the blood purification therapy. The patient was discharged from our hospital on day 23. His final serum MTX concentration was 0.04 nmol/mL (day 18), and his creatinine was 1.02 mg/dL (day 23). The clinical course is shown in Fig. 2. The patient later received an unrelated donor bone marrow transplantation and maintained CR.
Case 3
A 72-year-old woman (body surface area 1.44 m2) was diagnosed with primary central nervous system lymphoma 1 month prior to receiving chemotherapy. Induction chemotherapy, consisting of rituximab at 350 mg/m2, procarbazine at 100 mg/whole body, MTX at 2 g/m2 (over 2 h), and VCR at 1.4 mg/m2, was initiated 2 weeks prior to the occurrence of HD-MTX toxicity and led to a decrease in tumor size. Prior to the second course of therapy, the patient developed a urinary tract infection, which was treated with ceftriaxone 1 g once daily. During the second course of therapy, her serum creatinine increased to 2.4 mg/dL, and serum MTX concentration was 7.8 nmol/mL, although creatinine was 0.51 mg/dL on day 0 and urinary volume on day 1 was 4050 mL, and no urinary abnormalities had been noted. Blood tests revealed serum CPK 20 IU/L, BUN 22 mg/dL (BUN/Cr ratio was 10.8), uric acid 6.3 mg/dL, AST 22 IU/L, and ALT 25 IU/L on day 2. We performed plasma exchange with FFP four times and with 5% albumin three times and performed hemodialysis four times. The patient also experienced other complications induced by HD-MTX, including pancytopenia, and developed a fever during blood purification therapy. She was again treated with antibiotics. In this case, we could not easily decrease her serum MTX concentration below 0.5 nmol/L, so we repeatedly performed plasma exchange. The clinical course is illustrated in Fig. 3.
Despite intensive therapy, the patient’s condition continued to worsen owing to the aggravation of the lymphoma. Finally, she was transferred to hospice.
Summary of blood purification
The conditions of blood purification for the patients in these three cases are summarized in Table 1. MTX reduction rates accomplished with several modalities were estimated using MTX blood concentration before blood purification and on the following day; these results are shown in Table 2.
Literature review
AKI due to HD-MTX in hematological malignancies cases may not be rare; however, there have been not so many reported cases that needed blood purifications. We show the summary of previously reported cases as a literature review (Table 3).
Discussion and conclusions
Here, we present three cases of patients with hematological malignancies who experienced MTX toxicity. An abrupt increase of serum creatinine shortly after administration of HD-MTX is a distinctive feature of HD-MTX-induced AKI [16], suggesting that the major etiology of AKI was HD-MTX administration in these three cases.
The most commonly described mechanism of MTX nephrotoxicity is the crystallization of MTX in the renal tubular lumen; however, other mechanisms have also been proposed, such as constriction of the afferent capillary and direct effects on the mesangial or tubular epithelial cells [16]. Usually, preventive strategies, such as increased hydration and high-dose leucovorin, allow renal recovery without hemodialysis during HD-MTX therapy [1, 3, 15]. Prolonged duration of a high serum concentration of MTX should be avoided [1], because MTX is highly cytotoxic and has multiple potential adverse effects, such as hepatotoxicity and gastrointestinal and hematologic toxicity. Therefore, long-term elevated serum MTX concentrations should be avoided and serum MTX should be kept less than 0.1 nmol/mL [10].
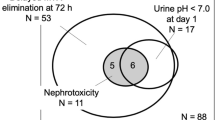
MTX renal toxicity has been graded according to the World Health Organization (WHO) criteria (grade 1, serum creatinine level < 1.5 × the upper limit of normal range [ULN]; grade 2, 1.5–3.0 × ULN; grade 3, 3.1–6.0 × ULN; grade 4, > 6.0 × ULN) [16]. The incidence of grade 3 or 4 renal failure in patients with osteosarcoma receiving HD-MTX therapy was found to be low (0.6%) [2], and the need for hemodialysis among patients with hematological malignancies was also low; only one patient received hemodialysis out of 194 patients treated with HD-MTX in a retrospective study [17]. Among the three cases presented here, two patients had grade 3 and one had grade 2 renal toxicity according to the WHO classification [16].
