Abstract
Atrial fibrillation (AF) is more common in patients with malignancies than in general population. The pathophysiological processes include the pro-inflammatory condition and the exaggerated inflammatory reaction to chemotherapy, radiotherapy, and surgery interventions. Thus, it is pivotal to decrease morbidity and mortality in this group by providing appropriate care and prevention. In this subset, the risk of thromboembolic and bleeding events is high and the common risk score such as CHA2DS2-VASc and HAS-BLED employed in non-oncologic patients have limited evidence in cancer patients. A paucity of evidence in the setting in individuals having both malignancies and atrial fibrillation entangle the clinician when it comes to therapeutic management. Tailored management is recommended of anticoagulation treatment could be difficult, and there is. In this review, we try to explain the mechanism of AF in cancer patients as well as its management in this setting.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is one of the most common supraventricular arrhythmias that affects more than 33 million people in the world, with a prevalence of 2–4%, expected to increase within the next decade [1, 2]. In specific population such as oncologic patients this prevalence increase, with a reported increment rate of 20% regardless of the kind of malignancy [3,4,5,6]. Particularly AF and cancer are inextricably linked as supported by consistent literature that underlines the bidirectional nature of this relationship [7, 8] A four fouled age-adjusted increase in the likelihood of incident AF during the first year was seen after cancer diagnosis [9]. The chance of detecting new AF peaked in the first 3 months following a malignancy diagnosis, declining gradually after six months [10]. Hematological cancers, intrathoracic cancers (e.g., pulmonary and esophageal malignancy) and central nervous system cancers are also related to a greater than two-fold incidence of AF [11]. Frequency of AF in multiple myeloma seems to be higher in patients older than 35 and increased sharply with age. In patients over 50 aged, liver malignancy appears to be a strongly related to AF occurrence, whereas pulmonary cancer showed strong correlation in patients aged less than 50 [12]. Fauchier et al. found that in oncologic patients AF is a strong predictor of all-cause death [13]. Indeed, the occurrence of AF is linked to a poorer outcome, either in presence of previous history of AF and/or in case of new diagnosis within 3 years cancer detection. The latter condition could be related to the pro-arrhythmogenic effects of some cancer treatment [14]. Furthermore, Ostenfeld et al. showed that within the first three months of AF detection, there is a fivefold increased risk for malignancy [15]. Moreover, 3 months after a new diagnosis of AF, the risk of cancer tripled and resulted consistent after one year. Rahman et al. showed that women with AF had a considerably greater risk of malignancy compared with those without AF [16]. Individuals with non-valvular AF and a newly discovered malignancy were included in a study by Kim et al. [11]. In this study stomach (approximately 20%), colorectal segment (about 15%), and lung cancer (about 5%) were the most prevalent solid tumor observed. Management of patients in whom AF and cancer coexist is complicated by elevated both bleeding and thrombotic risk [17] requiring tailored therapeutic strategy and multidisciplinary discussion that involves the cardiologist, the oncologist, and the patients itself. Reasons for elevated increased hypercoagulative state are unclear but evidence suggest that cancer and AF shared increased level of circulating pro-coagulative factors (von Willebrand factor, vascular endothelial growth factor, tissue factor, microvesicles, and neutrophil extracellular traps) [18], whereas chemotherapy is a recognized independent risk factor responsible for a six-fold increment in the risk of embolic events [19]. On the other hand, the increased bleeding risk that occurs in patients with cancer recognize direct cause such as erosion and tumor invasion, and indirect treatment related factors such as tissue injury due to chemotherapy or radiation therapy. Recently, Pastori et al., in a retrospective study observed that malignancy enhanced the incidence of severe bleeding and all-cause death in people with AF [20]. Different subtypes of malignancy had different relationship with cardio-embolic stroke, being higher in pancreatic, uterine and breast cancer and lower in lung and liver cancer, leukemia, and myeloma. The risk of bleeding appeared to outweigh the hazard of thromboembolic events especially in hematologic malignancies, liver, and met-astatic cancer, where thrombocytopenia frequently occurs [20]. The aim of this review is to report latest evidence in term of pathophysiologic mechanism and management of patients with AF in the setting of cancer patients, underlining unmet issues on the topic and future direction.
Pathophysiology
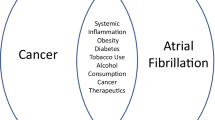
Numerous pathways have been hypothesized to determine mechanism of AF in cancer. Even though epidemiological data do not demonstrate causality, they underline the strong connection and shared risk factors between the two entities. Advanced age, smoke, metabolic syndrome, diabetes, chronic obstructive pulmonary disease (COPD), alcohol abuse, cirrhosis, arterial hypertension led to both atrial remodeling and pro-inflammatory state [21]. Tumors could cause AF either directly by invading the heart with primary tumors / metastatic extension, or indirectly by inducing fluid imbalance, hypoxia, electrolyte and metabolic imbalances, infection, anemia, autonomic nerve system dysfunction and paraneoplastic symptoms. Moreover, the relationship between shared metabolic risk factors and AF was inferred by the link between inflammatory biomarkers levels such as white blood cells, C-reactive protein (CRP), and ceruloplasmin and higher chance of developing preclinical and clinical AF. Inflammation also play a significant role in the pathophysiology of AF, which is a combination of structural, electrical, and functional atrial remodeling that involves the onset of AF [22]. In Fig. 1, the possible pathophysiological mechanisms that led to AF in cancer patients are reported briefly. Cancer treatments such as surgery intervention, radiotherapy (RT) and chemotherapy (CT) result in extremely high inflammatory state and promote the development of AF [23]. Perioperative AF (POAF) is highly prevalent in people with cancer. Patients with advanced cancer stages, cardiovascular (CV) comorbidities, older age, prolonged surgical time, and significant tissue excision appear to be more likely to experience POAF. Surgery-related AF risk could be explained by mechanical stimulation of the pericardium, inflammation damage, anesthetic drugs, and post-operative electrolyte imbalances. In a meta-analysis conducted by Inoue et al. the prevalence of POAF in patient with cancer is 13,5% (95% confidence interval (CI), 11.6–15.7%) [24]. Particularly, thoracic surgery for lung cancer is a significant risk factor for the onset of POAF. In this subgroup, the rate of POAF fluctuates from 9.9% to 14.9% [25, 26]. Additionally, abdominal (6–35%) and neck surgery are also linked to an elevated hazard of POAF [27, 28]. Different prophylactic treatments have been tested to reduce the incidence of POAF. A recent trial investigated the effects of prophylactic treatment with anti-inflammatory medication (i.e., colchicine) on the incidence of POAF in patients undergoing major non-cardiac thoracic surgery. The authors did not find any benefits from the administration of colchicine, while the risk of benign non-infectious diarrhea [29] resulted increased. Wang et al. provided a meta-analysis on pharmacological intervention to prevent POAF after lung surgery, showing that prophylactic beta-blocker reduced of 87% the risk of AF with no serious adverse events reported, but with no impact on 30-days mortality. Cardinale et al. proposed to screen patients undergoing thoracic surgery by dosing NT-pro-BNP values, since elevated pre and and/or post-surgery NT-pro-BNP appeared to be associated with increased risk of POAF compared thoracic surgery by dosing NT-pro-BNP values, since elevated pre and and/or post-surgery NT-pro-BNP appeared to be associated with increased risk of POAF compared with patients with normal values (64% versus 5%; P < 0.001) [30]. In those patients with high perioperative levels of NT-pro-BNP, the prophylactic treatment with metoprolol or losartan reduced the incidence of POAF compared to controls (6%, 12% and 40% respectively) [31]. In non-cancer patients, current ESC guidelines on management of patients with AF recommend prophylactic treatment with beta-blocker or amiodarone to prevent POAF in patients at higher risk of AF onset, considering potential adverse events and ambiguous effects on major adverse events [32, 33]. In patients with cancer, although with less evidence, these recommendations do not appear to differ, especially in the context of thoracic surgery [34].
