Abstract
Background
Petroclival meningiomas (PCMs) are technically challenging lesions. Although the standard retrosigmoid approach is one of the most effective approaches, this route may have some limitations for the tumors extending into the supratentorial region and middle cranial fossa. We provide a modified surgical technique called retrosigmoid-transtentorial approach (RTTA) to solve these problems. The feasibility and efficacy of the RTTA were studied, by analyzing neurological outcomes and considerations of surgical strategies.
Methods
We analyzed 64 of 71 PCM patients (90.1 %). All 64 patients had epicenter of tumor in the posterior fossa with varying degrees of extension into the supratentorial area and/or the middle fossa. A conventional retrosigmoid craniotomy was performed following which the tentorium was incised from the attachment of tumor toward the free edge, which improved exposure to the petroclival region by offering additional operative room without resection of the adjacent part of the petrous bone.
Results
The rate of gross total resection was 71.9 %. There was no incidence of intraoperative death. One patient died in the postoperative phase. The postoperative permanent morbidity rate was 25.4 %. Follow-up was done with the mean time of 60.7 ± 47.5 months. There were 6 recurrence, 8 progression and 7 death cases and the mean KPS score was 83.2 ± 13.4 in the last follow up.
Conclusions
The RTTA achieves the goal of applying a safe, quick, uncomplicated, and lesser invasive access to the petroclival region which is indicated for tumors located in the posterior fossa with some extension into the supratentorial area and/or the middle fossa.
Similar content being viewed by others
Background
Petroclival meningiomas (PCMs) account for 3 % to 10 % of posterior fossa meningiomas [1] which comprise of 14 to 40 % of all intracranial meningiomas [2]. They arise from the upper two thirds of the clivus, locate at the petroclival junction and are medial to the trigeminal nerve. They are regarded as the most formidable challenge to neurosurgeons because of their proximity to the brainstem and vital to neurovascular structures, which definitely determine its difficulty to perform gross total resection (GTR), and lead to high rates of surgical morbidity and mortality. Despite recent advances in diagnostic neuroimaging methods, microsurgical techniques, and cranial base approaches, together with intraoperative neurophysiological monitoring [3], many patients with PCMs are still suffering from residual tumor and permanent neurological deficits which greatly affect their quality of life.
In the past decade, several approaches have been applied to resect PCMs including translabyrinthine, transcochlear, total petrosal, retrosigmoid, subtemporal, orbitozygomatic, and combinations of the above [4–12] in the effort to remove tumors in different regions and try to improve the treatment outcome. Each approach has its advantages and disadvantages, apart from limitations and potential complications. Recent literatures reveal a tendency toward moving away from the complex petrosal approaches to favoring less aggressive cranial base approaches [6, 7, 13–15].
We advocate the principles for the surgical treatment of PCMs: shorter operating time, direct trajectory and minimal disruption of neurovascular and temporal bone structures. Therefore, we have preferred to use the RTTA as a minimally invasive treatment strategy to offer a favorable chance for total tumor removal and quality of life for the patients while minimizing the risk of postoperative complications and neurological deficits. RTTA offers a workhorse approach, and early tumor visualization of standard retrosigmoid approach, with direct trajectory and wider working corridor by incising tentorium.
The conventional suboccipital retrosigmoid approach has provided an effective alternative to transpetrosal approach for the treatment of PCMs since its description in the early 1900s. However this route appears to have some limitations for the lesions reaching the middle cranial fossa through the Meckel’s cave and the tumors extending toward the tentorium even rupturing into the supratentorial region through the tentorium cerebelli hiatus. It may preferentially provide a satisfactory exposure to the lesions mainly located in cerebellopontine angle (CPA) and lower clivus regions.
During the past decade, we also have frequently used the retrosigmoid approach, especially for the PCMs predominantly located in the posterior fossa. By improving our surgical techniques and comparing with other relevant approaches, we have learned that combining with the transtentorial approach, which means the tentorium is incised based on the retrosigmoid approach, can improve the extent of exposure to the petroclival region by adding operative interval without extensive bony resection of the suprameatal tubercle which provides a better opportunity to receive total tumor removal especially for the tumors mainly located in the posterior fossa with some extension into the supratentorial area and/or the middle fossa. We have termed this approach as the retrosigmoid-transtentorial approach (RTTA).
The purpose of this retrospective study is to evaluate the feasibility and efficacy of the RTTA, the clinical outcome as well as considerations of various surgical strategies. We have analyzed our surgical experience and operative technique in a series of 64 patients with PCMs, operated via the RTTA in our department from July 1991 to April 2010.