The risk of MTX-mediated nephrotoxicity has been shown to increase with advanced age, male sex, MTX dose, low baseline kidney function, and the use of certain antibiotics [18]. The most important factor in patients with hematological malignancies may be the use of concomitant drugs, because patients may receive many of these and the drugs themselves can sometimes directly cause AKI [18]. Moreover, the elimination of MTX from the kidney is affected by concomitant therapies, such as NSAIDs, beta-lactam antibiotics, trimethoprim-sulfamethoxazole combinations, gemfibrozil, and PPIs [1, 12, 19]. Among beta-lactam antibiotics, tazobactom and piperacillin particularly reduce the clearance of MTX [9, 12]; however, the beta-lactam antibiotics used in these three cases were mainly cefepime and ceftriaxone.
MTX is a relatively small molecule (molecular weight 454), and the protein binding rate and distribution volume are approximately 50% and 0.4–0.8 L/kg, respectively [9, 15, 19]. In the three cases presented here, plasma exchange was the main modality used to eliminate MTX, because it can remove both protein-binding MTX and non-binding MTX; in addition, the MTX concentration increase by rebound is not so rapid [8]. In all of our three cases, although only intermittent measurements of MTX concentration changes were available because of the lack of data, treatment with combined modalities seemed to be the most effective. Plasma exchange alone appeared to have lower efficacy in this retrospective survey, and the volume of replacement fluid might be related to the efficiency of MTX elimination (Table 2).
The most effective method of MTX removal remains to be proven. The blood purification modalities for the elimination of MTX are hemodialysis, plasma exchange, charcoal hemoperfusion, and a combination of these modalities [1, 2, 6, 9, 10, 15, 19,20,21,22]. The heterogeneous methods, variable outcomes, and lack of suitable controls of these reports make comparison difficult. With respect to HD-MTX-induced renal failure in patients with hematological malignancies, hemodialysis conditions differed among the cases (Table 3), and the hemodialysis prescription seemed to be insufficient for MTX elimination, especially in our cases. In our facility, hemodialysis is routinely initiated under the following conditions: 3 h and blood flow of 150 mL/min, and we use a small-size dialyzer to avoid dialysis disequilibrium syndrome. We should have modified the hemodialysis conditions to increase MTX elimination efficiency, namely, through the use of longer treatment times, higher blood flow, and larger dialyzer surface areas. Concerning the elimination rate for MTX, hemodialysis has been considered to be a good modality for eliminating MTX [2]; however, serum MTX concentration could increase rapidly to pre-treatment levels because of rebound [8, 10]. It is presumably correct that the combined modalities, such as simultaneous hemodialysis therapy and plasma exchange, are superior among these therapeutic options [1, 6, 10, 15]. Although Table 3 showed only one previously reported hematological malignancy case treated by simultaneous blood purification modalities [10], combined modalities may have an advantage in hematological malignancy cases because these patients need rapid MTX elimination to avoid worsening pre-existing complications.
Although the three cases we presented here involved no significant renal dysfunction before HD-MTX therapy, AKI occurred after HD-MTX therapy. Among the many risk factors for AKI, specific proteins and genes that affect MTX pharmacodynamics and metabolism should be considered. For instance, genome-wide association studies have found that methylenetetrahydrofolate reductase (MTHFR) can alter MTX metabolism [23]. The most common polymorphism of the MTHFR gene is C677T [24], and patients who have the C677T single-nucleotide polymorphism tend to not complete HD-MTX therapy due to adverse effects [25]. While we did not examine the polymorphisms associated with MTX metabolism, it is possible that the presented cases had genetic defects that caused MTX elimination.