RT contributes to fibrosis development of the atrial tissue and raises the risk of inflammatory responses in the endothelial compartment, including the coronaries, raising the risk of the onset of AF. For these reasons, people who receive left breast radiotherapy are more likely to develop AF [35]. The cumulative amount of radiation, the body surface area exposed, and the age of the subject at the time of radiation exposure are all significant risk factors correlated with myocardial damage. Additionally, in consecutive treatment protocol, RT and CT worked together synergistically for cardiotoxicity. An elevated rate of arrhythmias was seen in individuals receiving neo-adjuvant RT and CT versus those receiving only surgery in patients affected by locally advanced esophageal squamous cell carcinoma [36]. Table 1 reports anticancer medication that could potentially induce AF as referred in the pharmacovigilance database created by the World Health Organization [37]. A meta-analysis showed an incidence of AF events of 10.3% in those patients undergoing traditional treatment such as anthracyclines, while higher risk was found in those treated with targeted treatment like Bruton tyrosine kinase inhibitors (ibrutinib: 10–19%) or with alkylating agents (Melphalan: 22.5%). However, Font et al. considered only the incidence of symptomatic AF for the analysis, not considering undetected subclinical AF, often present in cancer patients [38], thus underestimating the actual burden of AF induced by chemotherapy [39]. CT-induced AF may appear acutely (within 24 h) following drug intake (gemcitabine or cisplatin) or it could occur in days, weeks, or even months after chemotherapy (ibrutinib) [23, 40]. Proteasome inhibitors like bortezomib or carfilzomib, prescribed in hematological cancers, showed a dose-dependent toxic effect that have been linked to increased CV reactivity, vascular impairment, and myocardial oxidative stress [41, 42]. Apart from its inhibition of cardiac BTK, ibrutinib also inhibits Tec protein tyrosine kinase (TEC) off-target. These targets have been demonstrated to be formed in cardiac cells with atrial cells expressing them more during AF then sinus rhythm [43]. Ibrutinib exhibited an atrial-specific toxicity in human cardiomyocytes through significant increase in left atrial fibrosis and impairment in atrial myocyte calcium channel regulation. Furthermore, these tyrosine kinases regulate the phosphoinositide 3-kinase–Akt pathway, an essential regulator of heart protection in stressful situations and could cause inflammatory and oxidative damage by increasing reactive oxygen species (ROS) levels [43,44,45]. AF has also been linked to other supportive therapy, which can trigger cardiac arrhythmias by different modalities. Nonsteroidal anti-inflammatory drugs (NSAIDs) and opiates, which are both linked to an elevated risk of developing AF, are frequently prescribed to cancer patients, particularly to those who are affected by end stage disease [46, 47]. However, pathophysiologic pathways specific to AF in cancer are still unclear.
Management
Treatment of reversible AF triggers in cancer such as electrolyte disruption (due to fever or sepsis), pain and hypoxemia, is recommended considering that reversion to sinus rhythm might happen as a result in some cases. Cardiac imaging assessment can help to detect additional potential features (acute ventricular dysfunction, pulmonary thromboembolism, pericardial effusion, cardiac tamponade, tumor invasion, and endocarditis) [48] and/or marked geometrical changes (left ventricular hypertrophy, atrial enlargements) that increase the chances to detect those cancer patients at higher risk of AF. Significant left atrial (LA) remodeling is common in AF and may implies advanced state of fibrosis. Thus, preventing atrial remodeling is essential through direct targeted and specialized management of the risk factors (obesity, sleep apnea, etc.) [49]. LA strain is highly sensitive to measuring increased LA stiffness and fibrosis. LA strain has also been shown to be a significant indicator of AF recurrence following cardioversion [50], and following ablation [51]. A recent investigation on pediatric patients revealed a substantial reduction in LA strain during anthracyclines treatment [52]. In a study conducted by Yaylali et al. breast cancer individuals treated with adriamycin, cyclophosphamide, paclitaxel atrial electromechanical delay correctly predicted AF onset [53]. Therefore, cardiac imaging may help to guide the management of the patients detecting those patients who are keener to AF onset/recurrence. However, further studies are required to understand the prognostic impact of those assessment on cardiovascular outcomes in patients with cancer. Once higher risk patients have been identified, lifestyle modifications are encouraged to reduce the CV risk and concomitantly reducing the risk of AF [33].
