Abstract
Background
Asymptomatic peripheral artery disease (PAD) increases the risk of mortality in non-hemodialysis patients. However, the association between asymptomatic PAD and mortality rate remains unclear in patients on hemodialysis.
Methods
This retrospective cohort study aimed to assess the prognostic significance of asymptomatic PAD in a population of 310 hemodialysis patients. Patients with an ankle–brachial index of < 1.00, or > 1.40 with a toe–brachial index of < 0.70, were diagnosed as having PAD. The San Diego Claudication Questionnaire was used to characterize leg symptoms in patients with PAD, and asymptomatic PAD was defined as the absence of symptoms in the legs or buttocks while walking. The mortality risk of asymptomatic PAD was assessed using the Cox proportional hazard model.
Results
The rate of PAD was 28.1%. Among 87 patients, those with PAD, 66.7% were asymptomatic. Fifty-eight patients died during a mean follow-up of 38.9 months. Multivariate analysis revealed hazard ratios of 1.963 (95% confidence interval (CI), 1.012 to 3.740; P = 0.046) and 3.237 (95% CI, 1.402 to 7.020; P = 0.007) in patients with asymptomatic PAD and symptomatic PAD, respectively, compared to patients without PAD. No significant difference was observed between patients with asymptomatic PAD and symptomatic PAD in terms of survival.
Conclusions
Hemodialysis patients with asymptomatic PAD have an elevated mortality risk compared to patients without PAD, with no significant difference compared to patients with symptomatic PAD.
Similar content being viewed by others
Background
Peripheral artery disease (PAD) is associated with an increased risk of all-cause and atherosclerotic mortality [1]. Leg symptoms are typical complaints of patients with PAD, and the severity of PAD is classified according to the degree of leg symptoms, such as the Fontaine and Rutherford classifications [2,3,4,5]. Although intermittent claudication and leg pain are well known and the most common symptoms of PAD, the majority of PAD patients are asymptomatic. In 2009, Diehm et al. [6] reported that the mortality risk among asymptomatic PAD patients did not differ significantly from that among symptomatic PAD patients in community-dwelling adults. However, few reports are available regarding the usefulness of medical therapy for asymptomatic PAD to derive a meaningful conclusion. The current American Heart Association/American College of Cardiology guidelines [7] and Society for Vascular Surgery guidelines [8] do not strongly recommend treatment interventions for patients with asymptomatic PAD.
The risk of atherosclerosis is elevated regardless of the stage of chronic kidney disease, especially among patients undergoing hemodialysis (HD patients) [9]. PAD is an important manifestation of systemic atherosclerosis and is observed commonly in HD patients. The prevalence of PAD is markedly high in HD patients, although approximately 68.7% are asymptomatic [10]. One reason for the high proportion of asymptomatic PAD among HD patients is that habitual physical activity levels are reportedly low in these patients [11], and given that HD patients often have peripheral neuropathy due to a high prevalence of diabetes [12], their leg symptoms such as pain and numbness are masked. As a result, these patients have a similar risk of poor prognosis. Previous studies in HD patients reported a relationship between PAD and elevated mortality risk [13, 14]; however, no report has focused on the presence of leg symptoms. Moreover, the mortality risk associated with asymptomatic PAD remains unclear in HD patients. Accordingly, the present retrospective cohort study aimed to examine the mortality risk of asymptomatic PAD in HD patients.
Methods
This retrospective cohort study (registry number UMIN000020830) was reported in accordance with the STROBE guidelines (Additional file 1) [15].
Study design and patients
This study was a single-center, retrospective, and longitudinal cohort study. We conducted a retrospective analysis of patient data from another prospective study. The study was performed in accordance with the Declaration of Helsinki and the protocol was approved by the Kitasato University Allied Health Sciences Research Ethics Committee (approval no. 2012-020), and informed consent was obtained from all the patients.
We performed a retrospective cohort study of 390 patients undergoing maintenance HD at Sagami Junkanki Clinic (HD treatment organization certified by the Japanese Society for Dialysis Therapy) between May 2012 and May 2015. Inclusion criteria in this study were age > 20 years and provided informed consent for another prospective study. The exclusion criteria were patients with an ankle–brachial index (ABI) > 0.99 with lower extremity peripheral revascularization and amputation at baseline. Patients were analyzed from the date of first measurement of ABI until death or end of follow-up on Oct 2016. The date and cause of death for patients who died were obtained from their medical records. Patients who left the facilities or underwent kidney transplantation were censored. The date of death or censoring was recorded for the time-to-event analysis of all-cause mortality.
