Abstract
Background
Peripheral arterial disease (PAD) has much impact on mortality in hemodialysis (HD) patients. Ankle-brachial index (ABI) and skin perfusion pressure (SPP) are useful tools to detect PAD in HD patients. However, the prevalence of PAD in incident HD patients by ABI and SPP measurement has not been fully elucidated.
Methods
We examined both ABI and SPP in 185 consecutive patients with end-stage renal failure at the initiation of HD therapy. PAD was diagnosed by previous history, clinical symptoms, histories of endovascular peripheral intervention, bypass surgery, amputation due to PAD, and values of ABI and SPP. Cut-off value of ABI and SPP for diagnosing PAD was set at < 0.9 and < 50 mmHg, respectively.
Results
The percentage of limbs with ABI < 0.9 and SPP value < 50 mmHg among total limbs were 10.8 and 21.1%, respectively. Among 185 patients in incident HD patients, 45 patients were diagnosed as having PAD. ABI and SPP positively correlated (r = 0.311, p = 0.006). However, discrepancy between ABI and SPP values (normal or high ABI with low SPP, or low ABI with normal SPP) was also found. Among 45 incident HD patients with PAD, only 14 patients (31.1%) showed low ABI and low SPP values.
Conclusion
Measurement of both ABI and SPP might be necessary to improve the diagnostic accuracy of PAD. Prevalence of PAD in incident HD patients was proved to be very high.
Similar content being viewed by others
Background
Peripheral arterial disease (PAD) in lower extremities in hemodialysis (HD) patients has much impact on prognosis. Amputation rate of lower limbs in HD patients is reported to be 4.3/100 person year in USA [1]. Once PAD worsens to severe disease state, critical limb ischemia (CLI) which necessitates major amputation of lower limbs, the prognosis is severely poor, i.e., 1-year survival rate 30–50% [2, 3] and 5-year survival rate 14.4% [3]. Detecting early symptoms of PAD such as chillness and/or claudication are sometimes difficult and un-reliable in HD patients, and it is not unusual to find CLI as the first apparent symptom of PAD. Therefore, early detection of PAD would be extremely important to improve the prognosis of HD patients.
Ankle-brachial pressure index (ABI) is a useful screening test for detecting PAD. ABI value less than 0.9 is generally thought to have arterial stenosis or obstruction in lower limbs. However, we have previously reported that the sensitivity of ABI < 0.9 for detecting PAD in HD patients was only 29.9% (specificity was 100%) [4]. Accurate ABI values may not be obtained and pseudo-normalize in cases of high arterial calcification or incompressible arteries [5]. In this regard, skin perfusion pressure (SPP) might be a superior tool to ABI in order to detect microcirculatory impairment more accurately. SPP is also applicable to the patients with edema or severe blood vessel calcification cases as SPP detects circulation of the subcutaneous tissue, and SPP can detect the limb ischemia more accurately [6,7,8,9]. SPP values less than 50 mmHg could detect PAD in HD patients with high sensitivity of 84.9% and high specificity of 78.6% as we previously reported [4].
Most previous epidemiological reports concerning the prevalence of PAD in HD patients depended on the ABI value [4,5,6, 9,10,11,12,13,14,15], and reports concerning SPP as a diagnostic tool for PAD in HD patients are limited [4, 6, 7, 9, 16]. Furthermore, the prevalence of PAD at the initiation of HD therapy has not been so far elucidated. Therefore, we examined ABI and SPP in 185 consecutive incident HD patients to compare the results of two diagnostic methods, to evaluate the relationship between ABI and SPP, and to clarify the prevalence of PAD at the initiation of HD.
Methods
Patients
This study was performed in a single HD center in Shonan Kamakura General Hospital. Eligible subjects comprised all consecutive patients from December 2003 to November 2008 who newly started HD in our hospital. Patients who could not be evaluated by SPP due to involuntary movement were excluded. Patients who had history of previous major amputation of lower legs were registered as a subject, although they did not have SPP data.