Methods
Patient population
In this institutional retrospective study 71 patients with PCMs (including 5 patients previously underwent operation or radiosurgery) were treated surgically from July 1991 to April 2010 by the same senior authors. Sixty four patients (90.1 %), 48 females and 16 males, were treated via the RTTA. The mean age was 47.0 ± 11.2 years and ranged from 15 to 68 years (Table 1). The main presenting symptoms and signs were nonspecific headache (32 cases, 50.0 %), facial numbness and pain (22 cases, 34.4 %), tinnitus and hearing loss (33 cases, 51.6 %), disequilibrium (37 cases, 57.8 %) and lower cranial nerve symptoms (32 cases, 50.0 %) (Table 2). The mean symptom duration was 32 ± 30 months, ranging from 1 week to 180 months (Table 1).
All of patients underwent pre-operative evaluations with computed tomography (CT) and/or magnetic resonance imaging (MRI) with or without gadolinium contrast enhancement. The tumor size was defined as the greatest diameter of contrast-enhancing area on MRI and the mean of the maximum diameters of the tumors was 44.4 mm. According to Sekhar et al.’s criteria [16], tumors were classified into 4 groups by size. There were no tumors with diameter less than 10 mm. Four patients (6.2 %) had medium tumors (diameter range from 10 to 24 mm), 28 patients (43.8 %) had large tumors (diameter range from 25 to 44 mm), and 32 patients (50.0 %) had giant tumors (diameter larger than 45 mm) (Table 1). The PCMs were divided into three subtypes according to their main locations and extension. TypeI, clivus (28 cases, 43.8 %): the tumor grew up from the petroclival fissure into the contralateral area, and tumor body was located in the middle and upper clivus (Fig. 1). Type II, petroclival (13 cases, 20.3 %): the tumor originated from the petroclival fissure but mainly grew to the homolateral area, and tumor body was located in the middle clivus and CPA (Fig. 2). Type III, petroclivosphenoidal (23 cases, 35.9 %): the tumor originated from the middle and upper clivus and extended to the parasellar region, middle cranial fossa, petrous apex and sphenoclival fissure, then invaded to dorsum sellar through the tentorial hiatus (Fig. 3). Sixty-four patients (100 %) exhibited brainstem compression of various degrees; 17 patients (26.6 %) had radiographic evidence of cavernous sinus (CS) invasion; 32 patients (50.0 %) had radiographic evidence of Meckel’s cave invasion and 18 patients (28.1 %) had radiographic evidence of the expansion of supratentorial ventricular system. (Table 1)
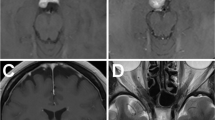
Contrast of MRI in clivus type case to show total resection by RTTA. Preoperative axial (a), coronal (b) and sagittal (c) MRI show a large petroclival meningioma located in the dorsum sellae and upper middle clivus with a long “dural tail sign” along the middle clivus and the tumor extended toward the supratentorium compressing brain stem. Postoperative axial (d) coronal (e) and sagittal (f) MRI show a complete tumor removal without recurrence including the supratentorial region
Contrast MRI in petroclival type case to show total resection by the RTTA. Preoperative axial (a, b), sagittal (c) and coronal (d) MRI show a very large petroclival meningioma located mainly in the posterior fossa, invading into the whole clivus and the tentorium, extending toward the supratentorial region. Postoperative axial (e) and sagittal (f) MRI show a complete tumor removal without recurrence
Contrast of MRI in petroclivosphenoidal type case to show gross total resection by RTTA. Preoperative axial (a), coronal (b) and sagittal (c) MRI show a very large petroclival meningioma located mainly in the posterior fossa, extending through Meckel’s cave into the middle fossa, reaching the posterior wall of the CS. Postoperative axial (d) coronal (e) and sagittal (f) MRI show a complete tumor removal without recurrence, including the extensions into Meckel’s cave and the posterior CS
Several surgical approaches, including the RTTA (64 cases, 90.1 %), pterional or extended pterional approach (3 cases, 4.2 %), frontal-temporal-orbital-zygomatic approach, retrosigmoid combined with pterional approach, retrosigmoid combined with transverse sinus transtentorial approach and presigmoid approach (1 case, 1.4 % each) were utilized to remove the tumors, depending on the location and extension of the lesion. The 64 cases treated by the RTTA included all 3 types of tumors mentioned above. These tumors predominantly located in the posterior fossa arising from the petroclival area with some degree of extension upward to the supratentorial region and/or part of the middle fossa. The medical records and radiolographic images of the patients were carefully reviewed retrospectively.