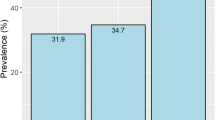
Among 390 patients undergoing HD between May 2012 and May 2015, 62 patients did not provide informed consent. Therefore, 328 patients followed up were included in this retrospective study. Additionally, we excluded 13 patients with missing data, and 5 patients with an ABI > 0.99 with lower extremity peripheral revascularization and amputation from this study. Thus, 310 HD patients were analyzed. Of these, 87 (28.1%) patients had PAD, including 58 (66.7%) with asymptomatic PAD (Fig. 1).
Baseline characteristics
Baseline characteristics of patients, including age, sex, body mass index, dialysis vintage (time since initiation of dialysis), cause of end-stage renal disease (ESRD), diabetes, comorbidities (e.g., coronary artery disease, cerebrovascular disease, and spinal stenosis), medications (e.g., angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, beta-blockers, lipid-lowering agents, antiplatelet agents, and anticoagulant agents), smoking status (ever or never), systolic blood pressure, diastolic blood pressure, pulse pressure, pulse rate, levels of serum albumin, creatinine, calcium, phosphorus, hemoglobin, hematocrit, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, and triglycerides, and geriatric nutritional risk index (GNRI) were obtained from medical records at the time of study entry. Blood data were measured immediately before the hemodialysis session. GNRI was calculated from the patient’s serum albumin, body weight, and height as follows: GNRI = (14.89 × albumin) + (41.7 × (body weight/body weight at BMI of 22)) [16].
Diagnosis of PAD
PAD was diagnosed based on the ankle–brachial index (ABI) and toe–brachial index (TBI), which were obtained using a blood pressure pulse-wave inspection apparatus (Form3; Omron Colin, Tokyo, Japan) that allows for simultaneous measurement of arm (without dialysis access) and ankle or toe blood pressure. All patients were examined at rest in the supine position by clinical laboratory technologists using the same apparatus. ABI was calculated as the ratio of ankle systolic blood pressure to arm systolic blood pressure and TBI as the ratio of toe systolic blood pressure to arm systolic blood pressure. The lowest values of ankle and toe pressure were used for the calculations. ABI is generally used as a tool for detecting PAD, with a cutoff value of < 0.90 for detecting PAD in the general population [3]. However, since ABI values are high in HD patients and may be influenced by vascular calcification [17], an ABI cutoff of < 0.90 could result in underdiagnosing PAD in this patient population. Therefore, given that current guidelines specify a normal ABI range to be 1.00 to 1.40 and recommend follow-up for those with an ABI of > 1.4, with a TBI of < 0.7 indicating PAD [7], PAD was diagnosed in the present study using an ABI of < 1.00, or > 1.4 and a TBI of < 0.7, in HD patients.
Leg symptoms
A structured interview was conducted to assess leg symptoms in patients. Leg symptoms in patients with PAD were characterized using the San Diego Claudication Questionnaire [18, 19], which is derived from the Rose Claudication Questionnaire [20], and is used to classify symptoms as intermittent claudication, leg pain on exertion and rest, atypical exertional leg pain/carry on (i.e., exertional leg symptoms that do not begin at rest and do not stop the individual while walking), atypical exertional leg pain/stop exertional leg symptoms (i.e., exertional leg symptoms that do not begin at rest, prevent the individual from walking, and do not involve the calves or resolve within 10 min of rest), or the absence of clinical symptoms based on the response to the question, “Do you get pain in either the legs or buttocks while walking?” [21]. Symptomatic PAD was defined as PAD with exertional leg symptoms (i.e., intermittent claudication, leg pain on exertion and rest, and atypical exertional leg pain), while asymptomatic PAD was defined as PAD with no clinical symptoms [21].
Statistical analysis
The results of normally distributed continuous variables are expressed as the means ± standard deviation, and non-normally distributed variables are presented as the median (25th and 75th percentiles). Patients were divided into four groups according to PAD diagnosis and leg symptoms: non-PAD, total PAD (asymptomatic and symptomatic PAD), asymptomatic PAD, and symptomatic PAD groups. In addition, differences between non-PAD and total PAD groups and between asymptomatic PAD and symptomatic PAD groups at baseline were investigated exploratively with χ2 tests, t tests, and Mann-Whitney U tests, respectively. The cumulative incidence of death was calculated in all groups using the Kaplan–Meier method, and comparisons were performed using the log-rank test.