Clinical information about age; sex; smoking history; comorbidity including hypertension, diabetes mellitus, dyslipidemia, ischemic heart disease (IHD), and stroke; and drug use including ARB, statin, medication of anti-platelet drugs including cilostazol, aspirin, sarpogrelate, and prostaglandin I2 analogue was recorded by medical records and interviews to the patients. Laboratory data including hemoglobin, blood glucose, serum albumin, calcium, phosphate, total cholesterol, triglyceride, high-density lipoprotein, low-density lipoprotein, and C-reactive protein were evaluated using in-hospital laboratory in our hospital. All patients gave informed consent, and local ethical committee approved the study (ethical No. TGE00797-024) and was performed in accordance with the principles of the Declaration of Helsinki.
ABI/SPP
ABI was measured by ABI-form (Colin, Komaki, Japan) as previously reported [4, 5]. ABI-form simultaneously measures unilateral branchial pressure in the arm without an arterio-venous fistula and ankle blood pressure by oscillometric method. ABI was calculated as the ratio of ankle systolic pressure divided by brachial systolic pressure.
SPP was measured by using a Laser Dopp PV3000® (Kaneka, Osaka, Japan) according to the method described by Castronuovo et al. [17]. Briefly, the laser Doppler skin perfusion pressure transducer consists of a laser Doppler probe secured within the bladder of a blood pressure cuff that contains a transparent polyvinylchloride window so that microcirculatory perfusion measurements can be made during cuff deflation. Two points were set to measure SPP in each patient, (a) a point between first and second metatarsal bones in instep and (b) a front middle point in sole. After inflating the cuff pressure at first to stop skin perfusion, then deflate the cuff pressure and measure the point of cuff pressure when skin perfusion restarted. SPP was expressed as pressure of restarting the skin perfusion. Each patient had four SPP data, i.e., right and left leg in instep and sole. SPP value less than 50 mmHg of at least one point among these 4 points was necessary to diagnose PAD. Measurement of SPP was performed within 1 h after an HD session, and ABI test was also performed on the same day after an HD session.
Validation of SPP measurement has been clearly reported [4]. We previously evaluated the diagnostic accuracy of ABI and SPP to detect PAD in HD patients using contrast-enhanced computed tomography as standard images. As a result, ABI value < 0.9 can detect PAD with 100% specificity and SPP value < 50 mmHg with specificity of 76.9%. Japanese Society for Dialysis Therapy Guideline suggested that ABI 1.0 might be used as cutoff value to detect PAD in maintenance HD patients because of high vascular calcification [18]. However, there is no evidence that ABI 1.0 could be also applied in incident HD patients. Therefore, we used ABI cutoff value 0.9, cutoff value in the general population and 100% specificity in maintenance HD patients for PAD, in this study.
Definition of PAD
Patients were interviewed about symptoms of PAD including chillness, numbness, claudication, and resting pain. Previous histories of intervention (percutaneous peripheral intervention or bypass surgery) or amputation due to PAD were also recorded. All patients then underwent physiological examination about skin color, warmth, pulse exam of femoral artery, popliteal artery, dorsal artery, posterior tibial artery, and skin lesion including ulcers and gangrenes. The symptomatic information, previous histories of intervention or amputation, and physiological examinations including ABI and SPP were used to assign a limb ischemia. When patients filled at least one criteria among (1) ABI < 0.9, (2) SPP < 50 mmHg, (3) apparent previous history of intervention or amputation of lower limbs due to PAD, and (4) apparent clinical symptoms including claudication, resting pain, or ulcer due to ischemia, they were defined as having PAD.
In patients who were diagnosed as having PAD, severity was categorized from Ito IV by Fontaine’s clinical severity classification [19].
Statistical analysis
All data are expressed as mean ± SD. Unpaired t test or Mann-Whitney U test were used for group comparisons. Categorical data were analyzed by means of chi-square test. Univariate analysis was performed using Spearman’s rank correlation coefficient. Stepwise logistic regression analysis was performed based on a forward-backward procedure to define the independent variables related to PAD. Statistical analyses were done using statistical software (JMP 10: SAS Institute, JAPAN, and SPSS 10.0 software: SPSS Inc., Chicago, IL, USA) for Windows personal computer.
