Abstract
Background
The management of renal anemia in the pre-dialysis period has been remarkably improved by long-acting erythropoiesis-stimulating agents (ESA). However, many incident dialysis patients cannot achieve target hemoglobin (Hb) levels (> 10 g/dL) and sometimes require blood transfusions. Anemia at the time of dialysis initiation is reportedly correlated with cardiomegaly and early cardiovascular events. Here, we investigated whether this V-shaped depression in Hb level at dialysis initiation adversely affects short-term prognosis.
Methods
The medical charts of 166 patients who underwent initial dialysis were retrospectively reviewed for Hb level, ESA treatment status, dry weight (DW), cardiothoracic rate (CTR), and brain natriuretic peptide (BNP) level at dialysis initiation and 1 year later. Patients were subdivided into three groups according to the tertile of Hb levels. The risk of mortality within 1 year after initiation was analyzed using multivariable-adjusted Cox proportional hazard model.
Result
Mean Hb level at initiation was 8.6 ± 1.3 g/dL despite the administration of sufficient ESA. After initiation, Hb levels rapidly increased and the Hb time course showed a V-shape with the bottom at initiation. Hb level, CTR, and log BNP showed a significant negative correlation. The Hb level and CTR 1 year after initiation did not correlate with Hb levels at initiation. Lower Hb levels at initiation as a V-shaped depression do not adversely affect 1-year mortality rate by multivariable-adjusted Cox proportional hazard model.
Conclusion
Hb level around dialysis initiation showed a V-shaped depression despite ESA use. Our findings suggest that the V-shaped Hb depression at initiation does not affect short-term prognosis.
Similar content being viewed by others
Background
Before the advent of erythropoiesis-stimulating agents (ESA), renal anemia arose as an almost obligatory complication of chronic kidney failure requiring blood transfusions and early dialysis initiation for patients with end-stage kidney disease (ESKD). With ESA use, the management of renal anemia in the pre-dialysis period has been dramatically improved, reducing blood transfusion rates and postponing dialysis initiation by symptomatically relieving anemia and creating a renal protective effect. However, it has been reported that hemoglobin (Hb) decreases to 8.4 g/dL during the initial dialysis phase despite recombinant human erythropoietin (rHuEPO) treatment [1].
This study aimed to evaluate the influence of Hb depression at dialysis initiation on short-term prognosis. Our principal goal was to investigate if a V-shaped Hb depression at initiation has any effect on short-term prognosis.
Methods
Patients
We retrospectively reviewed the charts of inpatients who underwent initial dialysis between January 2012 and May 2015 at Dokkyo Medical University Saitama Medical Center (Koshigaya, Saitama, Japan). After initiation, all patients were treated in an ordinary outpatient clinic according to Japanese guidelines.
This was an observational study and approved by the Dokkyo Medical University Saitama Medical Center Ethics Committee (2017/1716). Observations and inspections were conducted according to the items and methods below, and the collected data were used in the study.
Basic patient information included date of initiation, age, sex, primary disease, main reason for dialysis initiation, body weight, and blood pressure at initiation. Laboratory values including levels of Hb, blood urea nitrogen (BUN), creatinine (Cr), potassium (K), calcium (Ca), phosphorus (P), albumin, Fe, ferritin, and C-reactive protein; estimated glomerular filtration rate (eGFR); total iron binding capacity (TIBC); and transferrin saturation (TSAT) were collected from 6 months prior to 1 year after initiation. Data on B-type natriuretic peptide (BNP) level were collected at initiation, while data on cardiothoracic rate (CTR) were collected at initiation and 1 year after initiation.
State of ESA administration included ESA drug name and dosage. Erythropoietin resistance index (ERI) (IU/kg/week/g/100 mL) = weekly ESA dose (unit as Epo conversion rate)/(Hb [g/dL] × body weight [kg]). Considering the use of different ESA, the dose conversion ratio was rHuEpo to darbepoetin to epoetin beta pegol of 1:200:240 [2, 3]. The usual ESA treatment interval is once a month, although some patients received it biweekly.