Rhythm- or rate-control
AF should be managed by a multidisciplinary team according to patient's symptoms, age, CVD, preferences, and the ongoing cancer therapy. Individual therapeutic strategy (rate, rhythm control, ablation) should consider possible interactions between anti-arrhythmic and cancer medications, absolute contraindications to anticoagulant therapy and hemodynamic instability requiring prompt electrical cardioversion [33]. A particular care must be used in patients at risk of POAF. In patients at low CV risk a cardiac rhythm strategy could be considered, while in patients with high CV risk or in those managed by pro-arrhythmic cancer medications, a rate control management is recommended since sinus rhythm persistence is difficult to achieve. Additionally, in elderly patients and/or those with CVD or enlarged left atrium, a rate control strategy appears reasonable, particularly for those with a poor prognosis for malignancy (including patients receiving palliative therapy). For the rate control strategy beta-blockers (e.g. metoprolol considering the fewer interactions) are generally recommended. Non-dihydropyridine calcium channel blockers may be used cautiously considering the higher risk of drug interactions by CYP 3A4 pathway with different CT drugs. Moreover, when digoxin use is indicated for rate control (HF or intolerance to beta-blockers), a careful risk benefit assessment is indicated considering the competitive mechanism on P-glycoprotein, resulting in higher digoxin levels and possible toxic effects [54]. Dronedarone moderately affects both these pathways and for this reason it is not recommended. Amiodarone is often used to manage cancer-related arrhythmias, especially in individuals with concomitant CV diseases. This drug could raise the levels of other CT drugs due to actions on CYP3A4 and P-glycoprotein. The interactions between anti-arrhythmic drugs and CT medications are reported in Table 2. In non-oncologic patients ablation should be evaluated in paroxysmal or persistent AF to improve outcome and symptoms, particularly when medical treatment failed to improve clinical condition [33]. Recent trials demonstrated benefits of AF catheter ablation also in patients with heart failure with reduced ejection fraction (HFrEF) [55] and in those with end-stage heart failure (i.e., patients evaluated for heart transplantation) [56]. The indication for ablation treatment is not clearly established in individuals with AF and concomitant malignancy. When rate or rhythm management results inefficacious or detrimental (due to interactions or intolerance), catheter ablation could represent an option, subordinate to prognosis assessment discussed by the multidisciplinary teams. There are very few studies that have assessed AF ablation in cancer patients. A retrospective analysis of 15 patients with persistent AF following pneumonectomy for cancer showed that the treatment was safe and successful, with 20% recurrence after one year follow-up [57]. However, a retrospective trial comparing catheter ablation for AF in patients with and without cancer revealed that obesity but not cancer was considered an increased risk factor for recurrence at 12 months, underlining that catheter ablation in oncologic population is as safe and effective as in general population [58]. Post cardioversion anticoagulation should be continued for at least 4 weeks or more, depending on bleeding/ embolic risk and risk of recurrence (see below) [4].
Anticoagulation
Anticoagulation in cancer patients should be managed according to a detailed and individualized strategy, considering thrombotic and bleeding risk, type of cancer, potential drug interactions and patient's choice. The assessment of the patient's therapeutic objectives and preferences, current health condition, and prognosis are pivotal to re-duce adverse events and suboptimal treatment adherence. In patients with pre-existing AF managed by anticoagulants, the antithrombotic strategy should be reviewed, either for surgical reason or CT drug interactions. Cancer-related hypercoagulability may lead AF patients to thrombus development with a related fivefold risk of thromboembolism [4, 59]. Pharmacological interactions need to be examined when anticoagulation is used in patients with active cancer, through individualized considerations of the benefits and risks. While validated score for prediction of venous thromboembolic events and thromboprophylaxis in cancer patients has been tested [60,61,62,63,64], risk assessment for both thrombotic and bleeding events is slippery since common risk scores (CHADS2, CHA2DS2-VASc, HAS-BLED) do not include malignancy as a risk factor for individuals with AF. CHA2DS2-VASc score was able to predict prognosis in patients with cancer, when AF was discovered 3 years after the diagnosis, but was not accurate to predict thromboembolic events [65]. In a retrospective observational study from Spain CHA2DS2-VASc and HAS-BLED were tested on 16,056 patients with AF (7.1% of whom had previous history of cancer) as predictors of embolic and bleeding events during a period of about 5 years. The authors found that traditional scores failed to predict both embolic and bleeding events in cancer patients (Hazard Ratio—HR 1.14, 95% CI 0.98 to 1.32; p = 0.076 for CHA2DS2-VASc and HR 1.08, 95% CI 0.99 to 1.17; p = 0.070 for HAS-BLED). However, they found that in cancer patients with AF the identification of patients at low embolic risk was possible when CHA2DS2-VASc score was equal to zero, highlighting its accuracy in identifying those patients in whom anticoagulation should be avoided [65].
Therefore, the lack of evidence supporting the use of conventional risk score and a well-validated risk score specifically for patients with cancer led to an under prescription of anticoagulation treatment due to concerns regarding fatal adverse events in such a fragile population. Previous evidence found that 1/3 of individuals with cancer and AF received nontherapeutic dosages of low-molecular-weight heparins (LMWHs) whereas 1/4 of individuals did not receive any anticoagulant therapy [66]. This founding could be partially addressed by the high bleeding risk (HAS-BLED > 3) that results in in almost half (44.3%) of individuals with the standard anticoagulant indications (CHADS2-VASc > 2) and cancer as underlined by Fradley et al. [54].
Bleeding risk increases further if patients need to undergo surgical or interventional procedures or need to combine antiplatelet therapy [7], in the setting of acute coronary syndromes [67, 68]. The ABC stroke risk score [69] (age, biomarkers, and clinical history) and the HEMORR2HAGES score [70] (hepatic or renal dysfunction, alcohol, cancer, age > 75 years, platelet count, multiple bleeding events, hypertension, anemia, genetic factors, fall risk, and stroke) are tools that might be used to identify individual risk of bleeding in complex circumstances. Among the reasons of anticoagulation treatment withheld cerebral metastases, CKD, prior and current bleeding history, CT, thrombocytopenia (< 25.000 platelets, mainly in hematological malignancies) and/or simultaneous use of NSAIDs were the most frequent ones.
In patients with valvular AF or CKD, the vitamin K antagonists (VKAs) may represent the first choice [71] even though CT medications could impact coagulation profile and liver function, reducing or increasing the plasma concentration of the anticoagulant, hindering the possibility to obtain optimal therapeutic range (time of on-target INR > 65%). Fluctuation in INR values represent a challenge in the daily practice particularly in patients with high bleeding risk due to the tumor's localization (central nervous system, urinary system, upper or lower gastrointestinal tract).
Moreover, other common conditions as poor nutrition, vomiting, liver dysfunction, thrombocytopenia, and the necessity for surgery, decreases the probability of an optimal therapeutic range increasing the risk of VKAs related adverse events compared to non-oncologic patients [72]. LMWH are preferable to VKAs during active cancer treatment with better profile in terms of drug interactions and stable anticoagulation. Prolonged administration of LMWH is considered safe and efficacious, however the reduced quality of life and difficulties related to the long term subcutaneous treatment may affect its prescription, considering that many CT regimens are administered for several months. Additionally, their efficacy has never been established and the dose for the stroke prevention during AF has not been determined in the context of cancer patients, with ambiguous results in terms of prevention of embolic events and death when LMWH were compared to Direct oral anticoagulants (DOACs) [73]. Almost all the evidence about the efficacy and safety of LMWH in cancer patients refer to the setting of treatment or prophylaxis of VTE. Individuals at higher risk of bleeding, such as those with active gastrointestinal or genitourinary malignancy, gastrointestinal mucosal abnormalities, low platelet counts (between 25,000 and 50,000 × 109/mcL), gastrointestinal toxicity, or severe renal dysfunction, should be managed by LMWH at the recommended or therapeutic dosage [33, 74, 75]. Interactions of VKAs and LMWH with CT drug are summarized in Table 3.