There have been reports showing the efficacy of carboxypeptidase-G2 (CPDG2), which cleaves MTX, in the treatment of MTX toxicity [2, 19, 21]. We could not use CPDG2 in these cases because it has not been approved in Japan CPDG2 whereas it was approved in the United States in 2012. As shown in Table 3, the average numbers of blood purification therapy in the cases used CPDG2 were lower (4.3 times) than those of the cases not used it (6.5 times); however, there was no significant difference.
Here, we report three cases of HD-MTX-induced renal failure in patients with hematological malignancies. Because prolonged elevation of serum MTX concentrations will damage cells, MTX should be eliminated and prompt recognition and effective treatment are essential in patients undergoing therapy who experience numerous complications. Although the efficacy of MTX elimination provided by the various modalities remains unknown, the use of combined methods, such as hemodialysis and plasma exchange, appears superior and desirable.
Abbreviations
- AKI:
-
Acute kidney injury
- ALT:
-
Alanine aminotransferase
- Ara-C:
-
Cytarabine
- ASP:
-
L-asparaginase
- AST:
-
Aspartate aminotransferase
- BUN:
-
Blood urea nitrogen
- CPDG2 :
-
Carboxypeptidase-G2
- CPK:
-
Creatine phosphokinase
- CR:
-
Complete remission
- FFP:
-
Fresh frozen plasma
- H2 blocker:
-
Histamine receptor blocker
- HD-MTX:
-
High-dose methotrexate
- MTHFR:
-
Methylenetetrahydrofolate reductase
- MTX:
-
Methotrexate
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- PPI:
-
Proton pump inhibitor
- ULN:
-
Upper limit of normal range
- VCR:
-
Vincristine
- WHO:
-
World Health Organization
References
Howard SC, McCormick J, Pui C-H, Buddington RK, Harvey RD. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21:1–12.
Widemann BC, Balis FM, Kempf-Bielack B, Bielack S, Pratt CB, Ferrari S, et al. High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma: incidence, treatment, and outcome. Cancer. 2004;100:2222–32.
Schmiegelow K. Advances in individual prediction of methotrexate toxicity: a review. Br J Haematol. 2009;146:489–503.
Ferreri AJ, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374:1512–20.
Relling MV, Stapleton FB, Ochs J, Jones DP, Meyer W, Wainer IW, et al. Removal of methotrexate, leucovorin, and their metabolites by combined hemodialysis and hemoperfusion. Cancer. 1988;62:884–8.
Wall SM, Johansen MJ, Molony DA, DuBose TD, Jaffe N, Madden T. Effective clearance of methotrexate using high-flux hemodialysis membranes. Am J Kidney Dis. 1996;28:846–54.
Windsor RE, Strauss SJ, Kallis C, Wood NE, Whelan JS. Germline genetic polymorphisms may influence chemotherapy response and disease outcome in osteosarcoma: a pilot study. Cancer. 2012;118:1856–67.
Cecyn KZ, Lee J, Oguro T, Petrilli AS, Bordin JO. Use of plasma exchange in methotrexate removal in a patient with osteosarcoma and acute renal insufficiency. Am J Hematol. 2003;72:209–11.
Chan WKY, Hui WF. Sequential use of hemoperfusion and single-pass albumin dialysis can safely reverse methotrexate nephrotoxicity. Pediatr Nephrol. 2016;31:1699–703.
Fujikura E, Akiu M, Miyauchi K, Yoshida M, Aoki S, Yamamoto T, et al. Blood purification therapies in methotrexate-induced acute kidney injury: four case reports. Ren Replace Ther. 2017;3:48.
Buchen S, Ngampolo D, Melton RG, Hasan C, Zoubek A, Henze G, et al. Carboxypeptidase G2rescue in patients with methotrexate intoxication and renal failure. Br J Cancer. 2005;92:480–7.