A total of 166 incident patients were followed up for 1 year; of them, 12 died (7.2%), 6 were lost to follow-up, 4 underwent transplantation, and 144 remained on dialysis (Fig. 1).
Patients were subdivided into three groups according to the tertile of Hb levels and compared Hb levels and CTR at 1 year after initiation.
The risk of mortality within 1 year after initiation was analyzed using multivariable-adjusted Cox proportional hazard model.
Statistical analyses
Statistical analysis was performed using SPSS version 23 (SPSS Inc., Chicago, IL, USA). All results are expressed as mean ± SD. To measure intergroup differences, paired t tests, the Kruskal-Wallis test, and Fisher’s exact test were used as appropriate. The risk of mortality was analyzed using multivariable-adjusted Cox proportional hazard model. The correlation between the two groups was determined using Pearson’s correlation coefficient. Values of P < 0.05 were considered statistically significant.
Results
Patient characteristics
This study comprised 166 patients who started undergoing dialysis between January 2012 and May 2015. Men accounted for 67% of the subjects, and the mean age was 61.5 ± 14.3 years. The patient characteristics are shown in Table 1. The ESKD was caused by diabetic nephropathy (89 cases, 49%), nephrosclerosis (41 cases, 25%), chronic glomerulonephritis (13 cases, 8%), and polycystic kidney (PKD) (4 cases, 2.4%). The primary reasons for dialysis initiation included uremia (57 cases, 34%), uremia with edema (47 cases, 28%), congestion or dyspnea (33 cases, 20%), and edema (15 cases, 9%). The mean blood pressure was 147 ± 21/76 ± 16 mmHg. The mean body weight was 62.6 ± 15.7 kg. Of the total patients, dialysis was initiated in 125 (75.3%) using arteriovenous fistulae (AVF) and in 41 (24.7%) using a catheter. The mean Hb was 8.56 ± 1.3 g/dL. The mean Fe was 61 ± 31 /dL, TIBC was 212 ± 44 μg/dL, and ferritin was 305 ± 315 ng/dL. The mean BUN was 103 ± 33 mg/dL; Cr was 11.1 ± 4.3 mg/dL. The mean eGFR was 4.5 ± 1.8 mL/min/1.73 m2. The mean K was 4.7 ± 1.0 mEq/L. The mean corrected Ca was 8.5 ± 1.1 mg/dL, and P was 7.6 ± 2.1 mg/dL. The mean intact parathyroid hormone was 186 ± 107 pg/mL. The mean BNP was 954 ± 2136 ng/mL (4~18,258). The mean CTR was 54.8 ± 6.0%.
A total of 129 patients (78%) were treated with long-acting ESA (epoetin beta pegol in 89 and darbepoetin in 40). The mean ESA dosage was 4871 ± 3259 U/week (Epo conversion rate: Hb [g/dL] × body weight [kg]). The mean ERI was 9.95 ± 7.35 IU/kg/week/g/100. Forty-six patients (28%) were treated with an oral iron supplement.
The Hb distribution at initiation was normal. At initiation, the associations between Hb level and both CTR and log BNP were significantly negative (R = − 0.175, P = 0.025; R = − 0.29, P < 0.01) (Fig. 2).
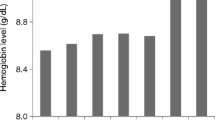
The time course of Hb levels and percentages of ESA usage in 105 patients obserbed from 6 months prior to initiation are shown in Fig. 3. At 6 months prior to initiation, the Hb level was 9.7 ± 1.2 g/dL with 62% of patients treated with ESA. Although the ESA usage rate gradually increased up to nearly 100% toward the initiation, the Hb level gradually decreased to 9.3 ± 1.2 g/dL at 1 month prior to initiation and then dramatically dropped to 8.6 ± 1.3 g/dL at initiation. After initiation, Hb level rapidly increased to > 10 g/dL and a V-shaped curve was seen at the bottom at the time of initiation.
