Abstract
Background
Hepatitis C virus (HCV) infection a major disorder that is not only a liver-related disease but also a cardiovascular complication in renal transplant recipient. Interferon-based therapy is a contraindication after transplantation because interferon is possibly induced to graft rejection. Only limited anecdotal evidence exists on the antiviral efficacy and tolerability of HCV direct acting antivirals (DAAs) in patients with chronic kidney disease (CKD), including renal transplant recipients. Then, we report a successful treatment case of chronic hepatitis C virus genotype 1b infection in a patient with compensated cirrhosis after renal transplantation using daclatasvir-asunaprevir combination therapy and reviewed the literature.
Case presentation
A 64-year-old female renal transplantation (RT) recipient complicated with compensated cirrhosis due to hepatitis C virus (HCV) genotype 1b infection was treated with interferon (INF)–ribavirin-free combinations of DAAs such as daclatasvir plus asunaprevir. She was medicated with daclatasvir 60 mg once a day and asunaprevir 100 mg twice a day for 24 weeks. Her initial HCV RNA was log10 6.2 IU/ml, and HCV RNA was not detected from 10 weeks. She achieved a sustained virological response at 24 weeks (SVR24). During this therapy, her serum creatinine and tacrolimus trough level were slightly elevated, but those abnormalities were returned to the basal level by decreasing the dose of extended-release tacrolimus. No other adverse event requiring discontinuation of the treatment occurred.
Conclusions
Almost all DAA treatments of HCV infection in renal transplant recipients have been sofosbuvir-based antiviral treatment, but we considered that daclatasvir-asunaprevir combination therapy is suitable to renal-impaired recipient because sofosbuvir is contraindicated in patients whose estimated GFR is <30 ml/min/1.73 m2, and both of daclatasvir and asunaprevir are mainly primarily metabolized by the liver. We showed excellent effect daclatasvir-asunaprevir combination therapy to a renal transplant recipient with chronic HCV infection and reviewed the literature.
Similar content being viewed by others
Background
Chronic hepatitis C virus (HCV) infection progresses to hepatic dysfunction, cirrhosis, and hepatocellular carcinoma, and the target of the therapy is elimination of the virus [1]. Since Omata et al. [2] reported the effectiveness of interferon (IFN) in 1991, it has been the standard therapy for chronic HCV infection until the appearance of direct acting antivirals (DAAs) [3]. However, the use of IFN is prohibited in renal transplantation (RT) patients because IFN could induce acute rejection. Kidney Disease Improving Global Outcomes (KDIGO) recommends IFN therapy for recipients with HCV infection before RT [4]. Therefore, there has been no choice of treatment in post-transplant patients with chronic HCV infection. Here, we report the successful treatment of a patient with oral IFN- and ribavirin-free DAAs (daclatasvir plus asunaprevir) for compensated cirrhosis due to HCV genotype 1b infection after RT. These new DAAs may change the natural history of HCV infection after RT. However, there is as yet no evidence to support the use of DAAs in the RT setting because the understanding of DAA metabolism and drug–drug interaction is insufficient. In addition, there have been no large studies of DAA treatment in this patient group [5].
Case presentation
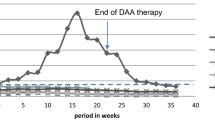
A 64-year-old female was diagnosed with chronic glomerulonephritis in March 1976 and soon started hemodialysis. She showed intermittent elevation of transaminase and thrombocytopenia at the start of hemodialysis. We considered that these abnormalities were caused by chronic non-A and non-B hepatitis, which is now called type C hepatitis, because she did not show abnormal neurological signs, jaundice, or ascites. She had never received a transfusion and was not a drug addict. Therefore, the infection route of hepatitis C virus (HCV) was unknown. She underwent hemodialysis three times a week for about 26 years, and she then received a cadaveric RT in April 2003. Before transplantation, she underwent an examination by upper gastrointestinal endoscopy, which revealed no esophageal varices or other abnormalities. After transplantation, her