Surgical technique
Different surgical approaches have been used to expose and remove the tumors depending on the location and epicenter of the tumor, direction of tumor extension, tumor size, patient’s age, medical comorbidities, and proposed extent of resection. We selected the RTTA for majority of the patients, as a safe alternative to lateral approaches.
The patient is placed into the lateral-decubitus position with the head slightly rotated in order to bring up the mastoid process on the affected side. Intraoperative facial and trigeminal electromyographic responses and somatosensory evoked potentials are assessed. An inverted L-shaped incision is performed from just anterior to the mastoid, curving up and posteriorly across the superior nuchal line, and descending at the level of the cervical spine. Then a standard retrosigmoid soft tissue dissection is taken. The bone is exposed from the asterion superiorly to the foramen magnum inferiorly, and from the mastoid process to several centimeters posterior to the sigmoid sinus. The edges of the transverse, sigmoid sinuses and their junction are exposed widely after craniotomy.
An incision is made in the dura with the edges being based on the transverse and sigmoid sinuses. With gentle retraction of the cerebellum from the petrous bone, the arachnoid of the cerebellomedullary cistern are opened to allow the egress of cerebrospinal fluid (CSF). Once the cerebellum is relaxed substantially, the attachment of the tumor and inferior surface of the tentorium is exposed through the CPA. The petrosal vein is identified and well preserved.
In our experience, the cranial nerves (CNs) VII-VII complex is usually located dorsal to the petroclival meningioma and displaced downward and backward by the tumor. After identifying CNs VII-VII complex and the lower CNs by intraoperative electromyography, the characteristics of the tumor including its location with regard to the arachnoid planes, consistency and vascularity is assessed. Vascular supplies of the tumor usually originate from the petrous bone dura to tentorium.
First attention is given to the tumor base. We completely or partially cut off its blood supply to reduce bleeding. Subsequently, we perform tumor debulking from the intervals between CNs VII-VII complex and tentorium, between CNs VII-VII complex and CN V, and between CNs VII-VII complex and the lower CNs, carefully separating the tumor from the CNs and brainstem. Tumor dissection from the brain stem is the most important step. The arachnoid plane and the brain stem veins should be preserved as much as possible. If the tumor is tightly adherent to the neurovascular structures, rendering separation difficult, some tumor capsules may have to be left to prevent significant morbidity.
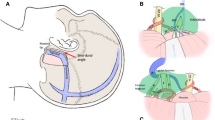
Once the tumor in the posterior fossa is removed, the resection is advanced into the direction of the middle fossa and supratentorial area. CN IV is identified, dissected, and mobilized along its course anterior to the tentorial rim. If components of the tumor rupture into the supratentorial area and/or invade into some part of middle fossa, the tentorium medial to CN V and superior to the petrous apex is opened as widely as possible near the petrous ridge while carefully protecting CN IV, which helps expose the tumor located in supratentorium and middle fossa. The tentorium is incised from the outside to the inside, beginning at 0.5-1.0 cm behind the petrous ridge to preserve the posterior roots of CN IV and CN V and to avoid injuring the superior petrosal sinus. After incising the tentorium, we own a newly exposed space by adding the interval between CN V and CN III through which the tumor located in supratentorium and middle fossa can be removed. The integrity of surrounding nerves and vessels could be preserved their integrity as much as possible without extensive bony resection of the suprameatal tubercle. If there are signs of tumor invasion, the tentorium is resected together with the tumor (Figs. 4, 5 and 6).
Intraoperative pictures demonstrating surgical steps of tumor resection via RTTA. a Expose the tumor in the superior and medial clival region after cerebellar retraction via the retrosigmoid approach. b Coagulate the tumor basement, perform intratumoral decompression, and then incise the tentorium after removing the tumor located in the posterior fossa. c Separate the adhesion between the tumor and brain stem. d Remove the the suprotentorial tumor by piecemeal resection through incised tentorium cerebelli hiatus. e Separate the adhesion between the suprotentorial tumor and CN III. f Remove the tumor totally and keep well the neurovascular structures integrity (Tu: Tumor, PCNs: Posterior Cranial Nerves, PV: Petrosal Vein, Te: Tentorium, BS: Brain Stem)
After resecting the tumor and ensuring hemostasis, the dura mater is closed watertight, and the craniotomy and skin wound are closed in the usual fashion. We do not regularly place drains.
Statistical analysis
Statistical analysis was performed using the IBM SPSS Statistics 21.0. A univariate analysis was used to evaluate the main factors affecting rate of GTR (Chi-Square Test) or Fisher’s Exact Test. (If expected count number <5). A p value <0.05 was considered significant difference.