Time course of hemoglobin (Hb) and erythropoiesis-stimulating agent (ESA) usage rate between 6 months before and after dialysis initiation. Despite the ESA usage rate gradually increasing to nearly 100% as initiation approached, the Hb level gradually decreased until 1 month prior to initiation and then dramatically decreased to 8.6 g/dL at initiation. After initiation, Hb level rapidly increased to > 10 g/dL and exhibited a V-shaped curve with the bottom at the time of initiation
We compared the Hb level, CTR, ESA dose, and ERI between the time of initiation and 1 year later in 144 patients (Fig. 4). Hb level at 1 year after initiation showed a significant increase compared with the level at initiation (10.6 ± 1.0 vs 8.6 ± 1.3 g/dL, P < 0.001). CTR showed a significant decrease compared with that at initiation (49.3 ± 5.0 vs 54.8 ± 6.0%, P < 0.001). ESA dose at 1 year after initiation showed an insignificant decrease compared with that at initiation. (4232 ± 3044 vs 4872 ± 3259 U/week, P = 0.068). ERI showed a significant decrease compared with that at initiation (7.74 ± 6.92 vs 9.95 ± 7.35, P < 0.001) (Fig. 4).
Changes in hemoglobin (Hb), cardiothoracic rate (CTR), erythropoiesis-stimulating agent (ESA) dosage, and erythropoietin resistance index (ERI) from initiation to 1 year after initiation Hb at 1 year after initiation showed a significant increase, and CTR and ERI showed a significant decrease compared with initiation. ESA dose at 1 year after initiation showed an insignificant decrease compared with the initiation
The patient characteristics of three groups according to the tertile of Hb levels at initiation are shown in Table 2. There was a significant difference only in Hb and CTR at initiation between the three groups. The mean Hb level at initiation and 1 year after among three groups and the association between the Hb levels at initiation and 1 year later are shown in Fig. 5. There were no differences in Hb level among the three groups at 1 year after initiation. Thus, there was no significant correlation between Hb levels at initiation and 1 year later.
Changes in hemoglobin (Hb) levels 1 year after initiation in the three groups according to the tertile of Hb levels and association between at initiation and 1 year after initiation. There were no significant differences in Hb at 1 year after initiation between the three groups, and there was no significant correlation between Hb at initiation and 1 year after initiation
Changes in CTR at 1 year after initiation among the three groups are shown in Fig. 6. CTR showed significant differences at initiation but no significant differences at 1 year after initiation among the three groups.
Changes in cardiothoracic rate (CTR) from dialysis initiation to 1 year after initiation in the three groups according to the tertile of Hb levels. Though there was a significant difference in CTR among the three groups at initiation, this significant difference could not be observed at 1 year after
Twelve patients were dead. Three deaths in higher and middle tertile group and 6 in lower tertile group. The cause of death was pneumonia in three patients, cardiovascular event including sudden death in three patients, and cerebral infarction, gastrointestinal bleeding, colon cancer, subdural hemorrhage, hyperkalemia, and myelodysplastic syndrome (MDS) in each one. The results of multivariate Cox proportional hazard model indicated that eGFR at initiation was the only significant risk factor for mortality within 1 year after dialysis initiation (hazard ratio 1.419, P = 0.006) (Table 3).
Discussion
Nevertheless, the Hb level gradually decreased at 1 month prior to initiation and then dramatically decreased at initiation despite the administration of the long acting ESA. ERI also increased as patients approached the initiation of dialysis. Kawahara reported that the monthly Hb decreased gradually during 6-month period before initiation of dialysis, whereas ESA dose and ERI increased as patients approached the initiation of dialysis [4]. Our data showed a tendency similar to that reported by Kawahara. It could be thought that the point at which a maximum ERI was reached would be the ideal timing for the initiation.
Regarding the V-shaped depression of Hb at dialysis initiation, it was previously not observed after use of long-acting ESA in a limited number of cases [5]. Kataoka reported that this depression was observed even after use of long-acting ESA in 72 incident dialysis patients [6]. The difference in this study was thought to be due to both number of patients and initiation timing. Our result supported Kataoka’s observation of V-shaped depression at initiation even after long-acting ESA use. Since almost all patients in our study underwent dialysis initiation with serious symptoms such as uremia, edema, and dyspnea at a mean Cr level of 11.1 mg/dL, the timing of dialysis initiation was relatively late, so anemia was considered to be progressing.