DOACs represented the first choice of oral anticoagulation in AF patients [33]. In the daily practice, DOACs are usually preferred over VKAs in the occasion of sudden withheld (invasive surgery, major bleeding), considering the pharmacokinetic profile, quick onset and offset and presence of reversal medication, despite their prescription among cancer patients remains "off-label". In fact, the randomized clinical trials assessing the safety and efficacy of DOACs compared to VKAs in AF patients were not designed to evaluate safety and efficacy in cancer population and are at least effective and safe as LMWH.
However, subgroup analyses including cancer population are available, since the diagnosis of cancer was not considered as an exclusion criteria per se and in some cases occurred during the follow up phase of the studies.
This sub analysis revealed that DOACs in patients with cancer are safer and, at least, as efficacious to VKA, with a better safety profile [76]. The number of patients with cancer in these trials and their cancer-related exclusion criteria are reported in Table 4.
In the Rivaroxaban Once-daily, Oral, Direct Factor Xa Inhibition Compared with Vita-min K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF trial) [77], history of cancer was not explicitly reported among the exclusion criteria. However, concurrent disease with an estimated lifespan of less than 2 years, diseases that increase the risk of bleeding, and a number of platelets less than 90 000/mL at the screening visit were in the exclusion criteria and for this reason many cancer patients were not included. A secondary analysis [78] conducted in those patients with a history of cancer (4,5% of the participants showed “actively treated cancer”) found that there is not significant differences in the risk of ischemic stroke/ thromboembolism (IS/TE) (HR 0.86, 95% CI 0.55–1.33; P = 0.50) but greater risk of any bleeding (HR 1.30, 95% CI 1.16–1.47; P < 0.0001) compared to individual with no history of cancer. However, no significant differences were found in terms of efficacy (rate of IS/TE, HR 0.52, 95% CI 0.22– 1.21; P = 0.21) or safety outcomes (major or non-significant bleeding, HR 1.09, 95% CI 0.82–1.44; P = 0,79) between rivaroxaban and warfarin anticoagulation [78]. The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial (ARISTOTLE trial) conditions with increased bleeding risk, planned major surgery, severe comorbidities with life expectancy ≤ 1 year, platelet less than 100,000/mm3 and hemoglobin level less than 9 g/dL were considered exclusion criteria like limiting the recruitment of patients with cancer [79]. Sub analysis was also available in this trial since 6,8% of the recruited patients had history of cancer either active cancer (12.7%) or previous history of cancer treatment (87.3%). A secondary analysis on these patients [80] revealed no differences in IS/TE (HR 0.93, 95% CI 0.63–1.37; P = 0,7104) and any bleeding (HR, 1.10; 95% CI, 0.99–1.22; P = 0,0718) between the individuals with background/active cancer compared to those without. Individuals with remote cancer experienced comparable incidence of ischemic events as those with active disease. However, mortality from any cause seemed to be more common in the active cancer cohort. Both cancer and non-cancer patients experienced apixaban's greater efficacy compared to warfarin in preventing IS/SE (HR 1,09; 95% CI, 0,53–2,26; P = 0,3671). In the Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation Thrombolysis in Myocardial Infarction 48 trial (EN-GAGE AF-TIMI 48 trial) [81] patients with active cancer (diagnosed within 5 years) or under anticancer therapy were excluded from the recruitment at the time of randomization. However, 5,5% of these patients have developed cancer post-randomization and a sub-analysis was conducted including these patients [82]. According to the secondary analysis of the ENGAGE AF-TIMI 48 trial [82], there was no difference in the incidences of IS/SE among individuals who had and did not have cancer (HR, 1.08; 95% CI, 0.83–1.42; P = 0.55). Furthermore, edoxaban is more efficient in IS/SE prevention in the higher dose arm (edoxaban 60 mg) compared to warfarin (HR, 0.60; 95% CI, 0.31– 1.15 vs HR, 0.89; 95% CI, 0.76–1.05; P = 0,25) and with similar efficacy of warfarin in the lower dose arm (edoxaban 30 mg) (HR, 0.87; 95% CI, 0.47–1.59 vs HR, 1.15; 95% CI, 0.99–1.34; P = 0.38), as well as having a similar safety profile. In Randomized Evaluation of Long-Term Anticoagulation Therapy trial (RELY trial) [83], likewise, patients with recent diagnosis of cancer (< 6 months) and life expectation < 3 likewise, patients with recent diagnosis of cancer (< 6 months) and life expectation < 3 years were not included and secondary analyses was not performed. However, in the Anticoagulants for Reduction in Stroke: Observational Pooled Analysis on Health Out-comes and Experience of Patients trial (ARISTOPHANES trial) 9% of the patients had active cancer and 24% of these patients use dabigatran. Individuals on dabigatran and those taking warfarin had comparable rates of IS/SE (HR 0.88; 95% CI 0.54- 1.41; P < 0,001) and major bleeding (HR 0.76; 95% CI: 0.57–1.01; P = 0,058) [84]. Furthermore, dabigatran had a similar IS/SE risk compared to apixaban and lower major bleeding compared to rivaroxaban. Moreover, in this sub-analysis apixaban was related to a decreased incidence of IS/SE and major bleeding among individuals with AF and active malignancy, while rivaroxaban was linked to comparable risks in warfarin users. Additionally, in comparison with dabigatran, patients managed by apixaban showed a reduced risk of IS/SE compared to rivaroxaban users [79]. Shah et al. also demonstrated that in patients with AF and cancer, the rate of IS is similar in patients in therapy with dabigatran compared with patients in therapy with warfarin (HR 0.89; 95% CI 0.56–1.42; P = 0,63) [85]. Furthermore, they observed that the risk of serious bleeding was analogue with dabigatran (HR 0.96, 95% CI 0.72–1.27; P = 0,75) and rivaroxaban (HR 1.09, 95% CI 0.79–1.39; P = 0,59) compared to warfarin, but significantly lower with apixaban (HR 0.37, 95% CI 0.17–0.79; P = 0,01) in patients with AF and active cancer [85]. In a meta-analysis conducted by Yang and colleagues DOACs reduce the risk of IS/SE, VTE, serious bleeding, and all-cause mortality in individuals with AF and cancer and the lowest risk of IS/SE was found in apixaban users [74]. These results were confirmed by Mariani and colleagues' recent meta-analysis, revealing that DOACs were correlated with lower incidence of any stroke (RR 0.84; 95% CI 0.74–0.95; P = 0.007) or thromboembolism (RR 0.65; 95% CI 0.52–0.81; P = 0.001), lower