Results
Patients
There were 193 incident HD patients during 5-year study period in HD center in our hospital. Among them, 8 patients (4.1%) had involuntary movement of the legs and were excluded from this study due to inability to measure SPP. As a result, 185 patients were finally evaluated. History of amputations of lower extremities due to PAD at the start of HD was three patients in unilateral major amputation and one patient with minor amputation. There was no patient with bilateral major amputation.
Table 1 shows the basic characteristics of 185 patients (130 men, 55 women) with a median age of 70 years old [interquartile range (IQR) 61–78]. Almost half of the patients had diabetes (100/185: 54.5%), and 43.1% of the patients had ischemic heart disease. Median values of ABI and SPP among all patients were 1.18 (IQR 1.05–1.27) and 73 (IQR 52–85) mmHg, respectively.
Distribution of ABI and SPP value and the prevalence of PAD
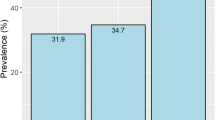
Distribution of ABI and SPP in incident HD patients was shown in Fig. 1. While limbs with ABI value less than 0.9 were 10.8%, 21.1% of limbs showed SPP values less than 50 mmHg among total limbs. On the other hand, limbs with ABI value more than 1.3 were 19.6% among total limbs at the initiation of HD therapy (Fig. 1). If ABI value was set at 1.0, 19.6% of patients showed ABI < 1.0.
Prevalence of PAD at the initiation of HD assessed by ABI and SPP, histories of revascularization and amputation were 24.3% (45/185). Fontaine severity classification among incident HD patients with PAD is shown in Table 2. Among 45 patients with PAD, almost 70% of patients (31/45) were asymptomatic. The number of patients who have already been diagnosed as having PAD before starting HD was 29 patients (15.7%).
Relationship between ABI and SPP
SPP and ABI positively correlated (r = 0.311, p = 0.006; Fig. 2). Fourteen patients (7.6%) showed ABI < 0.9 and SPP < 50 mmHg, and 140 patients (75.6%) showed ABI ≧ 0.9 and SPP ≧ 50 mmHg, respectively. However, discrepancy between ABI and SPP values (normal or high ABI with low SPP, or low ABI with normal SPP) was also found. Six patients (3.2%) showed ABI < 0.9 and SPP ≧ 50 mmHg, and 25 patients (13.5%) showed ABI ≧ 0.9 and SPP < 50 mmHg, respectively.
Risk factors for PAD in incident HD patients
When patients were divided into two groups, i.e., PAD group and non-PAD group, there were significant differences in the prevalence of ischemic heart disease, total cholesterol, and LDL cholesterol between two groups. Ischemic heart disease was more prevalent in PAD patients compared with non-PAD patients (60 vs. 33.6%; p < 0.001) (Table 3). Multiple regression analysis, using significant variables in univariate analysis as independent variables, showed that only ischemic heart disease was an associating factor for PAD at the initiation of HD (Table 4). Both older age or diabetes mellitus were not independent associating factors for PAD in patients with end stage renal failure.
Discussion
We have clearly provided the evidence that the prevalence of PAD in incident HD patients was high (24.3%) by ABI and SPP measurements along with ischemic leg symptoms and histories of re-vascularization or amputation due to PAD. Previous epidemiological reports using ABI and SPP could not fully elucidate such high rate of PAD in incident HD patients. Risk factors for PAD include old age, male gender, smoking, diabetes mellitus, homocysteine, and C-reactive protein [20,21,22,23]. Furthermore, renal failure itself was an independent risk factor for PAD [23]. Patients at the initiation of HD have not only renal failure but also several risk factors as mentioned above. Therefore, it may be well appreciated that the prevalence of PAD is already very high at the initiation of HD.
The symptoms of PAD in its early stage are not so clinically prevalent or even might be asymptomatic so that patients do not complain of the symptoms related to PAD. Elderly HD patients might not walk long enough to complain of apparent intermittent claudication. Therefore, PAD might be missed when only judged by clinical symptoms. ABI has been widely used in previous reports as screening tool for PAD in HD patients. However, it has been well known that measurement of ABI might be strongly influenced in cases of highly calcified or incompressible arteries [5, 10].
