Abstract
Background
Pituitary apoplexy is a neurosurgical emergency and is a known yet rare complication of pituitary macroadenoma. Patients typically present with visual field defects, headache and altered sensorium. There are multiple risk factors for this complication and a thorough drug history is essential to exclude iatrogenic causes of disease. We present an extremely rare case of newly diagnosed pituitary insufficiency unveiled by ibrutinib therapy (a Bruton tyrosine kinase inhibitor). Furthermore, after initial withdrawal of ibrutinib because of the erroneous diagnosis of Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH), its re-administration led to the development of classical pituitary apoplexy 4 months after treatment was restarted.
Case presentation
A male patient in his 60s with a background of chronic lymphocytic leukaemia (CLL) on ibrutinib and venetoclax presents with acute confusion and deranged electrolytes. He is found to be hyponatraemic and is diagnosed with Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH) and treated with fluid restriction. He represents again 3 weeks later with hyponatraemia and further investigations reveal pituitary insufficiency and macroadenoma. He was restarted on ibrutinib and venetoclax at the time of discharge.
Four months later, he presents with sudden retro-orbital headache associated with vomiting. Clinical findings include cranial nerve III, IV and XI palsy. Humphrey’s visual field examination revealed a left visual field index (VFI) of only 1% while the right was 64% with temporal hemianopia. Both pupils were mid-dilated and poorly reactive to light. MRI pituitary with contrast showed features of pituitary apoplexy and optic nerve compression. He was urgently referred to the neurosurgical team and underwent an emergency trans-sphenoidal hypophysectomy with circumferential excision of the macroadenoma. Post-operative recovery was uneventful with marked improvement in vision bilaterally.
The patient was restarted on ibrutinib and venetoclax 2 weeks post-operatively. Approximately 1 year post-treatment, he remains in radiological, clinical and biochemical remission from CLL and all medications have been withdrawn.
Conclusions
This is a unique and rare case of pituitary macroadenoma apoplexy following the commencement of ibrutinib for CLL. Central nervous system haemorrhage is a rare side effect of ibrutinib due to its platelet dysfunction effects. A thorough assessment is required to assess the risks and benefits of using ibrutinib in patients with pituitary macroadenoma to avoid serious complications.
Similar content being viewed by others
Background
Pituitary apoplexy is a neurosurgical emergency. It is a known yet rare complication of pituitary macroadenoma [1]. Patients typically present with visual field defects, headache and altered sensorium [1]. There are multiple risk factors for this complication and a thorough drug history is essential to exclude iatrogenic causes of disease [1]. We present an extremely rare case of newly diagnosed pituitary insufficiency unveiled by ibrutinib therapy (a Bruton tyrosine kinase inhibitor). Furthermore, after the initial withdrawal of treatment of ibrutinib because of the erroneous diagnosis of Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH), its re-administration led to the development of classical pituitary apoplexy 4 months after treatment was restarted. There is only one similar reported case in the United States and it is unclear if the patient was restarted on ibrutinib.
Case presentation
A male patient in his 60s with progressive chronic lymphocytic leukaemia (CLL) despite standard therapy. A combination of ibrutinib and venetoclax have been used in patients with refractory CLL [2] as in this case. His past medical history included trisomy 12 and primary hypothyroidism. A month later he presented to the Emergency Department (ED) with confusion and hyponatraemia. Paired serum and urine sodium and osmolality showed a serum sodium 120 mmol/L, urine sodium 77 mmol/L, serum osmolality 265 mOsm/kg and urine osmolality 792 mOsm/kg. He was diagnosed with Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH) and both ibrutinib and venetoclax were stopped during the admission. Hyponatraemia resolved following fluid restriction and both chemotherapy agents were restarted on discharge. Three weeks later, he presented with symptoms of nausea, diarrhoea and hypotension. Bloods again demonstrated hyponatraemia with sodium of 122 mmol/L. A full pituitary profile revealed pituitary insufficiency. Serum 09:00 cortisol was 15 nmol/L with adrenocorticotropic hormone (ACTH) of 11 ng/L. Other blood results included: testosterone 0.42 nmol/L, luteinising hormone (LH) 13 IU/L, follicular stimulating hormone (FSH) 1.7 IU/L, thyroid stimulating hormone (TSH) 0.12 mU/L, thyroxine 12 pmol/L and serum insulin-like growth factor 1 (IGF-1) was normal. Treatment with hydrocortisone was commenced. MRI pituitary revealed a pituitary macroadenoma as shown in Fig. 1. He was discharged feeling “like a new man” on hydrocortisone 15/5/5 mg daily. The haematology team were anxious to stop his treatment and ibrutinib was commenced at a lower dose.