clinical course was very good except for mild liver injury and thrombocytopenia. HCV antibody positivity was first detected on screening for infections in April 1991, when this examination became commercially available in Japan. For the first time, we checked HCV RNA, which was positive in April 2008. Upper gastrointestinal endoscopy to screen for malignancy, which was carried out in June 2014, revealed esophageal varices at the upper esophagus. At that time, her height was 141.1 cm, body weight 39.2 kg, body temperature 36.5 °C, and blood pressure 125/60 mmHg. Her consciousness was clear, and neurological examination was normal. Her palpebral conjunctiva was not anemic, and bulbar conjunctiva was not icteric. Her abdomen was not distended, and hepatomegaly was not present. Her spleen was palpable about 6 cm below the left hypochondrium. Lower abdominal wall vein dilatation (so called Caput medusae) was present. There were no vascular spiders, palmar erythema, digital clubbing, or asterixis. Urinalysis revealed 1+ proteinuria, but occult blood was negative. The amount of proteinuria over 24 h was 0.25–0.35 g. Other laboratory findings were as follows: leukocytes 5400/μl, erythrocytes 423 × 104/μl, hemoglobin 11.7 g/dl, hematocrit 35.7 %, platelets 6.7 × 104/μl, blood urea nitrogen 36.9 mg/dl, serum creatinine 1.30 mg/dl, e-GFR 32.6 ml/min/1.73 m2, uric acid 5.2 mg/dl, total protein 6.5 g/dl, albumin 3.9 g/dl, AST 64 IU/l (normal value 8–38 IU/l), ALT 65 IU/l (normal value 40–40 IU/l), γ~GTP 47 IU/l (normal value <70 IU/l), total bilirubin 0.78 mg/dl, ALP 430 IU/l (normal value 100–335 IU/l l), i-PTH 222 pg/ml (normal value 10–65 pg/ml), hepaplastin test 101.3 % (normal value 70–130 %), PT 85.6 % (normal value 70–140 %), type IV collagen∙7S 7.6 ng/ml (normal value <6.0), procollagen III peptide 1.9 U/ml (normal value <1.0 U/ml), HCV RNA 6.2 log10 IU/ml, and HCV genotype 1b. We examined the patient for pretreatment resistance-associated variants. There were no variants in the L31M and Y93H regions. A computed tomography (CT) scan revealed splenomegaly and a tiny quantity of ascites (Fig. 1). There were no findings of liver atrophy or tumor (Fig. 2). We diagnosed well-compensated liver cirrhosis (Child–Pugh grade A; score 6 points). After the local ethics committee approval and written informed consent, we decided to treat her HCV chronic infection with the interferon (IFN)free DAAs daclatasvir plus asunaprevir because of the risk of acute renal rejection after IFN treatment. We prescribed daclatasvir 60 mg once daily plus asunaprevir 100 mg twice daily for 24 weeks. Her other prescription medications were extended-release tacrolimus 2 mg/day, mycophenolate mofetil 500 mg/day, prednisolone 2 mg/day, valsartan 40 mg/day, alfacalcidol 0.25 μg/day, cinacalcet 25 mg/day, febuxostat 40 mg/day, ursodeoxycholic acid 600 mg/day, menatetrenone 15 mg/day, and acetaminophen 800 mg/day. Her HCV RNA level decreased rapidly and was undetectable from 10 weeks after the start of treatment, and she achieved a sustained virologic response (SVR) at 24 weeks (SVR24). Her slightly elevated transaminase level was normalized quickly. Slightly elevated serum creatinine and trough level of tacrolimus were decreased after dose adjustment of extended-release tacrolimus from 2 to 1.5 mg/day (Fig. 2). There were no other adverse events. A follow-up CT scan was performed after 24 weeks’ treatment with daclatasvir and asunaprevir and showed persistent splenomegaly, but no more ascites.
Discussion
About 170–180 million people are chronically infected with HCV worldwide [6]. The prevalence of infection in kidney transplant recipients is significantly higher than in the general population [7], and it is associated with increased morbidity and mortality [8]. RT recipients have an HCV infection rate of 5–15 % in developed countries, with substantially higher rates reported in the developing world. After RT, HCV-RNA-positive recipients have an increased risk of cirrhosis, hepatocellular carcinoma, and death. Infection with HCV is also an independent risk factor for graft loss and is associated with proteinuria, chronic rejection, transplant glomerulopathy, posttransplant diabetes, and HCVassociated glomerulonephritis. Therefore, prevention and management of HCV infection is a critical factor in RT therapy [5].