Results
Surgical outcomes
All operations were performed in one stage. The tumors were pathologically graded according to the WHO classification [17] for all 64 patients: 61 cases were Grade I, 2 cases were Grade II and 1 was Grade III. Gross total resection (GTR) (Simpson Grade II) was achieved in 46 patients (71.9 %). This was confirmed by intraoperative view and postoperative MRI (Figs. 1, 2 and 3). Subtotal resection (STR) (Simpson Grade III) was done in 7 cases (10.9 %). Partial removal (Simpson Grade IV) was done in the remaining 11 cases (17.2 %). In the 3 subtype of PCMs, the GTR of Clivus, Petroclival and Petroclivosphenoidal was 92.9 %, 61.5 % and 52.2 % separately (Table 3). Anatomical preservation of CNs was achieved in all patients. The main factors affecting whether to archive GTR include tumor size (p = 0.018), subtype (p = 0.004), presence or absence of invasion into the CS (p = 0.008), and extent of brainstem compression (p = 0.005) (Table 3).
Postoperative complications
One patient (1.6 %) died from thromboembolism secondary to pre-existing rheumatic heart disease on postoperative 4th day. Postoperative complications were observed in 36 cases (56.3 %). The majority of the complications consisted of new CN functional deficits (24 cases, 37.5 %), including 9/28 cases (32.1 %) of Clivus, 4/13 cases (30.8 %) of Petroclival and 11/23 cases (47.8 %) of Petroclivosphenoidal separately. Hematoma within the tumor cavity, epidural hematoma and pulmonary infection were 2 cases separately. (Table 1) The most common symptoms of neuronal functional deficits were hemiplegia (23 cases, 36.5 %), disequilibrium (18 cases, 28.6 %) and impairment of CN III-VII and the lower CNs (Table 2).
Sixty of the 64 patients had recorded followed up for 4 to 132 months (60.7 ± 47.5). Ten cases with temporary hemiplegia recovered after 6–12 months; 7 cases with balance dysfunction recovered. Recovery from CN deficits were: oculomotor nerve in 11 cases; trigeminal nerve in 15 cases; abducens nerve in 7 cases; lower CNs in 9 cases. In addition 9 of the 20 patients with facial palsy (House-Brackmann Grade 3 or 4) recovered to House-Brackmann Grade 2 after 3–6 months. Postoperative KPS at discharge and at recent evaluation was 73.2 ± 15.6 and 83.2 ± 13.4 respectively. And KPS score was divided into 3 levels: excellent (KPS ≥ 80), fine (60 ≤ KPS < 80) and poor (KPS < 60). The distribution of KPS in 64 patients in the immediate postoperative period was 51.6 % excellent, 37.5 % fine, and 10.9 % poor. The KPS levels had improved to 67.9 % excellent, 30.2 % fine and decreased to 1.9 % poor in 53 cases at the most recent follow-up evaluation (Fig. 7 ).
Recurrence and tumor progression
Seven patients died from various reasons in the 60 patients who got follow-up. Four died of meningioma progression after STR or partial section. The other 3 had GTR and died of un-meningioma diseases such as diabetes, heart failure and gastric cancer.
Forty-four of the 46 patients who had GTR were followed up (mean followed-up time 60.7 ± 47.5 months), and 6 had recurrence (13.6 %). The mean time to recurrence was 24.7 months postoperatively. There was 16 patients (2 patients of Clivus, 4 patients of Petroclival and 10 patients of Petroclivosphenoidal) got observed (mean followed-up time 52.0 months) in 18 STR and partial section patients. The tumor progressed in 8 cases (including 6 cases of Petroclivosphenoidal and 2 cases of Petroclival) with 4 cases (all of Petroclivosphenoidal) resulting in death. Another 8 patients (including 4 cases of Petroclivosphenoidal, 2 cases of Clivus and Petroclival, each) received gamma knife radiosurgery, of which 3 cases had tumor progression (2 cases of Petroclivosphenoidal and 1 case of Petroclival).
Discussion
Petroclival meningiomas (PCMs) are defined as meningiomas growing from the dura along the upper two-thirds of the clivus and the petroclival fissure within the internal auditory meatus which may infiltrate or encase cranial nerves and arteries of the posterior circulation [4–7, 18, 19]. Extension of the tumor at the site of its attachment into the supratentorial compartment, the CS, and the CPA are common [13]. Therefore the resection of PCMs continues to pose a significant technical challenge for neurosurgeons, and carries a relatively high risk for surgical morbidity and mortality. With the advances in skull base techniques as well as improvement in neuroimaging and intraoperative electrophysiology, the complex petroclival region has become more accessible and the surgical results and the prognosis of patients have been improved relatively. Obviously, different surgical approaches have been used to expose and remove the tumors according to the location of the epicenter of the tumor, direction of tumor extension, tumor size, patient age, medical comorbidity, and proposed radicality of resection. Significantly, personal experience, preferences, and the microneurosurgical technique can also affect the choice of surgical approach [13]. Each approach has its own set of indications and limitations.