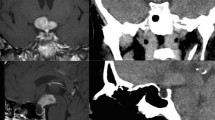
Four months later, the patient presented with a 3-day history of sudden retro-orbital headache associated with vomiting, lethargy and anorexia. Examination findings included left cranial nerve III, IV and XI palsy, left-sided ptosis with blurred vision and diplopia. Left eye examination demonstrated blurred vision and diplopia. Both pupils were mid-dilated and poorly reactive to light; this was more marked on the left. Visual field test by confrontation revealed right temporal hemianopia and near-complete vision loss in the left eye. An urgent CT head followed by an MRI pituitary revealed a 30% increase in the size of the tumour as well as features suggestive of pituitary apoplexy as shown in Fig. 2. The tumour was invading the left cavernous sinus and showed post-contrast unenhanced areas suggestive of necrosis. There was evidence of blood degradation products consistent with a subacute bleed. There was stretching and compression of the optic chiasm. Features were consistent with pituitary apoplexy on an underlying macroadenoma. Humphrey’s visual field examination revealed a left visual field index (VFI) of only 1% with optic disc pallor, while the right was 64% with temporal hemianopia as shown in Fig. 3.
Subsequent imaging 3 months later demonstrating pituitary apoplexy. A T1 coronal MRI showing pituitary macroadenoma (2.6 × 2 × 2.3 cm) with evidence of apoplexy and stretching and compression of the optic chiasm. B T1 sagittal MRI with contrast. C T2 axial MRI without contrast. D non-contrast CT head
Intravenous hydrocortisone was commenced and the patient urgently referred to the neurosurgical team. He underwent emergency trans-sphenoidal hypophysectomy with circumferential excision of the apoplectic macroadenoma on day seven of the presentation. Follow-up post-hypophysectomy MRI 6 months later showed optic chiasm decompression and a 13-mm residual tumour as shown in Fig. 4.
Post-trans-sphenoidal hypophysectomy, the patient recovered well and had a marked improvement in the right eye and 50% recovery of vision in the left eye. The patient was restarted on ibrutinib and venetoclax 2 weeks following surgery as it was felt that the benefit-to-risk ratio favoured continuation of the treatment and there was little pituitary tissue remaining. The patient had a pituitary MRI at three and 6 months post-operatively which showed a good surgical response.
CLL therapy was withdrawn a year later, following radiological, clinical and biochemical remission. Approximately 1 year post-CLL treatment with ibrutinib and venetoclax, the patient was in complete remission with negative bone marrow for the first time since diagnosis.
Discussion and conclusions
Central nervous system (CNS) haemorrhage is a rare side effect of ibrutinib. A randomised phase 3 trial comparing ibrutinib and chlorambucil in the treatment of CLL assessed the risk of major haemorrhage. Of the ibrutinib arm and following a median 17.4-month exposure, 4% (6 patients) developed a major haemorrhage; only 2 (1.3%) of which were CNS haemorrhages. Those patients had other comorbidities and cardiovascular risk factors predisposing them to this [3]. A literature search revealed that this complication is a rare occurrence; there have been no reported cases of pituitary apoplexy secondary to ibrutinib therapy in the United Kingdom (UK), making this the first case of its type to be published in the UK. There was only one similar case reported in the United States of America [4]. It is not known whether the treatment was restarted for that patient. Apoplexy is a known complication of pituitary macroadenoma and a thorough assessment is required to assess the risks and benefits of using ibrutinib in patients with pituitary macroadenoma [1].
Studies have shown that ibrutinib increases the risk of bleeding [5]. Ibrutinib is a Bruton tyrosine kinase (BTK) inhibitor which causes apoptosis and reduced chemotaxis of CLL cells [5]. Ibrutinib increases the risk of bleeding through its thrombocytopenic effect as well as platelet dysfunction associated with tyrosine kinase inhibition [6, 7]. BTK is involved in platelet signalling through cell-surface receptors expressed on platelets. These mediate platelet adhesion through von Willebrand factor. By inhibiting BTK platelet aggregation is suppressed thus increasing the risk of bleeding [7]. This patient was thrombocytopenic with a nadir platelet count of 89 × 109/L. A combination of thrombocytopenia along with the increased risk of bleeding associated with ibrutinib as described are likely to have caused pituitary apoplexy in this patient’s case.
Ibrutinib has Vascular Endothelial Growth Factor (VEGF) inhibition effects; a study on mice found that mice administered with OSI-930 (a tyrosine kinase receptor inhibitor including VEGF receptors 1 and 2) developed pituitary haemorrhage. All mice who received the treatment for 6 days developed pituitary haemorrhage and 66.7 of those who received it for 3 days developed this side effect. It is thought that OSI-930 disrupts the vascular supply of the pituitary gland by reducing the capillary network thus resulting in haemorrhage formation [8].
