Abstract
Background
Ventricular assist device (VAD) is usually attached by an inflow cannula to the apex of the systemic left ventricle (LV), but very few cases with implantation of the VAD in the morphologic right ventricle (RV) have been described.
Case presentation
We describe the case of a 41-year-old male who developed severe systemic RV failure related to a Mustard procedure he had as an infant for treatment of TGA. His heart failure was refractory and irreversible, and therefore, he underwent VAD implantation for systemic RV support. Although the patient developed pulmonary congestion on postoperative day (POD) 5, he was discharged on POD 60. He is now looking forward to receiving heart transplantation.
Conclusions
Placement of a VAD for systemic RV failure could be a life-saving treatment in adult patients with heart failure due to congenital heart disease.
Similar content being viewed by others
Background
After an atrial switch operation (Mustard or Senning operation) for correction of transposition of the great arteries (TGA) or in patients with congenitally corrected TGA (CCTGA), discordant atrioventricular and ventriculoarterial connections are both present. Owing to this double discordance, a reconnection of the heart vessels is necessary to allow for proper blood circulation. This can be done with the Mustard or Senning procedure, which will help prevent cyanosis. Patients who undergo such arterial switch procedures are surviving to adulthood, but they often develop RV heart failure a few decades later. Implantation of ventricular assist device (VAD) is a useful therapy for patients with end-stage heart failure as a bridge to heart transplantation. However, adult congenital heart disease (ACHD) patients with TGA post-Mustard procedure or CCTGA have unique anatomical and physiological characteristics that can make clinical management or surgical procedures more challenging.
Case presentation
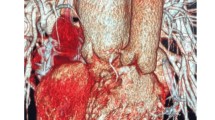
The patient was a 41-year-old man (height 174.2 cm, body weight 60 kg) with dextro-TGA who underwent balloon atrioseptostomy treatment at birth because of desaturation. He also had a Mustard procedure (interatrial baffle) at 10 months of age and required surgical repair for pulmonary vein stenosis at 2 years of age. At 41 years of age, he developed severe systemic RV failure and pulmonary edema (Fig. 1). He was treated with inotropic agents, mechanical ventilation, intra-aortic balloon pumping, and continuous hemodiafiltration. His hemodynamic status was stabilized with these treatments, but he remained inotrope-dependent, and his condition gradually worsened despite optimal medical therapy. After a medical team preoperative meeting, he was scheduled for a VAD implantation of the RV as a bridge to orthotropic cardiac transplantation.
Preoperative cardiac catheterization indicated elevated pulmonary arterial wedge pressure and severely impaired systemic RV function with infusion of dobutamine (3 μg/kg/min) and carperitide (24 ng/kg/min), a recombinant atrial natriuretic peptide with vasodilating and diuretic activity (Table 1). Echocardiography identified the dilated RV, preserved pulmonary ventricle (morphological left ventricle), and increased baffle flow velocity (Table 1). Chest radiography and computed tomography scan showed a severely dilated RV (Figs. 1 and 2).
In the operating room, a continuous monitoring of electrocardiography, SpO2, systemic arterial pressure via a right radial artery catheter, bispectral index (BIS; Medtronic, Minneapolis, MN), and regional cerebral oxygen saturation at the right and left forehead (INVOS™ 5100C, Somanetics, USA) was initiated before anesthesia induction. After administration of 100% oxygen, general anesthesia was induced slowly by intravenous administration of 3 mg of midazolam, 0.5 mg of fentanyl, and 50 mg of rocuronium. Additionally, dobutamine (3.3 μg/kg/min), nicorandil (1.1 μg/kg/min), which has the dual properties of a nitrate and a potassium channel opener, and carperitide (33 ng/kg/min) were continuously administered to minimize anesthetic-induced cardiac depression. During anesthesia induction and tracheal intubation, his circulatory and respiratory status remained stable. After tracheal intubation, a transesophageal echocardiography probe was inserted. Then, a central venous catheter and right heart catheter were inserted via the right internal jugular vein. The latter catheter (Swan Ganz catheter) was guided to the right pulmonary artery under fluoroscopic control because of his unique anatomy with interatrial baffle.