incidence of major bleeding (RR 0.68; 95% CI 0.50–0.92; P = 0.01), resulting to be non-inferior compared to VKAs [86] in patients with cancer and AF. A meta-analysis by Papanastasiou and colleague, found that in patients with AF receiving DOAC, the bleeding occurrence during the treatment increased the chance of cancer detection by 6 times (OR 6.12, 95% CI 4.47–8.37; P < 0,01) [87]. The P-glycoprotein (P-gp) pathway (primarily for apixaban and rivaroxaban) and the cytochrome P450 pathway (through CYP3A4) in the liver have an impact on the effects of all DOACs [70]. P-gp plays a crucial role in DOACs pharmacokinetic reducing their intestinal absorption and accelerating their liver and renal excretion. So, all DOACs must be used with caution in patients treated with drugs that strongly induce or inhibit CYP3A4 and P-gp, while the interactions by P-gp alone should only affect the patients in apixaban and rivaroxaban treatment. The interaction of DOACs with CT drugs are reported in Table 3. Less convincing data supports the security and effectiveness of DOACs as a preventative measure against stroke and thromboembolism in individuals with AF and specific active malignancy. Administration of DOACs in individuals with luminal gastrointestinal malignancies or individuals with active gastrointestinal mucosal anomalies such as ulcers, gastritis, esophagitis, or colitis, is discouraged based on significant bleeding evidence [88]. Concerning kidney function, all DOAC should be contraindicated in individuals with an estimated glomerular filtration rate (eGFR) < 15 ml/min/1.73 m2, except for Dabigatran that is contraindicated with eGFR < 30 ml/min/1.73 m2. Finally, it is essential that the initial anticoagulant prescription is routinely assessed and modified according to malignancy stages, possible alterations to the CT regimen, or variations in patients bleeding/thrombotic risk balance. The choice of the anticoagulant and its dosage is summarized in Fig. 2.
Management of atrial fibrillation in cancer patients. AF atrial fibrillation, CHA2DS2VASc score (congestive heart failure/LV dysfunction, hypertension, age > 75, diabetes mellitus, stroke/TIA, vascular disease, age 65–74, sex), CKD chronic kidney disease, DOAC direct oral anticoagulant, GI gastrointestinal, GU genitourinary, HAS-BLED score (hypertension, abnormal liver or renal function, stroke, bleeding, la-bile international normalized ratio, INR, elderly with age > 65, drugs or alcohol); HF heart failure, INR international normalized ratio, LMWH, low molecular-weight heparin, LV left ventricular, LVEF left ventricular ejection fraction, PLT platelets, TEE transesophageal echocardiography, TIA transient ischemic attack, TTE, transthoracic echocardiography, VKAs vitamin K antagonists
A web-based survey conducted on 960 physicians (82.4% cardiologist, 75.5.5 from Europe) by the Council of Cardio-Oncology of the European Society of Cardiology revealed that in 62.6% of cases DOACs were preferred over LMWH and warfarin, in all type of cancer but non-operable gastrointestinal cancer. Traditional risk score for thrombotic and bleeding risk were considered appropriate, despite concerns about the lack of validated literature for cancer patients [61].
Percutaneous left atrial appendage (LAA) closure
According to ESC guidelines [33], individuals with a high embolic hazard and conditions that exclude long-term anticoagulation (i.e., high bleeding risk) may consider percutaneous left atrial appendage closure as a secure and efficacious alternative to anticoagulant therapy. Analogously, in cancer patients who have absolute contraindication to anticoagulation, and in those who have an average lifespan of more than a year, LAA closure should be considered as a treatment option, even though there is lack of strong data to support this approach. In the context of malignancies high bleeding risk features such as intracranial metastases, thrombocytopenia (platelets count below 50,000), liver or renal dysfunction, elevated INR chemotherapy interaction (CYP system and P-glycoprotein cell transport enzyme) and active GI bleeding, may guide management decision, where LAA closure device offers efficacious and safe management solution.
based on anecdotal experience reported in literature on LAA closure in patients with cancer. In those patients in who percutaneous LAA closure is recommended focused imaging for LAA assessment is required, addressing LAA morphology and presence of thrombus apposition [89]. Transesophageal echocardiography (TEE) is estimated to be the gold standard technique for the evaluation of LAA's flow patterns related to cardioembolic cerebrovascular accidents (i.e. LAA mean flow velocities below 20 cm/Sec in atrial fibrillation and LAA, spontaneous echo-contrast), morphology and identification of LAA thrombi, with a sensitivity of 93–100% and specificity of 99–100% and minimal complications in patients with AF [90]. While 2D TEE gives higher resolution pictures for its superior frame rate, 3D TEE overcomes some disadvantages of 2D TEE (i.e. limited scanning planes, mental reconstruction), enabling navigation within cardiac chambers that allows meticulous stereotactic examination of the LAA, especially in complex anatomy [91]. Even though TEE is a generally safe procedure in experienced hand, it is not always available, and it came with absolute (i.e. esophagogastrectomy, recent upper GI surgery, active GI bleeding) and relative contraindication (i.e. history of GI surgery, esophagitis, coagulopathy) that need to be considered in the oncologic setting [92]. In presence of contraindication, inadequate imaging or in doubt cases, additional cardiac imaging examination such as cardiac computed tomography (CCT) or cardiovascular magnetic resonance (CMR) can be required. CCT has been demonstrated to be a reliable alternative to TEE offering a comprehensive morphological evaluation of the LAA in pre-operative evaluation for occlusive device implant [93], with a sensitivity of 96% and specificity of 92% for LAA thrombus identification [94]. However, CT has some disadvantages to take into consideration in cancer patients including the use of nephrotoxic contrast agent and radiation exposure. Data about CMR's capacity to identify LAA thrombi are encouraging since it enables non-invasive tissue characterization distinguishing between old (lower signal intensity) and fresh (higher signal intensity) thrombus, showing high concordance in thrombi identification compared to TEE [95]. Negative aspects about widespread use of CMR in this setting include higher costs, extended study duration, potential hazards associated with gadolinium-based contrast agent and the presence of devices incompatible with CMR.