In our cohort, the average Hb was 8.6 ± 1.3 g/dL with a peak at 8.0–8.9 g/dL and showed a normal distribution. Asakawa et al. demonstrated the same tendency in 2249 Japanese initiation patients with a normally distributed mean Hb level of 8.7 ± 1.6 g/dL with a peak at 8.0–8.9 g/dL [7] and a negative relationship between Hb and CTR [8]. Koibuchi et al. also reported the same correlation between them [9]. In our patients, there was a significant negative relationship between Hb and CTR at initiation. Moreover, the negative relationship between Hb and log BNP was also observed in our patients. Negative correlations of Hb with CTR and BNP are considered to be associated with fluid volume status. With progression of renal failure, the progression of renal anemia and over fluid volume status is presented. In addition, at the time of initiation, it also exhibits dilutable Hb reduction due to over fluid volume status. Regarding the relationship between anemia and cardiovascular parameters, a negative correlation has been noted between anemia and cardiac hypertrophy [10]. Moreover, correlations exit between the LVMI coefficient and both the survival rate and incidence of cardiac events. Meanwhile, there are also reports that low Hb levels during hemodialysis cause cardiac hypertrophy [11]. Accordingly, a persistent lower Hb of dialysis patients could be a high-risk factor for cardiovascular events. However, this V-shaped depression of Hb was corrected promptly after initiation and a shift to a stable state of dialysis was achieved within a few months. Although a lower level of Hb at initiation presents a higher CTR or BNP level, which is considered high-risk factors of cardiovascular events [12], CTR at 1 year after initiation showed a significant decrease in relation to Hb levels and the fluid volume reduction induced by dialysis.
Death was considered much more likely in the early stage after initiation, and the mortality rate immediately post-hemodialysis has been noted to be high even by global standards [13, 14]. Regarding the mortality within 1 year after initiation, it seems to be higher in the lower Hb tertile group in six cases, compared with the middle tertile and higher tertile, in three cases each, but multivariate Cox proportional hazard model indicated that Hb at initiation was not significant risk factor. One of the reasons for the lack of differences by Hb level would be the very good 1-year survival rate after initiation as over 90% in Japan recently, and our cohort size may not have been big enough to recognize as a significant difference. We think that one reason for this good survival rate in Japan is that almost all of the incident dialysis patients were admitted to the hospital for initiation in this dangerous period. And after discharge from the hospital at initiation, the very low mortality rate in Japan during the stable dialysis period was proven by Dialysis Outcomes and Practice Patterns Study (DOPPS) [11].
A statistical analysis from Japan [15] indicated a 10% mortality rate in the first year for 35,864 incident dialysis patients in 2014, most commonly caused by infection, followed by heart failure, malignancy, cerebrovascular disease, and myocardial infarction. The high probability of attribution to cardiovascular disease is well known, reaching 25%. In this study, 12 patients died. Our results are consistent with the annual dialysis data report from the Japanese Society for Dialysis Therapy (JSDT).
In the 1-year follow-up survey of 29,716 patients among 36,173 Japanese incident patients in 2007, a comparison of mortality by cohorts regarding as Hb level at initiation showed no significant differences in cohorts in Hb level from 7 to 11 g/dL [16].
Yamagata et al. investigated the ideal timing for dialysis initiation to determine whether eGFR was associated with better mortality after initiation in 9695 incident dialysis patients in 2007 based on these data and reported a lowest 1-year odds ratio (OR) of mortality in patients with an eGFR of 4–6 mL/min/1.73 m2, but the OR was identical among groups with an eGFR of 2–8 mL/min/1.73 m2. The average Hb of patients with an eGFR of 4–6 mL/min/1.73 m2 was 8.4 g/dL, while that of those with an eGFR of 2–8 mL/min/1.73 m2 was equivalent to 8.0–8.6 g/dL [17].