The presigmoid transpetrosal approach is considered to be a popular approach for PCMs performed by many neurosurgeons and mainly applied to tumors located in the posterior fossa and clivus areas. This approach offers a wide view of surgical field, short trajectory lateral access, wide exposure of CNs and main arteries of posterior circulation and higher preservation chance of the vein of Labbe, eliminating by less retraction of temporal lobe [13]. However this approach requires advanced anatomic knowledge and surgical training. It is time-consuming may cause more morbidities due to a large surgical wound [18]. Similarly, the combined transpetrosal approach which was once considered to be the first option for PCMs [16] can provide a much wider vision and shorter distance to access to the petroclival area, when they significantly grow into both the middle and posterior fossae equally. Besides the disadvantages similar to a presigmoid transpetrosal approach it also increases a potential risk of damage to the facial nerve, hearing loss, and injury to the vein of Labbe [20] which lead to slow recovery and poor neurological outcomes.
With the advent of minimally invasive techniques, much effort has been directed towards avoiding extensive exposure of the petrous bone structures [15, 21]. The traditional retrosigmoid approach was described in the early 1900s, offering lesser morbidity [13, 14, 22, 23], compared with the transpetrous or basal subtemporal approaches. We have elected to choose the RTTA, wherein the tentorium is incised from the attachment of tumor toward the free edge following the conventional suboccipital retrosigmoid craniectomy. And we considered that the RTTA was one of simple, effective and practical approaches for PCMs. The advantages are obvious including: 1) Less trauma to patient, simpler approach for operator and less time consumption [18]. 2) Avoiding the injury of the traction of vein of Labbe and decreasing the iatrogenic complications [13, 20]. 3) More abundant exposure of operative sight without more traction of cerebellum and venous sinus handling [20] to expose the deep regions of superior petrosal sinu, petrous apex, edge of tentorium and clivus and if needed, accompany with the incising of tentorium, the exposure can be extended to the whole region of clivus from dorsum sellae to foramen magnum region. 4) Earlier exposure and handling of brain-tumor boundary and basement of tumor without necessarily extensive resection of the petrous bone, drilling of the suprameatal bone and exposing the internal structures of petrosal bone [13].
We retrospectively summarized our experience with 71 cases of surgically treated PCMs, of which 64 cases (90.1 %) was done via the RTTA. In this group, the rate of GTR was in 71.9 %. Of the three subtype of PCMs mentioned above, those with the clivus type achieved GTR at the rate of 92.9 % (26 cases in 28 patients); 61.5 % (8 cases in 13 patients) of those with the petroclival type achieved GTR; on the other hand, 52.2 % (12 cases in 23 patients) of the petroclivosphenoidal type achieved and GTR (Table 3). And encouragingly the rate of GTR of our group was not lower or even higher than other approaches [10, 24–26]. We found that most of the patients recovered significantly and satisfactorily. Excellent KPS (KPS ≥ 80) was obtained in 51.6 % of 64 patients postoperatively and improved to 67.9 % of 53 patients at the time of the latest follow-up evaluation, meanwhile, the poor KPS decreased to 1.9 % at the most recent follow-up evaluation (Fig. 7 ). The recurrence is just 13.6 % of 44 patients who had GTR were followed up. These data has proved the quality of life has been improved obviously with a favourable prognosis compared with other approaches [10, 24–26].