In their recent research, Shabtaie et al. observed that LAA closure in individuals with malignancy was accomplished with good technical efficacy and provided decrease in stroke incidence or death without a higher hazard of bleeding compared to non-cancer individuals [96]. Data are confirmed in another recent study conducted in a tertiary center with follow up at 3 years following the LAA closure, showing no difference in all-cause death (HR 1.3, 95% CI 0.72–2.35; P = 0.38), serious bleeding events (HR 1.2, 95% CI 0.45–3.33; P = 0.68) or stroke (HR 0.64, 95% CI 0.19–2.21; P = 0.49) in patients with cancer compared to patients without cancer [97]. In a retrospective analysis conducted by Isogai and colleagues on more than 15,000 patients with AF who underwent LAA closure, divided by cancer history (2.4% with active cancer, 15.1% with prior history of cancer), no significant difference was found for the composite outcome (in-hospital mortality, ischemic stroke/transient ischemic attack, systemic embolism, bleeding necessitating blood transfusion, pericardial effusion/cardiac tamponade or embolized device extraction) in patients with active cancer (aOR = 0.99; 95% CI = 0.51–1.93, p = 0.973) or prior cancer (aOR = 0.94, 95% CI = 0.70 to 1.28, p = 0.704). On the other hand, active cancer was substantially linked to an increased chance of in-hospital ischemic stroke/TIA (aOR = 3.06, 95% CI = 1.17 to 8.01, p = 0.023). This observation might be explained by a possible hypercoagulability associated with active malignancy [98]. Since no data on the impact on thrombogenic state during malignancies on device related thrombosis, after LAA closure antiplatelet or short-term anticoagulation treatment (if tolerated) must be tailored on patients bleeding and thrombotic risk [99].
Conclusions
Patients with cancer are keener to experience AF, because of shared risk factors (inflammatory state) and detrimental effects of some cancer treatments (surgery, CT, RT). Rate/rhythm control strategy have to be tailored based on the clinical context (patient's symptoms, age, CVD, and possible interactions with the ongoing cancer therapy). Risk assessment for thromboembolic and bleeding events is puzzling since malignancy is not taken into consideration as predisposing risk factors in the shared risk stratification scores like CHA2DS2VASc and HASBLED, currently applied in cancer population. For a more accurate assessment of the thrombotic and bleeding hazard, an individual stratification tool is required, and further studies are required for clinical validation. The choice of anticoagulant treatment shows additional difficulties because of the absence of dedicated literature. Recently randomize trial on efficacy and safety of switching frail patients with AF from VKA to DOACs fail to reduce thromboembolic events at the expense of higher risk of bleeding events in those who were switch to DOAC treatment [100] puzzling furtherly the management of anticoagulation treatment in frail populations.
Drug interactions and specific clinical conditions like thrombocytopenia, renal and liver dysfunction, are features that frequently come along with malignancies and need to be addressed when anticoagulation is required. In individuals with a high embolic hazard and conditions that exclude long-term anticoagulation consider percutaneous left atrial appendage closure as a secure and efficacious alternative. In conclusion, AF results in a rise in their comorbidity and mortality, mining the potential benefit on the outcome of recent anticancer treatment, making the management of this condition a pivotal aspect towards which high efforts in research should be spent to determine evidence-based recommendation and to enhance tailored management.
Availability of data and materials
Not applicable. No new data were generated or analyzed in support of this research.
References
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American heart association council on epidemiology and prevention statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics-2019 update: a report from the american heart association. Circulation. 2019 Mar 5;139(10):e56-e528.https://doi.org/10.1161/CIR.0000000000000659. Erratum in: Circulation. 2020 Jan 14;141(2):e33. PMID: 30700139.
Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 Study. Circulation. 2014 Feb 25;129(8):837–47.https://doi.org/10.1161/CIRCULATIONAHA.113.005119. Epub 2013 Dec 17. PMID: 24345399; PMCID: PMC4151302.
Santoro C, Capone V, Canonico ME, Gargiulo G, Esposito R, Sanna GD, Parodi G, Esposito G. Single, dual, and triple antithrombotic therapy in cancer patients with coronary artery disease: searching for evidence and personalized approaches. Semin Thromb Hemost. 2021;47(8):950–61. https://doi.org/10.1055/s-0041-1726298. Epub 2021 Jul 14 PMID: 34261150.
Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, Cutter DJ, de Azambuja E, de Boer RA, Dent SF, Farmakis D, Gevaert SA, Gorog DA, Herrmann J, Lenihan D, Moslehi J, Moura B, Salinger SS, Stephens R, Suter TM, Szmit S, Tamargo J, Thavendiranathan P, Tocchetti CG, van der Meer P, van der Pal HJH; ESC Scientific Document Group. 2022 ESC guidelines on cardio-oncology developed in collaboration with the european hematology association (EHA), the european society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS). Eur Heart J. 2022 Nov 1;43(41):4229–4361.https://doi.org/10.1093/eurheartj/ehac244. Erratum in: Eur Heart J. 2023 May 7;44(18):1621. PMID: 36017568.
O’Neal WT, Lakoski SG, Qureshi W, Judd SE, Howard G, Howard VJ, Cushman M, Soliman EZ. Relation between cancer and atrial fibrillation (from the REasons for Geographic And Racial Differences in Stroke Study). Am J Cardiol. 2015;115(8):1090–4. https://doi.org/10.1016/j.amjcard.2015.01.540. Epub 2015 Jan 31. PMID: 25711434; PMCID: PMC4380860.
Hu YF, Liu CJ, Chang PM, Tsao HM, Lin YJ, Chang SL, Lo LW, Tuan TC, Li CH, Chao TF, Chung FP, Liao JN, Chen TJ, Chen SA. Incident thromboembolism and heart failure associated with new-onset atrial fibrillation in cancer patients. Int J Cardiol. 2013;165(2):355–7. https://doi.org/10.1016/j.ijcard.2012.08.036. (Epub 2012 Sep 16 PMID: 22989607).
Guzzetti S, Costantino G, Vernocchi A, Sada S, Fundarò C. First diagnosis of colorectal or breast cancer and prevalence of atrial fibrillation. Intern Emerg Med. 2008;3(3):227–31. https://doi.org/10.1007/s11739-008-0124-4. (Epub 2008 Mar 5 PMID: 18320149).
Conen D, Wong JA, Sandhu RK, Cook NR, Lee IM, Buring JE, Albert CM. Risk of malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiol. 2016;1(4):389–96. https://doi.org/10.1001/jamacardio.2016.0280. PMID:27438314;PMCID:PMC4957657.
Han H, Chen L, Lin Z, Wei X, Guo W, Yu Y, Wu C, Cao Y, He J. Prevalence, trends, and outcomes of atrial fibrillation in hospitalized patients with metastatic cancer: findings from a national sample. Cancer Med. 2021 Aug;10(16):5661–5670. https://doi.org/10.1002/cam4.4105. Epub 2021 Jul 7. Erratum in: Cancer Med. 2021 Oct;10(20):7441. PMID: 34235874; PMCID: PMC8366074.