Univariate and multivariate analyses were performed using Cox proportional hazards regression models to estimate the independent prognostic effect of PAD on survival after adjusting for confounders. Four separate models were used for comparisons: between non-PAD and total PAD groups (analysis included all patients), between non-PAD and asymptomatic PAD groups (patients with symptomatic PAD were excluded), between non-PAD and symptomatic PAD groups (patients with asymptomatic PAD were excluded), and between asymptomatic PAD and symptomatic PAD groups (patients without PAD were excluded).
We initially considered the following variables as potential confounders: age, sex, body mass index, dialysis vintage, smoking status, cause of ESRD, diabetes, coronary artery disease, cerebrovascular disease, and levels of creatinine, albumin, hemoglobin, HDL-C, and GNRI. These variables were selected based on an a priori determined model and evaluation of patient characteristics associated with mortality. Multicollinearity was tested by investigating the variance inflation factor and tolerance in multiple linear regression analysis. Two levels of multivariate analyses were performed: model 1, with adjustment for demographic characteristics (age, sex, and body mass index); and model 2, an augmented version of model 1 with adjustment for age, cause of ESRD, cerebrovascular disease, creatinine, and GNRI. Unadjusted and adjusted hazard ratios for death with 95% confidence intervals (95% CIs) were obtained. Statistical analyses using the t tests, Mann-Whitney U tests, χ2 tests, Kaplan–Meier method, and Cox proportional hazards regression models were performed using SPSS for Windows version 24.0 (IBM SPSS, Chicago, IL). In all analyses, P < 0.05 was considered statistically significant. The sample power was calculated with the number of patients in the asymptomatic PAD group, the hazard ratio for the non-PAD group compared to the asymptomatic PAD group, the median survival time in the non-PAD group, accrual time during which patients were recruited, additional follow-up time after the end of recruitment, and the ratio of non-PAD patients to asymptomatic PAD patients. The probability of type I error associated with the test of the null hypothesis was 0.05.
Results
Baseline characteristics
Table 1 summarizes the baseline characteristics of the study population comprising 183 (59.0%) men and 127 (41.0%) women. The median age was 66 (25th and 75th percentiles, 61, 74) years, median dialysis vintage was 5 (25th and 75th percentiles, 1, 13) years, and the most common cause of ESRD was diabetic nephropathy (34.5%) followed by glomerulonephritis (30.6%). Significant differences were observed between non-PAD and total PAD groups in age, cause of ESRD, prevalence of diabetes, coronary artery disease, cerebrovascular disease, spinal stenosis, use of beta-blockers, antiplatelet agents, and anticoagulant agents, smoking status, diastolic blood pressure, pulse pressure, ABI, TBI, and levels of creatinine, albumin, HDL-C, and triglycerides. A significant difference in dialysis vintage and ABI was observed between asymptomatic PAD and symptomatic PAD groups.
Kaplan–Meier estimate of patient survival
All patients were followed for up to 4 years. The follow-up period ranged from 2 to 48 months overall, with a median of 48.0 months for all groups, 48.0 months for the non-PAD group, 45.0 months for the total PAD group, 44.0 months for the asymptomatic PAD group, and 45.0 months for the symptomatic PAD group. Additional follow-up time after the end of recruitment was 17 months. In total, 58 patients died during the follow-up period due to the following causes: cardiovascular disease in 16, infection in 14, cancer in 7, cerebral vascular disease in 4, others in 7, and unknown in 10. Kaplan–Meier survival curves based on all-cause mortality for non-PAD and total PAD groups are shown in Fig. 2. The cumulative survival rate in the total PAD group was significantly lower than in the non-PAD group (log-rank χ2 = 19.890; P < 0.001). Kaplan–Meier survival curves based on all-cause mortality for non-PAD, total PAD, asymptomatic PAD, and symptomatic PAD groups are shown in Fig. 3. Cumulative survival rates were significantly lower in asymptomatic PAD and symptomatic PAD groups compared to the non-PAD group, but were not significant deference between asymptomatic PAD and symptomatic PAD groups (log-rank χ2 = 0.334; P = 0.564).