SPP is a useful tool for PAD screening. SPP can evaluate the microcirculation of lower limbs. The importance of SPP was first reported in critical limb ischemia with intractable wound. SPP values more than 30 or 40 mmHg have been reported to predict healing of intractable wound [9, 17]. Thus, the significance of SPP measurement has, at first, established a consensus in evaluating severe limb ischemia and as a predictor of wound healing. Besides in the cases of critical limb ischemia (CLI), usefulness to measure SPP has recently been expanding its area as diagnosing tool of early stage of PAD.
As for discrepancy between ABI and SPP values, there might be some possible explanations. In cases with normal or high ABI value (≧ 0.9) and low SPP value (< 50 mmHg), high arterial calcification might pseudo-normalize or even elevate the ABI value to abnormally high levels. Another possibility is the existence of below-ankle arterial lesions. ABI was measured using ankle pressure, and SPP in this study examined microcirculation of below ankle lesions (instep and sole). If patient has PAD only in below ankle arteries, normal ABI and abnormally low SPP values might be accepted. In cases with low ABI and normal SPP values, below knee arterial lesions (above ankle) with well-developed collaterals in foot might explain the discrepancy of ABI and SPP. In anyway, sole ABI value below 0.9 might miss considerable number of PAD patients with normal or high ABI and low SPP values as shown in Fig. 2. Measurements of both ABI and SPP might improve the diagnostic accuracy of PAD.
In our study, previous history of ischemic heart disease was significantly associated with the prevalence of PAD (Table 3). Sixty percent of incident HD patients with PAD had the history of ischemic heart disease. Therefore, PAD might be presented as one of the systemic atherosclerotic disease as shown in REACH registry [24]. We should check other atherosclerotic diseases including ischemic heart disease or cerebrovascular disease when PAD was diagnosed at the initiation of HD. In this point, early diagnosis of PAD might contribute to improve the prognosis of HD patients.
The rate of prescription of anti-platelet drugs was significantly different between PAD group and non-PAD group in our study (Table 3). Aspirin was prescribed in 48.9% of incident HD patients with PAD whereas only 22.6% of patients without PAD took aspirin. However, aspirin was mainly prescribed for the purpose of secondary prevention of ischemia events in the heart and brain. In other words, aspirin prescription was only made in 48.9% of PAD patients. Far less HD patients with PAD took cilostazol, sarpogrelate, or prostaglandin analogue. Other antiplatelet drugs should be considered to be prescribed in terms of PAD [25,26,27]. The final purpose of medication for PAD is to increase walking distance, prevent CLI and amputation, and improve the prognosis. Early diagnosis using ABI and SPP might contribute to early prescription and improved quality of life and prognosis.
Recently, low values of ABI and/or SPP were reported to be a prognostic factor of cardiovascular disease (CVD) and mortality. Otani et al. reported that both low ABI and SPP values were independent risk factors for mortality among HD patients [28]. Ishi et al. reported that abnormal ABI (< 0.9 or > 1.4) predicted mortality due to CVD [29] in HD patients. Therefore, ABI and/or SPP might be used not only as diagnostic tools of PAD but also one of prognostic factors in HD patients.
Although this is a single center, cross-sectional observational study, we have provided that the prevalence of PAD in incident HD patients was surprisingly high and most of these patients were asymptomatic. Both ABI and SPP measurement might be useful for diagnosing PAD in incident HD patients. Further studies are needed to clarify whether early diagnosis of PAD might improve the outcome of incident HD patients.
Conclusion
Measurement of both ABI and SPP might be necessary to improve the diagnostic accuracy of PAD. Prevalence of PAD in incident HD patients was proved to be very high.
References
Eggers PW, Gohdes D, Pugh J. Nontraumatic lower extremity amputations in the Medicare end-stage renal disease population. Kidney Int. 1999;56:1524–33.