Jakobsen CB, Lamberts M, Carlson N, Lock-Hansen M, Torp-Pedersen C, Gislason GH, Schou M. Incidence of atrial fibrillation in different major cancer subtypes: a nationwide population-based 12 year follow up study. BMC Cancer. 2019;19(1):1105. https://doi.org/10.1186/s12885-019-6314-9. PMID:31726997;PMCID:PMC6854796.
Kim K, Lee YJ, Kim TH, Uhm JS, Pak HN, Lee MH, Joung B. Effect of non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients with newly diagnosed cancer. Korean Circ J. 2018 May;48(5):406–417. https://doi.org/10.4070/kcj.2017.0328. Epub 2018 Feb 26. PMID: 29671285; PMCID: PMC5940645.
Yun JP, Choi EK, Han KD, Park SH, Jung JH, Park SH, Ahn HJ, Lim JH, Lee SR, Oh S. Risk of atrial fibrillation according to cancer type: a nationwide population-based study. JACC CardioOncol. 2021;3(2):221–32. https://doi.org/10.1016/j.jaccao.2021.03.006. PMID:34396327;PMCID:PMC8352078.
Fauchier L, Samson A, Chaize G, Gaudin AF, Vainchtock A, Bailly C, Cotté FE. Cause of death in patients with atrial fibrillation admitted to French hospitals in 2012: a nationwide database study. Open Heart. 2015;2(1) PMID:26688739;PMCID:PMC4680587. https://doi.org/10.1136/openhrt-2015-000290
Hussain M, Misbah R, Donnellan E, Alkharabsheh S, Hou Y, Cheng F, Crookshanks M, Watson CJ, Toth AJ, Houghtaling P, Moudgil R, Budd GT, Tang WHW, Kwon DH, Jaber W, Griffin B, Kanj M, Collier P. Impact of timing of atrial fibrillation, CHA2DS2-VASc score and cancer therapeutics on mortality in oncology patients. Open Heart. 2020;7(2) PMID:33243931;PMCID:PMC7692982. https://doi.org/10.1136/openhrt-2020-001412
Ostenfeld EB, Erichsen R, Pedersen L, Farkas DK, Weiss NS, Sørensen HT. Atrial fibrillation as a marker of occult cancer. PLoS ONE. 2014;9(8) PMID:25119880;PMCID:PMC4138009. https://doi.org/10.1371/journal.pone.010286
Rahman F, Ko D, Benjamin EJ. Association of atrial fibrillation and cancer. JAMA Cardiol. 2016;1(4):384–6. PMID:27438312;PMCID:PMC4957662https://doi.org/10.1001/jamacardio.2016.0582.
Canonico ME, Santoro C, Avvedimento M, Giugliano G, Mandoli GE, Prastaro M, Franzone A, Piccolo R, Ilardi F, Cameli M, Esposito G. Venous thromboembolism and cancer: a comprehensive review from pathophysiology to novel treatment. Biomolecules. 2022;12(2):259. https://doi.org/10.3390/biom12020259.PMID:35204760;PMCID:PMC8961522.
Chu G, Versteeg HH, Verschoor AJ, Trines SA, Hemels MEW, Ay C, Huisman MV, Klok FA. Atrial fibrillation and cancer - an unexplored field in cardiovascular oncology. Blood Rev. 2019;35:59–67. https://doi.org/10.1016/j.blre.2019.03.005. (Epub 2019 Mar 25 PMID: 30928168).
Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160(6):809–15. https://doi.org/10.1001/archinte.160.6.809. (PMID: 10737280).
Pastori D, Marang A, Bisson A, Menichelli D, Herbert J, Lip GYH, Fauchier L. Thromboembolism, mortality, and bleeding in 2,435,541 atrial fibrillation patients with and without cancer: a nationwide cohort study. Cancer. 2021;127(12):2122–9. https://doi.org/10.1002/cncr.33470. (Epub 2021 Feb 25 PMID: 33631041).
Leiva O, AbdelHameid D, Connors JM, Cannon CP, Bhatt DL. Common pathophysiology in cancer, atrial fibrillation, atherosclerosis, and thrombosis: jacc: cardiooncology state-of-the-art review. JACC CardioOncol. 2021;3(5):619–34. https://doi.org/10.1016/j.jaccao.2021.08.011. PMID:34988471;PMCID:PMC8702799.
Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12(4):230–43. https://doi.org/10.1038/nrcardio.2015.2. (Epub 2015 Jan 27 PMID: 25622848).
Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. 2014;63(10):945–53. https://doi.org/10.1016/j.jacc.2013.11.026. (Epub 2013 Dec 18 PMID: 24361314).
Inoue K, Tajiri K, Xu D, Murakoshi N, Ieda M. Risk factors and in-hospital outcomes of perioperative atrial fibrillation for patients with cancer: a meta-analysis. Ann Surg Oncol. 2023;30(2):711–21. https://doi.org/10.1245/s10434-022-12690-y. (Epub 2022 Oct 22 PMID: 36273057).
Imperatori A, Mariscalco G, Riganti G, Rotolo N, Conti V, Dominioni L. Atrial fibrillation after pulmonary lobectomy for lung cancer affects long-term survival in a prospective single-center study. J Cardiothorac Surg. 2012;10(7):4. https://doi.org/10.1186/1749-8090-7-4. PMID:22233837;PMCID:PMC3287133.
Nojiri T, Maeda H, Takeuchi Y, Funakoshi Y, Maekura R, Yamamoto K, Okumura M. Predictive value of preoperative tissue Doppler echocardiographic analysis for postoperative atrial fibrillation after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg. 2010;140(4):764–8. https://doi.org/10.1016/j.jtcvs.2009.11.073. (Epub 2010 Aug 9 PMID: 20691999).
Bhave PD, Goldman LE, Vittinghoff E, Maselli J, Auerbach A. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major noncardiac surgery. Am Heart J. 2012;164(6):918–24. https://doi.org/10.1016/j.ahj.2012.09.004. Epub 2012 Oct 26. PMID: 23194493; PMCID: PMC4096141.
Ryödi E, Metso S, Huhtala H, Välimäki M, Auvinen A, Jaatinen P. cardiovascular morbidity and mortality after treatment of hyperthyroidism with either radioactive iodine or thyroidectomy. thyroid. 2018 Sep;28(9):1111–1120.https://doi.org/10.1089/thy.2017.0461. Epub 2018 Jul 23. Erratum in: Thyroid. 2018 Dec;28(12):1731. Essi, Ryödi [corrected to Ryödi, Essi]; Saara, Metso [corrected to Metso, Saara]; Heini, Huhtala [corrected to Huhtala, Heini]; Matti, Välimäki [corrected to Välimäki, Matti]; Anssi, Auvinen [corrected to Auvinen, Anssi]; Pia, Jaatinen [corrected to Jaatin. PMID: 29882483.