These data were investigated in incident patients in 2007 when long-acting ESA could not be used. At the present time, long-acting ESA can be used, the anemia management around the initiation phase is improved, and Hb level at initiation is increasing [18]. The mortality rate 1 year after initiation showed some improvement compared with that in 2007 as 10.3% in 2014 vs 12.6% in 2007. The average age in our cohort was a little bit younger than that reported in the annual dialysis data report from the JSDT, and this is the one reason for our low mortality (7.2% in 2015).
Watanabe et al. recently reported that the 1-year survival rate was 95.36% in the rHuEPO and 90.36% in the non-treatment group despite the mean Hb at initiation being 8.35 g/dL in the rHuEPO and 8.25 g/dL in the non-treatment group [19].
In Japan, patients with a low Hb that could indicate a risk of cardiovascular events could safely initiate dialysis because the majority of initiation patients are hospitalized during high-risk period.
Finally, from an economical view for initiation patients, ESA costs before initiation require a corresponding self-burden vs almost no self-burden after initiation in Japan. It is economically beneficial for initiation patients when short-term Hb depression before initiation quickly catches up with ESA treatment after initiation without self-burden, and no difference is noted in short-term prognosis.
Study limitation
Our study has several limitations. First, this was a retrospective study, there were constraints regarding the number of the subjects and the length of the observation period. Our subject’s age was younger than the JSDT statistics; it had become sticking to the initiation point where the symptom appears. Second, comparison of the three cohorts examined important factors for survival rates such as age and diabetes and did not show any significant difference, but other comorbidities could not be considered. Third, cardiac function at initiation was not evaluated by UCG but only with CTR and BNP in our study.
Conclusion
In the dialysis initiation period, incident patients showed severe renal anemia that was resistant even to long-acting ESA as well as a high CTR and elevated BNP, both of which were considered high-risk factors of cardiovascular events. This was corrected promptly by dialysis initiation and a short-term V-shaped depression of Hb level had no effects on short-term prognosis including cardiovascular events under current ordinary hemodialysis treatment in Japan.
References
Akisawa T, Saito A, Gejyo F, et al. Impacts of recombinant human erythropoietin treatment during predialysis periods on the progression of chronic kidney disease in a large-scale cohort study (Co-JET study). Ther Apher Dial. 2014;18:140–8.
Bonafont X, Bock A, Carter D, Brunkhorst R, Carrera F, Iskedjian M, et al. A meta-analysis of the relative doses of erythropoiesis-stimulating agents in patients undergoing dialysis. Nephrol Dial Transplant Plus. 2009;2:347–35.
Vega A, Abad S, Verdalles U, et al. Dose equivalence between continuous erythropoietin receptor activator (CERA), darbepoiet and epoetin in patients with advanced chronic kidney disease. Hippokratia Med J. 2014;18:315–8.
Kawahara K, Minakuchi J, Yokota N, Suekane H, Tsuchida K, Kawashima S. Treatment of renal anemia with erythropoiesis-stimulating agents in predialysis chronic kidney disease patients: haemoglobin profile during the 6 months before initiation of dialysis. Nephrology. 2015;20(Suppl. 4):29–32.
Yoshiya K, Tsukuda M, Hara A, Anpuku T. The efficacy of continuous erythropoietin receptor activator (C.E.R.A) in renal anemia during dialysis initiation. Japanese J Clin Dial. 2013;29(9):115–7. (Japanese)
Kataoka H, Tsuchiya K, Naganuma T, Okazaki M, Komatsu M, Kimura T, Shiohira S, Kawaguchi H, Nitta K. Relationship between anaemia management at haemodialysis initiation and patient prognosis. Nephrology. 2015;20:14–21.
Asakawa T, Komatsu Y, Ando R, Joki N, Tanaka Y, Iwasaki M, Hase H, Ikeda M, Inaguma D, Sakaguchi T, Shinoda T, Koiwa F, Negi S, Yamaka T, Shigematsu T. Effect of long-acting erythropoiesis-stimulating agents on hemoglobin levels at the initiation of dialysis. Renal Replace Ther. 2016;2:12.