As we mentioned above, PCMs commonly extend into supratentorial compartment and some part of middle fossa. In particular, the large and giant PCMs always invade the tentorium or even rupture into tentorium cerebelli hiatus and extend toward dorsum sellae and suprasellar area. Therefore, incising the tentorium and removing tumors extended in supratentorial compartment appears to be a prudent, natural and necessary choice. Through the widened CPA, we have achieved excellent tumor decompression, especially when tumor mass displaces the brainstem providing increased access to the lower and upper petroclival surface [27]. Moreover, the large wide surgical corridor created by the lesion obviates performing an invasive transcochlear approach with facial transposition [18]. With the RTTA, the tentorium from the attachment of tumor or medial to the porous CN V is incised (Fig. 5). This can be extended through the free edge while paying close attention to the trochlear nerve, posterior cerebral artery and superior cerebellar artery when opening the free edge of the tentorium. It also can provide wide exposure of supratentorial compartment permitting to access to middle fossa freely without need of extensive suprameatal drilling or resection of petrous bone, thus avoiding some of the hazards of the transpetrosal approaches. After opening the tentorium, operative view and working angle are both enlarged, improving the exposure of these lesions. We testify the utility of RTTA for removing large and giant tumors from the experience of our cases, of which 93.8 % were large and giant tumors. Another major advantage of incising of the tentorium is creating a possibility of adding a new operative corridor between CN V and CN III (Fig. 6). Cutting of tentorium and exposing CN III offers working angle from the posterior fossa. Direct access to the dural attachment of tumors on the dorsal petrous bone provides easily disruption of tumor blood supply thereby providing a bloodless surgical field. If the tentorium is also invaded by the tumor, it can be resected together with tumor, offering an improved Simpson’s grade of resection. We believe that this approach can generate enough space to expose and resect tumors from multiple angles thus increasing the rate of GTR with preservation of neurovascular structures.
Obviously, the RTTA also contains some limitations: 1) As for the petroclivosphenoidal type of PMCs, the rate of GTR was only 52.2 % (12 cases in 23 patients), and the new postoperative CN functional deficits was up to 47.8 % and obtained higher rate of progress and death during follow-up. These meant the tumor could not be resected just only by RTTA when the main part of tumor located at middle cranial fossa or invaded into cavernous sinus, especially invading the internal structures of cavernous sinus. We need to choose the transpetrosal approach or combined other approaches to improve the rate of GTR. 2) This route appears to have limitations for the lesions reaching the middle cranial fossa through the Meckel’s cave and or extending above the tentorium through the tentorium cerebelli hiatus or infiltrating the tentorium itself. 3) The resection of tumor was mainly achieved through numerous neurovascular intervals via RTTA, therefore the risk of iatrogenic injury of neurovascular structures was relative higher. The main dysneuria in the group were varying degrees of cranial nerve palsy and hemiplegia (Table 2). As a consequence, the tumor-self “occupation” should be used possibly and the traction of neurovascular structures should be avoided by the microinstrument as far as possible. Sharp dissection of the arachnoid between the tumor and encapsulated cranial nerve in the early stage is necessary. The accomplishment of tumor resection within arachnoid interface and high-protection of arachnoid interface and vasa vasorum of cranial nerve are the key technique for the decreasing of neurovascular injury.
Samii at al. [28] introduced the retrosigmoid intradural suprameatal approach as a modification of traditional retrosigmoid approach to treat PCMs in 1983. Since then, several modifications of retrosigmoid approach having been invented to resect PCMs. Watanabe et al. [29] modified the suprameatal retrosigmoid approach by using the lateral oblique position and preferentially dividing the tentorium without extensive drilling of the suprameatal bone, reporting a GTR rate of 42 % in 26 patients. Similarly, Zhu et al. [30] introduced the keyhole approach in surgical treatment of PCMs which provided an easy and quick access to the supra- and infratentorial juxta-clival region without drilling of the petrous bone. They demonstrated GTR in 14 of 25 patients (56 %). Shaan et al. [31] proposed the extended retrosigmoid craniotomy for neoplastic lesions in the posterior fossa incorporating a component of posterior petrosal approaches by skeletonizing the sigmoid sinus, without requiring much petrosal drilling.
The overall rate of GTR of PCMs in literature is not very high, ranging 0-75 % [4–9, 13, 32–34], and in this series we incurred a 71.9 % GTR rate. The main limitations of achieving GTR include encasement of the vertebrobasilar arteries and perforating arteries [29], tumor blood supply and parasitization of adjacent arteries, degree of invasion to the subarachnoid space etc. [5] In addition to the factors, tumor size (p = 0.018), subtype (p = 0.004), presence or absence of invasion into the CS (p = 0.008), and extent of brainstem compression (p = 0.005) are significant prognostic factors. In this group, there were 17 patients (26.6 %) with tumors that had invaded the CS, including 8 (47.1 %) in whom GTR was achieved and 32 patients (50.0 %) with tumors that had invaded Meckel’s cave, including 18 (28.1 %) achieved GTR. It is impossible that all PCMs are removed totally, but it is very important to attempt to get GTR during the initial operation. Because the residual tumor has the ability to grow and mutate, the goal to attempt a total removal while preserving the patient’s neurological function is crucial. For this reason, it is important to plan and limit resection of these lesions, keeping a balance between guaranteeing good quality of life and total tumor resection.