HCV is a positive, single-stranded RNA virus, and it is divided into seven genotypes [9]. Genotype 1 is the most prevalent worldwide, accounting for approximately 60 % of infections [10]. Type lb infection is more closely associated with the development of liver cirrhosis and hepatocellular carcinoma than type 2a or 2b through its role in the progression of chronic liver inflammation to a cirrhotic stage [11]. IFN-based antiviral therapies are the standard approach to control HCV infection. However, KDIGO recommends that HCV-infected kidney transplant candidates are considered for treatment with standard IFN before transplantation because IFN has been associated with higher renal allograft rejection rates [4]. In 2014, a meta-analysis performed by Wei et al. demonstrated that IFN-based therapy for HCV infection post-RT has poor efficacy and limited safety. They identified 12 clinical trials (140 patients in total). The summary estimates for the SVR rate, dropout rate, and graft rejection rate were 26.6, 21.1, and 4 %, respectively. The most frequent side-effect requiring discontinuation of treatment was graft dysfunction (45.1 %) [12]. As described above, the use of IFN-based therapy is avoided in the treatment of chronic HCV-infected patients after RT, but IFN-free regimens are a great hope as a new treatment for RT recipients. DAAs show potential for greater efficacy in the eradication of HCV, and their reduced toxicity makes them an attractive therapeutic option after RT [5]. However, there are very few reports on DAA therapy in RT recipients. All existing reports involved sofosbuvir-based antiviral therapy (Table 1) [13–15]. Kamar et al. [13] reported the efficacy and safety of sofosbuvir-based antiviral therapy for treating HCV infection after RT. They treated 25 RT recipients with chronic HCV infection. At 4 and 12 weeks after completing DAA therapy, all had a SVR. The tolerance of anti-HCV therapy was excellent, and no adverse events were observed. Sawinski et al. [14] showed 20 consecutive RT recipients treated with IFN-free sofosbuvir-based antiviral treatment regimens for HCV. All patients cleared the virus while on therapy, and 100 % achieved a SVR at 12 weeks after completion of therapy. These DAAs were well tolerated, and less than half of the patients required calcineurin inhibitor dose adjustment during treatment. Hussein et al. [15] also demonstrated successful treatment of HCV genotype 4 in RT recipients with sofosbuvir and ribavirin. They treated three patients and achieved SVR12 without major adverse events. Sofosbuvir is mainly eliminated by the kidney; therefore, its use is contraindicated in patients with severe renal impairment (glomerular filtration rate <30 mL/min/1.73 m2) or chronic renal failure undergoing dialysis [16].
Daclatasvir plus asunaprevir has received its first global approval in this indication in Japan, which is the first all-oral, IFN-, and ribavirin-free regimen for HCV genotype 1b [17]. Kumada H. et al. demonstrated that 24-week treatment with daclatasvir and asunaprevir provides a highly effective option for non-transplantation patients who currently have no effective treatment options (ineligible for or intolerant of IFN-based therapy) and for those patients who did not achieve SVR with prior treatment. In addition, they described that 22 patients with virologic failure had NS5A polymorphisms L31 and/or Y93 prior to treatment. The present case showed no variants in the L31M and Y93H region. The patients had a complication with compensated cirrhosis, and Kumada H. et al. also reported that their patients with cirrhosis also achieved high rates of SVR24 (20/22, 90.9 %) [18]. Most RT recipients showed chronic kidney disease stage 3 or more [19]. Hence, it is very important to recognize DAA metabolism and drug–drug interactions, and we showed here effectiveness of DAA and the use of dose adjustments for renal or hepatic impairment (Table 2) [20]. Daclatasvir and asunaprevir are primarily metabolized by the liver. Therefore, it is suggested that there is no need to adjust the doses of these drugs according to renal function. Daclatasvir and asunaprevir are suitable drugs to renal transplant recipients with impaired real function. Asunaprevir is a substrate of CYP3A, P-gp, and OATP 1B1 and 2B1. It inhibits OATP 1B1, 1B3, and 2B1 and P-gp and CYP2D6 and induces CYP3A4. Daclatasvir and asunaprevir are both contraindicated in patients taking strong CYP3A4 inducers, such as rifampicin, rifabutin, phenytoin, carbamazepine, phenobarbital, systemic dexamethasone, and products including foods containing Hypericum perforatum (St. John’s wort) [21]. Asunaprevir is also contraindicated in patients taking cyclosporin [22].
Our patient had moderate renal and hepatic impairment (Child–Pugh grade A) and treatment with daclatasvir plus asunaprevir was highly effective in the elimination of HCV without any adverse reactions other than a slight increase in serum creatinine and the trough level of tacrolimus.
Conclusions
To the best of our knowledge, we report herein the first case of successful daclatasvir plus asunaprevir treatment for a patient who received a cadaveric RT complicated with compensated cirrhosis caused by HCV genotype 1b infection without any adverse effects. This new treatment may be considered for chronic HCV infection after RT.
Abbreviations
- DAAs:
-
Direct acting antivirals
- HCV:
-
Hepatitis C virus
- IFN:
-
Interferon
- KDIGO:
-
Kidney Disease Improving Global Outcomes
- RT:
-
Renal transplantation
- SVR24:
-
Sustained virological response at 24 weeks
References
Wise M, Bialek S, Finelli L, Bell BP, Sorvillo F. Changing trends in HCV-related mortality in USA. Hepatology. 2008;47:1128–35.