De MD, Garc J, Mart Y, Jos J, Burgaleta C. Severe acute renal failure following high-dose methotrexate therapy in adults with haematological malignancies: a significant number result from unrecognized co-administration of several drugs. Nephrol Dial Transplant. 2008;23:3762–6.
Kepka L, De LA, Ribrag V, Gachot B, Blot F, Theodore C, et al. Successful rescue in a patient with high dose methotrexate-induced nephrotoxicity and acute renal failure. Leuk Lymphoma. 1998;29:205–9.
Mohty M, Peyriere H, Guinet C, Hillaire-Buys D, Blayac J-P, Rossi J-F. Carboxypeptidase G2 rescue in delayed methotrexate elimination in renal failure. Leuk Lymphoma. 2000;37:441–3.
Kumar N, Shirali AC. What is the best therapy for toxicity in the setting of methotrexate-associated acute kidney injury: high-flux hemodialysis or carboxypeptidase G2? Semin Dial. 2014;27:226–8.
Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694–703.
May J, Carson KR, Butler S, Liu W, Bartlett NL, Wagner-Johnston ND, et al. High incidence of methotrexate associated renal toxicity in patients with lymphoma: a retrospective analysis. Leuk Lymphoma. 2014;55:1345–9.
Ganguli A, Sawinski D, Berns JS. Kidney diseases associated with haematological cancers. Nat Rev Nephrol. 2015;11:47890.
Connors NJ, Sise ME, Nelson LS, Hoffman RS, Smith SW. Methotrexate toxicity treated with continuous venovenous hemofiltration, leucovorin and glucarpidase. Clin Kidney J. 2014;7:590–2.
Sawada M, Fujiwara M, Shimada N, Tanaka N, Kuwakado K, Takeda N, et al. Methotrexate intoxicity treated with hemodialysis and charcoal hemoperfusion in 12-year-old boy. Nihon Shoni Jinzobyo Gakkai Zasshi. 2011;24:74–80.
Saland JM, Leavey PJ, Bash RO, Hansch E, Arbus GS, Quigley R. Effective removal of methotrexate by high-flux hemodialysis. Pediatr Nephrol. 2002;17:825–9.
Wu CC, Huang CF, Shen LJ, Wu FLL. Successful elimination of methotrexate by continuous veno-venous haemofiltration in a psoriatic patient with methotrexate intoxication. Acta Derm Venereol. 2015;95:626–7.
Lopez-Lopez E, Martin-Guerrero I, Ballesteros J, Garcia-Orad A. A systematic review and meta-analysis of MTHFR polymorphisms in methotrexate toxicity prediction in pediatric acute lymphoblastic leukemia. Pharmacogenomics J. 2013;13:498–506.
Tasbas O, Borman P, Karabulut HG, Tukun AYR. The frequency of A1298C and C677T polymorphisms of the methylentetrahydrofolate gene in Turkish patients with rheumatoid arthritis: relationship with methotrexate toxicity. Open Rheumatol J. 2011;5:30–5.
Turello R, Rentsch K, Di Paolo E, Popovic MB. Renal failure after high-dose methotrexate in a child homozygous for MTHFR C677T polymorphism. Pediatr Blood Cancer. 2008;50:154–6.
Availability of data and materials
The available data and materials are all included in the manuscript.
Author information
Authors and Affiliations
Contributions
MF, RK, SS, YS, and YMi cared for the patients and MK, SK, TU, YO, YMo, MN, HS, and TN provided blood purification therapy. MK prepared the manuscript. MN, HS, HM, and TN organized the comprehensive study project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethical Committee of Nagasaki University Hospital (No. 17032733) and informed consent was obtained from the patients or families.
Consent for publication
Informed consent was obtained from the patients or families.
Competing interests
The authors declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kitamura, M., Kitamura, S., Fujioka, M. et al. Methotrexate-induced acute kidney injury in patients with hematological malignancies: three case reports with literature review. Ren Replace Ther 4, 39 (2018). https://doi.org/10.1186/s41100-018-0180-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-018-0180-9