With the recognizing of the potential advantages of this approach compared with skull base approaches, we think the RTTA described here is an easier, safer and minimally invasive treatment but resolves much resection of PCMs especially is indicated for the following situations: a) the tumors has invaded into the whole clivus and extended from foramen magnum to dorsum sellae; b) the tumor originates from tentorial edge attaching to the petroclival fissure and petrous apex and extending toward the supratentorium; and c) the tumor is mainly located in the posterior fossa with the extension to the middle cranial fossa.
Conclusion
The retrosigmoid-transtentorial approach (RTTA) for the treatment of the PCMs provides favorable outcomes of neurological function and quality of life when GTR is attempted. This approach serves the goal of a safe and uncomplicated, less invasive access to the petroclival region for resection of PCMs, especially when the tumor mainly in posterior fossa has some extension into the supratentorial area and/or the middle fossa.
Abbreviations
CN, Cranial nerve; CPA, Cerebellopontine angle; CS, Cavernous sinus; CSF, Cerebrospinal fluid; CT, Computed tomography; GTR, Gross total resection; KPS, Karnofsky performance status; MRI, Magnetic resonance image; PCM, Petroclival meningioma; RTTA, Retrosigmoid-transtentorial approach; STR, Subtotal resection; WHO, World Health Organization
References
CASTELLANO F, RUGGIERO G. Meningiomas of the posterior fossa. Acta Radiol Suppl. 1953;104:1–177.
Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088–95. discussion 1088–95.
Bricolo AP, Turazzi S, Talacchi A, Cristofori L. Microsurgical removal of petroclival meningiomas: a report of 33 patients. Neurosurgery. 1992;31:813–28. discussion 828.
Couldwell WT, Fukushima T, Giannotta SL, Weiss MH. Petroclival meningiomas: surgical experience in 109 cases. J Neurosurg. 1996;84:20–8.
Sekhar LN, Wright DC, Richardson R, Monacci W. Petroclival and foramen magnum meningiomas: surgical approaches and pitfalls. J Neurooncol. 1996;29:249–59.
Samii M, Tatagiba M, Carvalho GA. Resection of large petroclival meningiomas by the simple retrosigmoid route. J Clin Neurosci. 1999;6:27–30.
Bambakidis NC, Kakarla UK, Kim LJ, Nakaji P, Porter RW, Daspit CP, et al. Evolution of surgical approaches in the treatment of petroclival meningiomas: a retrospective review. Neurosurgery. 2007;61:202–9. discussion 209–11.
Little KM, Friedman AH, Sampson JH, Wanibuchi M, Fukushima T. Surgical management of petroclival meningiomas: defining resection goals based on risk of neurological morbidity and tumor recurrence rates in 137 patients. Neurosurgery. 2005;56:546–59. discussion 546–59.
Ramina R, Neto MC, Fernandes YB, Silva EB, Mattei TA, Aguiar PH. Surgical removal of small petroclival meningiomas. Acta Neurochir (Wien). 2008;150:431–8. discussion 438–9.
Chang SW, Wu A, Gore P, Beres E, Porter RW, Preul MC, et al. Quantitative comparison of Kawase’s approach versus the retrosigmoid approach: implications for tumors involving both middle and posterior fossae. Neurosurgery. 2009;64:ons44–51. discussion ons51-2.
Li PL, Mao Y, Zhu W, Zhao NQ, Zhao Y, Chen L. Surgical strategies for petroclival meningioma in 57 patients. Chin Med J (Engl). 2010;123:2865–73.
Samii M, Gerganov V, Giordano M, Samii A. Two step approach for surgical removal of petroclival meningiomas with large supratentorial extension. Neurosurg Rev. 2010;34:173–9.
Goel A, Muzumdar D. Conventional posterior fossa approach for surgery on petroclival meningiomas: a report on an experience with 28 cases. Surg Neurol. 2004;62:332–8. discussion 338–40.
Samii M, Tatagiba M, Carvalho GA. Retrosigmoid intradural suprameatal approach to Meckel’s cave and the middle fossa: surgical technique and outcome. J Neurosurg. 2000;92:235–41.
Seifert V. Clinical management of petroclival meningiomas and the eternal quest for preservation of quality of life: personal experiences over a period of 20 years. Acta Neurochir (Wien). 2010;152:1099–116.
Sekhar LN, Jannetta PJ, Burkhart LE, Janosky JE. Meningiomas involving the clivus: a six-year experience with 41 patients. Neurosurgery. 1990;27:764–81. discussion 781.