Omata M, Yokosuka O, Takano S, Kato N, Hosoda K, Imazeki F, Tada M, Ito Y, Ohto M. Resolution of acute hepatitis C after therapy with natural beta interferon. Lancet. 1991;338:914–5.
Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet. 2015;385:1124–35.
KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int 2008;73(Suppl.109):S1–S99
Morales JM, Fabrizi F. Hepatitis C and its impact on renal transplantation. Nat Rev Nephrol. 2015;11:172–82.
Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–67.
Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G. Hepatitis C virus antibody status and survival after renal transplantation: meta-analysis of observational studies. Am J Transplant. 2005;5:1452–61.
Baid-Agrawal S, Pascual M, Moradpour D, Frei U, Tolkoff-Rubin N. Hepatitis C virus infection in haemodialysis and kidney transplant patients. Rev Med Virol. 2008;18:97–115.
Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, Simmonds P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes. Hepatology. 2014;59:318–27.
Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77–87.
Raimondi S, Bruno S, Mondelli MU, Maisonneuve P. Hepatitis C virus genotype 1b as a risk factor for hepatocellular carcinoma development: a meta-analysis. J Hepatol. 2009;50:1142–54.
Wei F, Liu J, Liu F, Hu H, Ren H, Hu P. Interferon-based anti-viral therapy for hepatitis C virus infection after renal transplantation: an updated meta-analysis. PLoS One. 2014;9:e90611.
Kamar N, Marion O, Rostaing L, Cointault O, Ribes D, Lavayssière L, Esposito L, Del Bello A, Métivier S, Barange K, Izopet J, Alric L. Efficacy and safety of sofosbuvir-based antiviral therapy to treat hepatitis C virus infection after kidney transplantation. Am J Transplant. 2015;16:1474–9.
Sawinski D, Kaur N, Ajeti A, Trofe-Clark J, Lim M, Bleicher M, Goral S, Forde KA, Bloom RD. Successful treatment of hepatitis C in renal transplant recipients with direct-acting antiviral agents. Am J Transplant. 2016;16:1588–95.
Hussein NR, Sidiq Z, Saleem M. Successful treatment of hepatitis C virus genotype 4 in renal transplant recipients with direct-acting antiviral agents. Am J Transplant. 2016. doi:10.1111/ajt.13767 [Epub ahead of print].
Pipili C, Cholongitas E. Pharmaceutical management of hepatitis B and C in liver and kidney transplant recipients. World J Gastrointest Pharmacol Ther. 2015;6:105–10.
Bristol–Myers Squibb. Japan approves first all-oral, interferonand ribavirin-free hepatitis C treatment, Daklinza (daclatasvir) and Sunvepra (asunaprevir) Dual Regimen [media release]. 7 July 2014 http://news.bms.com/press-release/japan-approvesfirst-all-oral-interferon-and-ribavirin-free-hepatitis-c-treatment-dakl
Kumada H, Suzuki Y, Ikeda K, Toyota J, Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K, Izumi N, Koike K, Takehara T, Kawada N, Sata M, Miyagoshi H, Eley T, McPhee F, Damokosh A, Ishikawa H, Hughes E. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083–91.
White C, Akbari A, Hussain N, Dinh L, Filler G, Lepage N, Knoll GA. Chronic kidney disease stage in renal transplantation classification using cystatin C and creatinine-based equations. Nephrol Dial Transplant. 2007;22:3013–20.
Hill L, et al. Hepatitis C virus direct-acting antiviral drug interactions and use in renal and hepatic impairment. Top Antivir Med. 2015;23(2):92–6.
Poole RM. Daclatasvir + asunaprevir: first global approval. Drugs. 2014;74:1559–71.
Bristol–Myers Squibb Company. Sunvepra capsules (asunaprevir) Japanese Prescribing Information. 2014
Acknowledgements
We would like to thank Dr. Mitsuhiro Kawano (Kanazawa University Hospital) for his critical reading of the manuscript.
Funding
None.
Availability of data and materials
We wish to share our data.
Authors’ contributions
RM and KM took care of patients and participated in the decision of treatment. RM prepared the manuscript. Both authors read and approved the final manuscript.
Competing interests
Miyazaki has received lecture fees from Bristol-Myers Squibb. Miyagi has no conflicts to report.
Consent for publication
We gained written informed consent for publication from this patient.
Ethics approval and consent to participate
After the local ethics committee approval, we obtained written informed consent from this patient.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Miyazaki, R., Miyagi, K. Successful treatment of chronic hepatitis C virus genotype 1b infection of a patient with compensated cirrhosis after renal transplantation using daclatasvir-asunaprevir combination therapy: a case report and literature review. Ren Replace Ther 2, 61 (2016). https://doi.org/10.1186/s41100-016-0075-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-016-0075-6