His anesthesia depth was controlled at a BIS of 40–60 by a continuous infusion of propofol (3–5 mg/kg/h), remifentanil (0.2–0.3 μg/kg/min), and rocuronium (7 μg/kg/min).
A standard median sternotomy was performed. Because his heart was heavily adherent, the cardiac surgeons had difficulty in adhesiolysis. The systemic aorta and systemic atrium were cannulated, and cardiopulmonary bypass (CPB) was instituted. At first, the patient’s aortic valve was replaced with a bioprosthetic valve for moderate aortic valve regurgitation. Subsequently, the trabeculae carneae of the RV, which could disturb the flow to the inflow cannula, were resected via the RV apex; the inflow cannula was positioned at the apex of the RV. The outflow cannula graft was anastomosed to the ascending aorta, and then a Jarvik 2000 VAD (Jarvik Heart, Inc., New York, NY, US) was implanted.
At the weaning of the CPB, infusion of dobutamine (3.3 μg/kg/min) and inhalation of nitric oxide (20 ppm), to decrease afterload of the pulmonary LV, was started. The CPB weaning was not difficult, and the VAD was successfully commenced at a pump speed of 10,000 rpm (3–5 L/min). The aortic cross-clamping, CPB, surgery, and anesthesia lasted 200, 296, 628, and 807 min, respectively. Estimated blood loss and urine output were 2051 and 2390 mL, respectively. Overall, 900 mL of crystalloid, 900 mL of colloid, 960 mL of packed red blood cells, 560 mL of fresh frozen plasma, and 200 mL of platelet concentrate were administered. After completion of operation, the patient was transferred to the intensive care unit with ventilator support under propofol sedation.
On postoperative day (POD) 2, the patient was weaned from mechanical ventilation. On POD 5, after our surgeons decreased the VAD dial from 3 to 2, the patient developed pulmonary congestion with respiratory distress, decreased arterial oxygen saturation, and elevated pulmonary artery pressure (PAP) (Table 2). Noninvasive positive-pressure ventilation (NPPV) was adopted, and intensified diuretic therapy was started. Additionally, the intensivists returned the VAD dial from 2 to 3. A few days later, he recovered from congestive heart failure, and then, NPPV was stopped. Following this, the patient did well hemodynamically and had good VAD performance. Another important point for postoperative management of VAD implantation is to prevent thrombosis. In this case, continuous infusion of heparin was administered for anticoagulation. The heparin infusion adjusted to an activated partial thromboplastin time (APTT) ration of 1.5–2.0 (or 40–50 s). Actually, the infusion rate or heparin was 15,000–20,000 units per day.
The patient was discharged from the hospital on POD 60. He is now eagerly awaiting heart transplantation. Table 1 shows his hemodynamic data, and Fig. 3 shows his chest radiography on POD 90.
Discussion
Many children with congenital heart disease (CHD) are now surviving into adulthood because of improved surgical repair techniques and management [1]; however, the number of adult congenital heart disease (ACHD) patients with heart failure is also increasing concomitantly [2].
Senning performed the first atrial switch procedure for TGA in 1958 [3], and Mustard developed an alternative operation, which resected the atrial septum and created an interatrial baffle to divert blood from both the superior vena cava and the inferior vena cava to the mitral valve and left ventricle [4]. Systemic RV dysfunction is a critical complication after a Senning or Mustard procedure because a morphological RV must support the systemic circulation instead of the morphological left ventricle [5]. Following an atrial switch operation, late death occurs in 16% of patients [6]. Additionally, the systemic RV is impaired in 9.1% of patients, and 52–55% of patients have a New York Heart Association functional class of II or III. Currently, the 20-year survival rate is 85.4% [7].