Dossa CD, Shepard AD, Amos AM, Kupin WL, Reddy DJ, Elliott JP, Wilczwski JM, Ernst CB. Results of lower extremity amputations in patients with end-stage renal disease. J Vasc Surg. 1994;20:14–9.
Aulivola B, Hile CN, Hamdan AD, Sheahan MG, Veraldi JR, Skillman JJ, Campbell DR, Scovell SD, LoGerfo FW, Pomposelli FB Jr. Major lower extremity amputation: outcome of a modern series. Arch Surg. 2004;139:395–9.
Okamoto K, Oka M, Maesato K, Ikee R, Mano T, Moriya H, Ohtake T, Kobayashi S. Peripheral arterial occlusive disease is more prevalent in patients with hemodialysis: comparison with the findings of multidetector-row computed tomography. Am J Kidney Dis. 2006;48:269–76.
Ohtake T, Oka M, Ikee R, Mochida Y, Ishioka K, Moriya H, Hidaka S, Kobayashi S. Impact of lower limbs’ arterial calcification on the prevalence and severity of PAD in patients on hemodialysis. J Vasc Surg. 2011;53:676–83.
Shimazaki M, Matsuki T, Yamauchi K, Iwata M, Takahashi H, Genda S, Ohata J, Nakamura Y, Inaba Y, Yokouchi S, Kikuiri T, Ashie T. Assessment of lower limb ischemia with measurement of skin perfusion pressure in patients on hemodialysis. Ther Apher Dial. 2007;11:196–201.
Tsai FW, Tulsyan N, Jones DN, Abdel-Al N, Castronuovo JJ Jr, Carter SA. Skin perfusion pressure of the foot is a good substitute for toe pressure in the assessment of limb ischemia. J Vasc Surg. 2000;32:32–6.
Davis M, Rajagopalan S. Is skin perfusion pressure a useful screening tool for peripheral arterial disease in patients on hemodialysis? Nat Clin Pract Nephrol. 2007;3:598–9.
Yamada T, Ohta T, Ishibashi H, Sugimoto I, Iwata H, Takahashi M, Kawanishi J. Clinical reliability and utility of skin perfusion pressure measurement in ischemic limbs—comparison with other noninvasive diagnostic methods. J Vasc Surg. 2008;47:318–23.
Leskinen Y, Salenius JP, Lehtimäki T, Huhtala H, Saha H. The prevalence of peripheral arterial disease and medial arterial calcification in patients with chronic renal failure: requirements for diagnosis. Am J Kidney Dis. 2002;40:472–9.
Kitaura K, Kida M, Harima K. Assessment of peripheral arterial disease of lower limbs with ultrasonography and ankle brachial index at the initiation of hemodialysis. Renal Fail. 2009;31:785–90.
Morimoto S, Yurugi T, Aota Y, Sakuma T, Jo F, Nishikawa M, Iwasaka T, Maki K. Prognostic significance of ankle-brachial index, brachial-ankle pulse wave velocity, flow-mediated dilatation, and nitroglycerin-mediated dilation in end-stage renal failure. Am J Nephrol. 2009;30:55–63.
Ogata H, Kumata-Maeta C, Shishido K, Mizobuchi M, Yamamoto M, Koiwa F, Kinugasa E, Akizawa T. Detection of peripheral artery disease by duplex ultrasonography among hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:2199–206.
Chen SC, Chang JM, Hwang SJ, Tsai JC, Liu WC, Wang CS, Lin TH, Su HM, Chen HC. Ankle brachial index as a predictor for mortality in patients with chronic kidney disease and undergoing haemodialysis. Nephrology. 2010;15:294–9.
Kato A, Takita T, Furuhashi M, Kumagai H, Hishida A. A small decrease in the ankle-brachial index is associated with increased mortality in patients on chronic hemodialysis. Nephron Clin Prac. 2010;114:c29–37.
Kondo Y, Muto A, Dardik A, Nishibe M, Nishibe T. Laser Doppler skin perfusion pressure in the diagnosis of limb ischemia in patients with diabetes mellitus and/or hemodialysis. Int Angiol. 2007;26:258–61.