Asakawa T, Joki N, Tanaka Y, Hayashi T, Hase H, Komatsu Y, Ando R, Ikeda M, Inaguma D, Sakaguchi T, Shinoda T, Koiwa F, Negi S, Yamaka T, Shigematsu T. Association between the hemoglobin level and cardiothoracic ratio in patients on incident dialysis. Cardiorenal Med. 2014;4:189–200.
Koibuchi K, Miyagi M, Arai T, Aoki T, Aikawa A, Sakai K. Comparing the efficacy of continuous erythropoietin receptor activator and darbepoetin alfa treatments in Japanese patients with chronic kidney disease during the predialysis period: a propensity-matched analysis. Nephrology. 2015;20(Suppl. 4):22–8.
London GM, Marchais SJ, Guerin AP, Fabiani F, Metivier F. Cardiovascular function in hemodialysis patients. Adv Nephrol. 1991;20:249–73.
Zoccali C, Benedetto FA, Mallamaci F, et al. Left ventricular mass monitoring I the follow-up of dialysis patients: prognostic value of left ventricular hypertrophy progression. Kidney Int. 2004;65:1492–8.
Koch M, Trapp R, Kohnle M, Aker S, Haastert B, Rump LC. B-type natriuretic peptide and severe heart failure at baseline predict overall mortality in incident dialysis patients. Clin Nephrol. 2010;73(1):21–9.
Robinson BM, Zhang J, Morgenstern H, Bradbury BD, Ng LJ, McCullough KP, Gillespie BW, Hakim R, Rayner H, Fort J, Akizawa T, Tentori F, Pisoni RL. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014;85(1):158–65.
Eckardt KU, Gillespie IA, Kronenberg F, Richards S, Stenvinkel P, Anker SD, Wheeler DC, de Francisco AL, Marcelli D, Froissart M, Floege J, ARO Steering Committee. High cardiovascular event rates occur within the first weeks of starting hemodialysis. Kidney Int. 2015;88(5):1117–25.
Masakane I, Taniguchi M, Nakai S, et al. Annual dialysis data report 2015, JSDT registry J. Jpn Soc Dial Ther. 2017;50(1):1–62.
The committee of Renal Data Registry of the Japanese Society for Dialysis. Therapy analysis on factors influencing the mortality of dialysis initiation patients in 2007 an overview of regular dialysis treatment in Japan (as 31 December 2007). Tokyo: Japanese Society of Dialysis therapy; 2008. p. 54–78.
Yamagata K, Nakai S, Masakane I, Hanafusa N, Iseki K, Tsubakihara Y. The committee of renal data ideal timing and predialysis nephrology care duration for dialysis initiation: from analysis of Japanese dialysis initiation survey. Ther Apher Dial. 2012;16(1):54–62.
Asakawa T, Komatsu Y, Ando R, et al. Effect of long-acting erythropoiesis stimulating agents on hemoglobin levels at the initiation of dialysis. Renal Replace Ther. 2016;2:12P.
Watanabe Y, Akizawa T, Saito T, et al. Effect of predialysis recombinant human erythropoietin on early survival after hemodialysis initiation in patients with chronic kidney disease: Co-JET study. Ther Apher Dial. 2016;20(6):598–607.
Acknowledgements
Not applicable
Funding
This study was not supported by any fundings.
Availability of data and materials
The datasets used in the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
SK designed and promoted the study. YK, HM, KN, AK, and AY participated in data collection. TT conceived of the study and participated in its design and coordination and helped draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This observational study was approved by the Dokkyo Medical University Saitama Medical Center Ethics Committee (2017/1716). All patients provided consent permitting data sampling and analysis at the time of initiation of the dialysis therapy.
Consent for publication
Not applicable
Competing interests
All authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kawamoto, S., Kaneko, Y., Misawa, H. et al. Lower Hb at the initiation of dialysis does not adversely affect 1-year mortality rate. Ren Replace Ther 4, 4 (2018). https://doi.org/10.1186/s41100-018-0145-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-018-0145-z