Implantation of VAD in a patient with ACHD, such as after an atrial switch operation for TGA, could be a particularly challenging procedure. A small number of cases describe the implantation of VAD for a morphologic RV [8,9,10,11,12]. Because the trabeculae carneae of the RV could disturb the blood flow to the inflow cannula, it must be resected via the RV apex before introduction of the inflow cannula to the apex of the RV, which was pointed out in this paper. Moreover, because cardiac surgery for ACHD involves the division of adhesions, the risk of bleeding, and challenging anatomy, VAD placement in ACHD patients is controversial [13].
The patient developed pulmonary congestion with increased PAP at POD 5. We presume that the preserved pulmonary LV function and decreased VAD output could easily induce over-filling of the systemic RV in patients after an atrial switch operation. In a previous report, Toyama et al. suggest that it is essential to adjust the systemic VAD flow according to the pulmonary ventricular function and the systemic ventricular filling in case of VAD implantation to a patient with CCTGA [14].
In fact, this case had congestive heart failure following the decrease in VAD dial by the surgeons from 3 to 2 on POD5. Our surgeons decreased the VAD dial in order to open aortic valve because they feared thrombosis at the aortic root. We thought that this procedure could induce pulmonary congestion. Actually, after the intensivists returned the VAD dial from 2 to 3, he recovered from congestive heart failure in combination with NPPV adaptation and the intensified diuretic therapy.
Although he underwent surgical repair for stenosis of his pulmonary veins at 2 years of age, there was the potential for residual pulmonary vein obstruction (PVO). In this case, PVO could be one of the major risk factors for pulmonary congestion. However, we unfortunately did not examine for details in this case, and so we could not deny or affirm the existence of PVO. In the perioperative management of VAD implantation for ACHD patients, we must assess the presence or absence of PVO preoperatively. PVO is a congenital or acquired cardiac malformation that is hard to diagnose clinically and could be properly diagnosed by echocardiography, and cardiac catheterization could reveal the site of stenosis morphologically. Additionally, because the volume of the atrium was limited after atrial baffle repair, excessive transfusion could easily induce pulmonary congestion.
Postoperative anticoagulant therapy of patients undergoing VAD implantation is one of the most important issues. Anticoagulation protocols change by each institution, implanted device, and individual patient. In many cases, to prevent clot formation, both warfarin and antiplatelet drugs, such as aspirin or dipyridamole, are administered for anticoagulation [15]. During warfarin therapy, the target prothrombin-international normalized ratio (PT-INR) ranges from 2.5 to 3.0. Aspirin is often given in doses from 80 to 325 mg per day. During anticoagulant therapy, we must pay attention to systemic bleeding complications.
VAD placement for systemic RV failure is a life-saving treatment in ACHD patients who encounter end-stage heart failure. Although these patients have unique anatomical and physiological characteristics that can make clinical management more challenging, systemic VAD implantations with systemic RV dysfunction have the potential to improve physiological conditions and can offer a bridge to heart transplantation.
Abbreviations
- ACHD:
-
Adult congenital heart disease
- CCTGA:
-
Congenitally corrected transposition of the great arteries
- CHD:
-
Congenital heart disease
- CPB:
-
Cardiopulmonary bypass
- LV:
-
Left ventricular
- NPPV:
-
Noninvasive positive-pressure ventilation
- PAP:
-
Pulmonary arterial pressure
- POD:
-
Postoperative day
- RV:
-
Right ventricular
- TGA:
-
Transposition of the great arteries
- VAD:
-
Ventricular assist device
References
Crumb SR, Cook SC, Cheatham JP, Galantowicz M, Feltes TF, Phillips A, et al. Quality outcomes of ACHD patients undergoing cardiovascular procedures and hospital admission in a free-standing children’s hospital. Int J Cardiol. 2011;146:326–9.
Stout KK, Broberg CS, Book WM, Cecchin F, Chen JM, Dimopoulos K, et al. Chronic heart failure in congenital heart disease: a scientific statement from the American Heart Association. Circulation. 2016;133:770–801.
Senning A. Surgical correction of transposition of the great arteries. Surgery. 1959;45:966–80.
Mustard WT. Successful two-stage correction of transposition of the great vessels. Surgery. 1964;55:469–72.