Multivariate analysis of the effect of asymptomatic PAD on mortality
The results of univariate Cox proportional hazards analysis of covariates to predict all-cause mortality are shown in Table 2. Total PAD, asymptomatic PAD, and symptomatic PAD groups had crude hazard ratios of 3.040 (95% CI, 1.816 to 5.089; P < 0.001), 2.809 (95% CI, 1.559 to 5.059; P = 0.001), and 3.515 (95% CI, 1.755 to 7.041; P < 0.001), respectively, compared to the non-PAD group. The results of multivariate Cox proportional hazards analysis of covariates to predict all-cause mortality are shown in Table 3. We developed two multivariate models to adjust for demographic and clinical characteristics. There was no multicollinearity among independent variables. In model 1, after adjusting for the effects of demographic characteristics, total PAD, asymptomatic PAD, and symptomatic PAD groups had hazard ratios of 2.796 (95% CI, 1.654 to 4.730; P < 0.001), 2.323 (95% CI, 1.257 to 4.183; P = 0.008), and 4.159 (95% CI, 1.970 to 8.179; P < 0.001), respectively, compared to the non-PAD group. In model 2, after adjusting for age, cause of ESRD, cerebrovascular disease, creatinine and GNRI, total PAD, asymptomatic PAD, and symptomatic PAD groups had hazard ratios of 2.273 (95% CI, 1.262 to 4.110; P = 0.006), 1.963 (95% CI, 1.012 to 3.740; P = 0.046), and 3.237 (95% CI, 1.402 to 7.020; P = 0.007), respectively, compared to the non-PAD group. No significant difference was observed between asymptomatic PAD and symptomatic PAD groups in terms of survival. The sample size in this study was sufficient as reflected by the sample power of 0.950.
Discussion
We examined all-cause mortality in a population of patients undergoing HD (n = 310). In total, 58 (18.7%) patients died during the observation period of up to 4 years. The mortality rate was significantly higher in HD patients with PAD compared to those without PAD, but no significant difference was found between patients with symptomatic PAD and those with asymptomatic PAD. This is the first study to report on the association between asymptomatic PAD and mortality risk in patients undergoing HD. Therefore, we considered that HD patients with asymptomatic PAD should not be underestimated.
The prevalence of PAD is higher in HD patients than in the general population. According to the Dialysis Outcomes and Practice Patterns Study (DOPPS), a prospective cohort observational study of adult HD patients, PAD prevalence rates in the total study population, in the USA/Canada, in Europe, in Australia/New Zealand, and in Japan were 25.3, 27.5–32.3, 17.5–38.0, 32.7, and 12.0%, respectively [13]. A large degree of variation in PAD prevalence was noted among 12 countries included in the DOPPS, with the highest rate of 38.0% in Belgium and lowest rate of 12.0% in Japan. DOPPS reports determined PAD by prior diagnosis of PAD, symptom, or surgical therapy for PAD. However, we provide prevalence of PAD by ABI and TBI. The prevalence of PAD in the present study was 28.1%, which is markedly higher than DOPPS reports. The diagnosis of PAD in HD patients is widely based on ABI; however, patients undergoing HD frequently show high ABI values due to medial artery calcification, despite limb ischemia [17]. TBI is a non-invasive and useful measure of PAD in patients with non-compressible arteries, which cause an artificial elevation of ABI [7], and its use is recommended by current guidelines for diagnosing PAD [3, 7]. The cutoff value of ABI for detecting PAD has been reported to be < 1.06 in HD patients, with high sensitivity and specificity of 80.0 and 98.0%, respectively [17]. In addition, since current guidelines specify a normal ABI range of 1.00 to 1.40 [22], we used a cutoff ABI of 1.00, rather than 0.9, for detecting PAD. The frequency distribution of ABI < 1.0 in previous study targeting Japanese HD patients was 25.1% of patients in Japanese HD patients, which is nearly similar those reported in this study [14]. Thus, the high prevalence of PAD in the present study population could be due to the ABI cutoff (< 1.00) used, in addition to including TBI for diagnosis.