Due to the rarity of this complication, there is no clear data about the probability of developing pituitary apoplexy in CLL with or without BTK inhibitor therapy, but there have been a handful of reported cases of patients being diagnosed with CLL or chronic myeloid leukaemia after presenting with pituitary apoplexy; thought to be due to thrombocytopenia associated with the underlying malignancy [9] or presenting with a pituitary mass [10].
Research has shown that patients with trisomy 12 (as in this case) are likely to be more sensitive to ibrutinib [11]. This patient had multiple risk factors that increased his risk of bleeding, thus it is likely that the patient developed pituitary macroadenoma apoplexy as a result ibrutinib therapy.
This is a unique and rare case of pituitary macroadenoma apoplexy following the commencement of ibrutinib for the treatment of CLL. A thorough assessment is required to assess the risks and benefits of using ibrutinib in patients with pituitary macroadenoma to avoid serious complications including haemorrhage which can be fatal. Patients with suspected pituitary apoplexy should be thoroughly and rapidly investigated as time is of the essence to avoid permanent vision loss.
Availability of data and materials
Not applicable.
Abbreviations
- SIADH:
-
Syndrome of Inappropriate Antidiuretic Hormone Secretion
- CLL:
-
Chronic lymphocytic leukaemia
- ED:
-
Emergency department
- VFI:
-
Visual field index
- BTK :
-
Bruton tyrosine kinase
- VEGF:
-
Vascular endothelial growth factor
References
Capatina C, Inder W, Karavitaki N, et al. Management of endocrine disease: pituitary tumour apoplexy. Eur J Endocrinol. 2015;172(5):R179–90. https://doi.org/10.1530/EJE-14-0794.
Jain N, Keating M, Thompson P, et al. Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med. 2019;381(8):788–9. https://doi.org/10.1056/NEJMoa1900574.
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. IBRUTINIB as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–37. https://doi.org/10.1056/NEJMoa1509388.
Guido P, Treasure M, DeCherney G. SUN-280 a brute of a case: pituitary apoplexy in a patient treated for chronic lymphocytic leukemia with ibrutinib. J Endocr Soc. 2020;4(Supplement_1):SUN-280. https://doi.org/10.1210/jendso/bvaa046.740.
Mock J, Kunk PR, Palkimas S, et al. Risk of major bleeding with Ibrutinib. Clin Lymphoma Myeloma Leuk. 2018;18(11):755–61. https://doi.org/10.1016/j.clml.2018.07.287.
Caron F, Leong D, Hillis C, et al. Current understanding of bleeding with ibrutinib use: a systematic review and meta-analysis. Blood Adv. 2017;1(12):772–8. https://doi.org/10.1182/bloodadvances.2016001883.
Wang J, Zhao A, Zhou H, et al. Risk of bleeding associated with Ibrutinib in patients with B-cell malignancies: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2020;11:580622. https://doi.org/10.3389/fphar.2020.580622.
Sugita Y, Takada S, Tanigaki K, et al. Inhibition of VEGF receptors induces pituitary apoplexy: an experimental study in mice. PLoS One. 2023;18(3):e0279634. https://doi.org/10.1371/journal.pone.0279634.
Gupta R, Bhattacharjee U, Lekshmon KS, Chaudhary S, Sharma P, Jandial A, et al. Acute lymphoblastic leukemia presenting as PITUITARY APOPLEXY: a case report and review of the literature. Case Rep Endocrinol. 2021;2021:1–5. https://doi.org/10.1155/2021/6086756.
Fain JS, Naeim F, Becker DP, Van Herle A, Yan-Go F, Petrus L, et al. Chronic lymphocytic leukemia presenting as a pituitary mass lesion. Can J Neurol Sci. 1992;19(2):239–42. https://doi.org/10.1017/s0317167100042335.
Amin N, Balasubramanian S, Saiya-Cork K, et al. Cell-intrinsic determinants of ibrutinib-induced apoptosis in chronic lymphocytic leukemia. Clin Cancer Res. 2017;23(4):1049–59. https://doi.org/10.1158/1078-0432.CCR-15-2921.
Acknowledgements
Not applicable.
Funding
No funding to declare.
Author information
Authors and Affiliations
Contributions
AG was the main writer of the manuscript and performed the literature search. AG was involved with the patient care during their hospital admission. RS was the consultant responsible for the care of the patient and contributed to revising the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written patient consent has been obtained.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gomaa, A., Skelly, R. Pituitary macroadenoma apoplexy as a rare complication of Bruton tyrosine kinase inhibitor in chronic lymphoid leukaemia. Chin Neurosurg Jl 9, 30 (2023). https://doi.org/10.1186/s41016-023-00345-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41016-023-00345-0