Warnes CA. Transposition of the great arteries. Circulation. 2006;114:2699–709.
Gelatt M, Hamilton RM, McCrindle BW, Connelly M, Davis A, Harris L, et al. Arrhythmia and mortality after the Mustard procedure: a 30-year single-center experience. J Am Coll Cardiol. 1997;29:194–201.
Hörer J, Schreiber C, Cleuziou J, Vogt M, Prodan Z, Busch R, et al. Improvement in long-term survival after hospital discharge but not in freedom from reoperation after the change from atrial to arterial switch for transposition of the great arteries. J Thorac Cardiovasc Surg. 2009;137:347–54.
Wiklund L, Svensson S, Berggren H. Implantation of a left ventricular assist device, back-to-front, in an adolescent with a failing Mustard procedure. J Thorac Cardiovasc Surg. 1999;118:755–6.
Stewart AS, Gorman RC, Pocchetino A, Rosengard BR, Acker MA. Left ventricular assist device for right side assistance in patients with transposition. Ann Thorac Surg. 2002;74:912–4.
George RS, Birks EJ, Radley-Smith RC, Khaghani A, Yacoub M. Bridge to transplantation with a left ventricular assist device for systemic ventricular failure after Mustard procedure. Ann Thorac Surg. 2007;83:306–8.
Joyce DL, Crow SS, John R, St Louis JD, Braunlin EA, Pyles LA, et al. Mechanical circulatory support in patients with heart failure secondary to transposition of the great arteries. J Heart Lung Transplant. 2010;29:1302–5.
Stokes MB, Saxena P, McGiffin DC, Marasco S, Leet AS, Bergin P. Successful bridge to orthotopic cardiac transplantation with implantation of a HeartWare HVAD in management of systemic right ventricular failure in a patient with transposition of the great arteries and previous atrial switch procedure. Heart, Lung Circ. 2016;25:e69–71.
Goldberg SW, Fisher SA, Wehman B, Mehra MR. Adults with congenital heart disease and heart transplantation: optimizing outcomes. J Heart Lung Transplant. 2014;33:873–7.
Toyama H, Takei Y, Saito K, Mori S, Ui A, Kobayashi N, et al. Ventricular assist device implantation in a patient with severe systemic right ventricular failure and pulmonary hypertension secondary to congenitally corrected transposition of great arteries. J Cardiothorac Vasc Anesth. 2018;32:436–40.
Kakker R, Ellis M, Fearon PV. Compartment syndrome of the thigh as a complication of anticoagulant therapy in a patient with a left ventricular assist device (Berlin Heart). Gen Thorac Cardiovasc Surg. 2010;58:477–9.
Acknowledgements
We would like to thank Editage (www.editage.jp) the for English language editing.
Funding
This case report was supported only by the institutional sources.
Availability of data and materials
The datasets generated or analyzed during this study are available from the corresponding author (KS) on reasonable request.
Author information
Authors and Affiliations
Contributions
KS was the primary anesthetist and drafted the manuscript. HT supervised anesthetic management. NA, AS, YE, and MY helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Authors’ information
KS is a M.D. and staff anesthesiologists of the Anesthesiology and Perioperative Medicine, Tohoku University School of Medicine. HT is a M.D., Ph.D., and lecturer of the Department of Anesthesiology, Tohoku University Hospital. NA is a M.D. and staff anesthesiologists of the Department of Anesthesiology, Tohoku University Hospital. AS is a M.D. and clinical fellow of the Department of Anesthesiology, Tohoku University Hospital. YE is a M.D., Ph.D., and Professor of Surgical Center and Supply, Sterilization, Tohoku University Hospital. MY is a M.D., Ph.D., and Professor of Anesthesiology and Perioperative Medicine, Tohoku University School of Medicine.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Saito, K., Toyama, H., Abe, N. et al. Implantation of ventricular assist device for systemic right ventricular failure in a patient with transposition of the great arteries and post-Mustard procedure: a case report. JA Clin Rep 4, 55 (2018). https://doi.org/10.1186/s40981-018-0194-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40981-018-0194-x