In the present study, HD patients with asymptomatic PAD had unfavorable prognoses compared with those without PAD. Previous studies showed that patients with asymptomatic PAD had significantly greater functional impairment and faster functional decline than those without PAD [18, 21, 23, 24]. Previously, we reported the prognostic significance of poor physical performance, including poor muscle strength and physical inactivity, on survival in a cohort of HD patients [25, 26]. Moreover, the first multicenter, randomized controlled trial conducted to examine the relative benefits of exercise training in patients with symptomatic PAD indicated that exercise training was associated with greater improvement of walking performance than primary aorto-iliac stent revascularization and optimal medical care [27]. Furthermore, exercise training has been shown to reduce atherosclerotic risk factors, including lipid disorders, insulin resistance, dysfunction of the cardiac autonomic system, and reduced maximal oxygen consumption, which also accelerate PAD progression [28,29,30]. However, it remains unclear whether exercise training interventions can improve or reverse the decline in physical performance among HD patients with asymptomatic PAD, although studies suggest that patients with PAD might benefit from adopting a more active lifestyle [8].
This study has several limitations. First, we conducted this study in Japan using a single-center, observational design, retrospective; and therefore, further multicenter studies are required for generalizability. Second, multivariate analysis was performed using Cox proportional hazards regression models to estimate the independent prognostic effect of asymptomatic PAD on mortality after adjusting for confounders. Differences in patient characteristics attributed to asymptomatic PAD in the present study were adjusted for only by observed factors. Therefore, additional information regarding characteristics that could potentially affect the prevalence of asymptomatic PAD in HD patients will be necessary. Thirdly, because of retrospective study and clinical problem, it was difficult to align the timing for performing ABI test. However, in previous study, Su HM et al. reported that the ABI remained constant before and after HD, or on the next dialysis-free day [31]. Additionally, Jimenez ZN et al. reported that they found no difference in ABI pre- and post-dialysis on the right or left side [32]. Finally, although we found that the mortality risk in patients with asymptomatic PAD was higher than that in patients without PAD, the underlying mechanisms remain unknown.
Conclusions
The prevalence of PAD is high in HD patients, and while most HD patients with PAD have no lower leg symptoms, these patients (i.e., asymptomatic PAD patients) have a significantly increased mortality risk compared to those without PAD. Moreover, the mortality risk of HD patients with asymptomatic PAD was no difference from patients with symptomatic PAD. These findings underscore the importance of screening and stratification of hemodialysis patients at risk of mortality as a part of routine care for HD patients regardless of whether or not patients present with leg symptoms. Additionally, further investigation is needed to establish treatment on asymptomatic and symptomatic PAD for HD patients.
Abbreviations
- ABI:
-
Ankle–brachial index
- CI:
-
Confidence interval
- DOPPS:
-
Dialysis Outcome and Patterns Study
- ESRD:
-
End-stage renal disease
- GNRI:
-
Geriatric nutritional risk index
- HD:
-
Hemodialysis
- HDL-C:
-
High-density lipoprotein cholesterol
- PAD:
-
Peripheral artery disease
- SD:
-
Standard deviation
- TBI:
-
Toe–brachial index
References
Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300(2):197–208.
Fontaine R, Kim M, Kieny R. Surgical treatment of peripheral circulation disorders. Helv Chir Acta. 1954;21(5-6):499–533.
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5–67.
Baker JD, Rutherford RB, Bernstein EF, Courbier R, Ernst CB, Kempczinski RF, et al. Suggested standards for reports dealing with cerebrovascular disease. Subcommittee on Reporting Standards for Cerebrovascular Disease, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery/North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg. 1988;8(6):721–9.
Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(3):517–38.
Diehm C, Allenberg JR, Pittrow D, Mahn M, Tepohl G, Haberl RL, et al. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009;120(21):2053–61.
Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69(11):e71–e126.
Society for Vascular Surgery Lower Extremity Guidelines Writing Group, Conte MS, Pomposelli FB, Clair DG, Geraghty PJ, JF MK, et al. Society for vascular surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg. 2015;61(Suppl):2S–41S.
Cheung AK, Sarnak MJ, Yan G, Dwyer JT, Heyka RJ, Rocco MV, et al. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000;58(1):353–62.
Matsuzawa R, Aoyama N, Yoshida A. Clinical characteristics of patients on hemodialysis with peripheral arterial disease. Angiology. 2015;66(10):911–7.
Johansen KL, Chertow GM, Ng AV, Mulligan K, Carey S, Schoenfeld PY, et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int. 2000;57(6):2564–70.
Ndip A, Rutter MK, Vileikyte L, Vardhan A, Asari A, Jameel M, et al. Dialysis treatment is an independent risk factor for foot ulceration in patients with diabetes and stage 4 or 5 chronic kidney disease. Diabetes Care. 2010;33(8):1811–6.