Radner H, Blumcke I, Reifenberger G, Wiestler OD. The new WHO classification of tumors of the nervous system 2000. Pathology and genetics. Pathologe. 2002;23:260–83.
Abdel AKM, Sanan A, van Loveren HR, Tew Jr JM, Keller JT, Pensak ML. Petroclival meningiomas: predictive parameters for transpetrosal approaches. Neurosurgery. 2000;47:139–50. discussion 150–2.
Nakamura M, Roser F, Dormiani M, Vorkapic P, Samii M. Surgical treatment of cerebellopontine angle meningiomas in elderly patients. Acta Neurochir (Wien). 2005;147:603–9. discussion 609–10.
Chen LF, Yu XG, Bu B, Xu BN, Zhou DB. The retrosigmoid approach to petroclival meningioma surgery. J Clin Neurosci. 2011;18:1656–61.
Bambakidis NC, Kakarla UK, Kim LJ, Nakaji P, Porter RW, Daspit CP, et al. Evolution of surgical approaches in the treatment of petroclival meningiomas: a retrospective review. Neurosurgery. 2008;62:1182–91.
Seoane E, Rhoton Jr AL. Suprameatal extension of the retrosigmoid approach: microsurgical anatomy. Neurosurgery. 1999;44:553–60.
Cheung SW, Jackler RK, Pitts LH, Gutin PH. Interconnecting the posterior and middle cranial fossae for tumors that traverse Meckel’s cave. Am J Otol. 1995;16:200–8.
Almefty R, Dunn IF, Pravdenkova S, Abolfotoh M, Al-Mefty O. True petroclival meningiomas: results of surgical management. J Neurosurg. 2014;120:40–51.
Li D, Hao SY, Wang L, Tang J, Xiao XR, Zhou H, et al. Surgical management and outcomes of petroclival meningiomas: a single-center case series of 259 patients. Acta Neurochir (Wien). 2013;155:1367–83.
Yamakami I, Higuchi Y, Horiguchi K, Saeki N. Treatment policy for petroclival meningioma based on tumor size: aiming radical removal in small tumors for obtaining cure without morbidity. Neurosurg Rev. 2011;34:327–34. discussion 334–5.
Samii M, Gerganov VM. Surgery of extra-axial tumors of the cerebral base. Neurosurgery. 2008;62:1153–66. discussion 1166–8.
Lan Q, Qian ZY, Chen J, Liu SH, Lu ZH, Huang Q. Microsurgical treatment of posterior cranial fossa tumors via keyhole approaches. Zhonghua Yi Xue Za Zhi. 2005;85:219–23.
Watanabe T, Katayama Y, Fukushima T, Kawamata T. Lateral supracerebellar transtentorial approach for petroclival meningiomas: operative technique and outcome. J Neurosurg. 2011;115:49–54.
Zhu W, Mao Y, Zhou LF, Zhang R, Chen L. Keyhole approach surgery for petroclival meningioma. Chin Med J (Engl). 2006;119:1339–42.
Raza SM, Quinones-Hinojosa A. The extended retrosigmoid approach for neoplastic lesions in the posterior fossa: technique modification. Neurosurg Rev. 2011;34:123–9.
Sekhar LN, Swamy NK, Jaiswal V, Rubinstein E, Hirsch Jr WE, Wright DC. Surgical excision of meningiomas involving the clivus: preoperative and intraoperative features as predictors of postoperative functional deterioration. J Neurosurg. 1994;81:860–8.
Mathiesen T, Gerlich A, Kihlstrom L, Svensson M, Bagger-Sjoback D. Effects of using combined transpetrosal surgical approaches to treat petroclival meningiomas. Neurosurgery. 2008;62:1213–23.
Tahara A, de Santana Jr PA, Calfat MMV, Panagopoulos AT, da Silva AN, Zicarelli CA, et al. Petroclival meningiomas: surgical management and common complications. J Clin Neurosci. 2009;16:655–9.
Acknowledgements
This work was supported by grants from the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (No.2014BAI04B02). We apologize to all researchers whose relevant contributions were not cited due to space limitations.
Authors’ contributions
All of authors have the equal contributions to the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All of the individual person’s data in the manuscript has been consented to publish which was obtained from the person in this study.
Ethics approval and consent to participate
This study on ethics has been approved and consented by the Medical Ethics Committee of Xiangya Hospital.
Disclosure
The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhao, Z., Yuan, X., Yuan, J. et al. Retrosigmoid-transtentorial approach for petroclival meningiomas: operative technique and clinical outcome. Chin Neurosurg Jl 2, 21 (2016). https://doi.org/10.1186/s41016-016-0040-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41016-016-0040-9