Castronuovo JJ Jr, Adera HM, Smiell JM, Price RM. Skin perfusion pressure of the foot is valuable in the diagnosis of critical limb ischemia. J Vasc Surg. 1997;26:629–37.
Hirakata H, Nitta K, Inaba M, Shoji T, Fujii H, Kobayashi S, Tabei K, Joki N, Hase H, Nishimura M, Ozaki S, Ikari Y, Kumada Y, Tsuruya K, Fujimoto S, Inoue T, Yokoi H, Hirata S, Shimamoto K, Kugiyama K, Akiba T, Iseki K, Tsubakihara Y, Tomo T, Akizawa T; Japanese Society for Dialysis Therapy. Japanese Society for Dialysis Therapy guidelines for management of cardiovascular diseases in patients on chronic hemodialysis. Ther Apher Dial 2012; 16: 387–435.
Fontaine R, Kim M, Kieny R. Surgical treatment of peripheral circulation disorders. Helv Chir Acta. 1954;21:499–533.
Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–5.
Fowkes FG, Housley E, Riemersma RA, Macintyre CC, Cawood EH, Prescott RJ, Ruckley CV. Smoking, lipids, glucose intolerance, and blood pressure as risk factors for peripheral atherosclerosis compared with ischemic heart disease in the Edinburgh Artery Study. Am J Epidemiol. 1992;135:331–40.
Lowe GD, Fowkes FG, Dawes J, Donnan PT, Lennie SE, Housley E. Blood viscosity, fibrinogen, and activation of coagulation and leukocytes in peripheral arterial disease and the normal population in the Edinburgh Artery Study. Circulation. 1993;87:1915–20.
MacGregor AS, Price JF, Hau CM, Lee AJ, Carson MN, Fowkes FG. Role of systolic blood pressure and plasma triglycerides in diabetic peripheral arterial disease. The Edinburgh Artery Study. Diabetes Care. 1999;22:453–8.
Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Röther J, Wilson PW; REACH Registry Investigators. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006; 11: 180–189.
Hidaka S, Kobayashi S, Iwagami M, Isshiki R, Tsutsumi D, Mochida Y, Ishioka K, Oka M, Maesato K, Moriya H, Ohtake T. Sarpogrelate hydrochloride, a selective 5-HT(2A) receptor antagonist, improves skin perfusion pressure of the lower extremities in hemodialysis patients with peripheral arterial disease. Ren Fail. 2013;35:43–8.
Ohtake T, Sato M, Nakazawa R, Kondoh M, Miyaji T, Moriya H, Hidaka S, Kobayashi S. Randomized pilot trial between prostaglandin I2 analog and anti-platelet drugs on peripheral arterial disease in hemodialysis patients. Ther Apher Dial. 2014;18:1–8.
Pande RL, Perlstein TS, Beckman JA, Creager MA. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011;124:17–23.
Otani Y, Otsubo S, Kimata N, Takano M, Abe T, Okajima T, Miwa N, Tsuchiya K, Nitta K, Akiba T. Effects of the ankle-brachial blood pressure index and skin perfusion pressure on mortality in hemodialysis patients. Intern Med. 2013;52:2417–21.
Ishii H, Takahashi H, Ito Y, Aoyama T, Kamoi D, Sakakibara T, Umemoto N, Kumada Y, Suzuki S, Murohara T. The association of ankle brachial index, protein-energy wasting, and inflammation status with cardiovascular mortality in patients on chronic hemodialysis. Nutrients. 2017;9(4):416.
Availability of data and materials
Data are available on request to the authors.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the ethical committee of Tokushukai Group ethical committee (No.TGE00797-024, 3 March 2017).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ishioka, K., Ohtake, T., Moriya, H. et al. High prevalence of peripheral arterial disease (PAD) in incident hemodialysis patients: screening by ankle-brachial index (ABI) and skin perfusion pressure (SPP) measurement. Ren Replace Ther 4, 27 (2018). https://doi.org/10.1186/s41100-018-0168-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-018-0168-5