Rajagopalan S, Dellegrottaglie S, Furniss AL, Gillespie BW, Satayathum S, Lameire N, et al. Peripheral arterial disease in patients with end-stage renal disease: observations from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Circulation. 2006;114(18):1914–22.
Ono K, Tsuchida A, Kawai H, Matsuo H, Wakamatsu R, Maezawa A, et al. Ankle-brachial blood pressure index predicts all-cause and cardiovascular mortality in hemodialysis patients. J Am Soc Nephrol. 2003;14(6):1591–8.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296.
Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–83.
Ohtake T, Oka M, Ikee R, Mochida Y, Ishioka K, Moriya H, et al. Impact of lower limbs’ arterial calcification on the prevalence and severity of PAD in patients on hemodialysis. J Vasc Surg. 2011;53(3):676–83.
McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286(13):1599–606.
Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1(1):65–71.
Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–58.
McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292(4):453–61.
Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, et al. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58(19):2020–45.
McDermott MM, Ferrucci L, Liu K, Guralnik JM, Tian L, Liao Y, et al. Leg symptom categories and rates of mobility decline in peripheral arterial disease. J Am Geriatr Soc. 2010;58(7):1256–62.
McDermott MM, Guralnik JM, Ferrucci L, Tian L, Liu K, Liao Y, et al. Asymptomatic peripheral arterial disease is associated with more adverse lower extremity characteristics than intermittent claudication. Circulation. 2008;117(19):2484–91.
Matsuzawa R, Matsunaga A, Wang G, Kutsuna T, Ishii A, Abe Y, et al. Habitual physical activity measured by accelerometer and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(12):2010–6.
Matsuzawa R, Matsunaga A, Wang G, Yamamoto S, Kutsuna T, Ishii A, et al. Relationship between lower extremity muscle strength and all-cause mortality in Japanese patients undergoing dialysis. Phys Ther. 2014;94(7):947–56.
Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125(1):130–9.
Matsuzawa R, Matsunaga A, Kutsuna T, Ishii A, Abe Y, Yoneki K, et al. Association of habitual physical activity measured by an accelerometer with high-density lipoprotein cholesterol levels in maintenance hemodialysis patients. ScientificWorldJournal. 2013;2013:780783.
Mustata S, Chan C, Lai V, Miller JA. Impact of an exercise program on arterial stiffness and insulin resistance in hemodialysis patients. J Am Soc Nephrol. 2004;15(10):2713–8.
Deligiannis A, Kouidi E, Tourkantonis A. Effects of physical training on heart rate variability in patients on hemodialysis. Am J Cardiol. 1999;84(2):197–202.
Su HM, Chang JM, Lin FH, Chen SC, Voon WC, Cheng KH, et al. Influence of different measurement time points on brachial-ankle pulse wave velocity and ankle-brachial index in hemodialysis patients. Hypertens Res. 2007;30(10):965–70.
Jimenez ZN, de Castro I, Pereira BJ, de Oliveira RB, Romao JE Jr, Elias RM, et al. When is the best moment to assess the ankle brachial index: pre- or post hemodialysis? Kidney Blood Press Res. 2012;35(4):242–6.
Acknowledgements
We thank all patients for their participation and commitment during the study. We also thank all investigators and contributors of our study.
Funding
This research was supported by a Kitasato University Research grant and JSPS KAKENHI grant number JP16K 16466.
Availability of data and materials
We decided not to share the data in our study because all data are thoroughly described and reflected in the accompanying tables and figures (all relevant data are within the paper).
Author information
Authors and Affiliations
Contributions
MH, RM, NA, YH, and AM conceived the research idea and study design. MH, KU, JY, KY, TW, and TS were involved for the data acquisition. MH, RM, NA, and AM were responsible for the data analysis/interpretation and MH and KH were responsible for the statistical analysis, while NA, YT, SN, AY and AM were responsible for the supervision or mentorship. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Kitasato University School of Allied Health Sciences and was conducted in accordance with the standards set forth by the latest revision of the Declaration of Helsinki. All patients received a detailed explanation of the study protocol and provided informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Table S1. STROBE Statement—checklist of items that should be included in reports of observational studies. (DOCX 38 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Harada, M., Matsuzawa, R., Aoyama, N. et al. Asymptomatic peripheral artery disease and mortality in patients on hemodialysis. Ren Replace Ther 4, 17 (2018). https://doi.org/10.1186/s41100-018-0159-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-018-0159-6