Conen D, Ke Wang M, Popova E, Chan MTV, Landoni G, Cata JP, Reimer C, McLean SR, Srinathan SK, Reyes JCT, Grande AM, Tallada AG, Sessler DI, Fleischmann E, Kabon B, Voltolini L, Cruz P, Maziak DE, Gutiérrez-Soriano L, McIntyre WF, Tandon V, Martínez-Téllez E, Guerra-Londono JJ, DuMerton D, Wong RHL, McGuire AL, Kidane B, Roux DP, Shargall Y, Wells JR, Ofori SN, Vincent J, Xu L, Li Z, Eikelboom JW, Jolly SS, Healey JS, Devereaux PJ; COP-AF Investigators. Effect of colchicine on perioperative atrial fibrillation and myocardial injury after non-cardiac surgery in patients undergoing major thoracic surgery (COP-AF): an international randomised trial. Lancet. 2023 Aug 25:S0140–6736(23)01689–6. https://doi.org/10.1016/S0140-6736(23)01689-6. Epub ahead of print. PMID: 37640035.
Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, Salvatici M, Veronesi G, Veglia F, Fiorentini C, Spaggiari L, Cipolla CM. Increased perioperative N-terminal pro-B-type natriuretic peptide levels predict atrial fibrillation after thoracic surgery for lung cancer. Circulation. 2007;115(11):1339–44. https://doi.org/10.1161/CIRCULATIONAHA.106.647008. (Epub 2007 Mar 5 PMID: 17339553).
Cardinale D, Sandri MT, Colombo A, Salvatici M, Tedeschi I, Bacchiani G, Beggiato M, Meroni CA, Civelli M, Lamantia G, Colombo N, Veglia F, Casiraghi M, Spaggiari L, Venturino M, Cipolla CM. Prevention of atrial fibrillation in high-risk patients undergoing lung cancer surgery: the presage trial. Ann Surg. 2016;264(2):244–51. https://doi.org/10.1097/SLA.0000000000001626. (PMID: 26764872).
O'Neal JB, Billings FT 4th, Liu X, Shotwell MS, Liang Y, Shah AS, Ehrenfeld JM, Wanderer JP, Shaw AD. Effect of preoperative beta-blocker use on outcomes following cardiac surgery. Am J Cardiol. 2017 Oct 15;120(8):1293–1297. https://doi.org/10.1016/j.amjcard.2017.07.012. Epub 2017 Jul 24. PMID: 28826895; PMCID: PMC5675103.
Kotalczyk A, Lip GY, Calkins H. The 2020 ESC guidelines on the diagnosis and management of atrial fibrillation. Arrhythm Electrophysiol Rev. 2021;10(2):65–7. https://doi.org/10.15420/aer.2021.07. PMID:34401177;PMCID:PMC8335854.
Fabiani I, Colombo A, Bacchiani G, Cipolla CM, Cardinale DM. Incidence, management, prevention and outcome of post-operative atrial fibrillation in thoracic surgical oncology. J Clin Med. 2019;9(1):37. https://doi.org/10.3390/jcm9010037. PMID:31878032;PMCID:PMC7019802.
Mery B, Guichard JB, Guy JB, Vallard A, Barthelemy JC, Da Costa A, Magné N, Bertoletti L. Atrial fibrillation in cancer patients: hindsight, insight and foresight. Int J Cardiol. 2017;1(240):196–202. https://doi.org/10.1016/j.ijcard.2017.03.132. (Epub 2017 Apr 6 PMID: 28390744).
Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, Mao W, Xiang J, Han Y, Chen Z, Yang H, Wang J, Pang Q, Zheng X, Yang H, Li T, Lordick F, D'Journo XB, Cerfolio RJ, Korst RJ, Novoa NM, Swanson SJ, Brunelli A, Ismail M, Fernando HC, Zhang X, Li Q, Wang G, Chen B, Mao T, Kong M, Guo X, Lin T, Liu M, Fu J; AME Thoracic Surgery Collaborative Group. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol. 2018 Sep 20;36(27):2796–2803.https://doi.org/10.1200/JCO.2018.79.1483. Epub 2018 Aug 8. PMID: 30089078; PMCID: PMC6145832.
Keramida K, Filippatos G, Farmakis D. Cancer treatment and atrial fibrillation: use of pharmacovigilance databases to detect cardiotoxicity. Eur Heart J Cardiovasc Pharmacother. 2021;7(4):321–3. https://doi.org/10.1093/ehjcvp/pvaa059. (PMID: 32633808).
Strickberger SA, Ip J, Saksena S, Curry K, Bahnson TD, Ziegler PD. Relationship between atrial tachyarrhythmias and symptoms. Heart Rhythm. 2005;2(2):125–31. https://doi.org/10.1016/j.hrthm.2004.10.042. (PMID: 15851283).
Font J, Milliez P, Ouazar AB, Klok FA, Alexandre J. Atrial fibrillation, cancer and anticancer drugs. Arch Cardiovasc Dis. 2023;116(4):219–26. https://doi.org/10.1016/j.acvd.2023.02.005. (Epub 2023 Mar 11 PMID: 37002156).
Alexandre J, Moslehi JJ, Bersell KR, Funck-Brentano C, Roden DM, Salem JE. Anticancer drug-induced cardiac rhythm disorders: current knowledge and basic underlying mechanisms. Pharmacol Ther. 2018;189:89–103. https://doi.org/10.1016/j.pharmthera.2018.04.009. (Epub 2018 Apr 24 PMID: 29698683).
Gavazzoni M, Vizzardi E, Gorga E, Bonadei I, Rossi L, Belotti A, Rossi G, Ribolla R, Metra M, Raddino R. Mechanism of cardiovascular toxicity by proteasome inhibitors: new paradigm derived from clinical and pre-clinical evidence. Eur J Pharmacol. 2018;5(828):80–8. https://doi.org/10.1016/j.ejphar.2018.03.022. (Epub 2018 Mar 15 PMID: 29550338).
Waxman AJ, Clasen S, Hwang WT, Garfall A, Vogl DT, Carver J, O'Quinn R, Cohen AD, Stadtmauer EA, Ky B, Weiss BM. Carfilzomib-Associated Cardiovascular Adverse Events: A Systematic Review and Meta-analysis. JAMA Oncol. 2018 Mar 8;4(3):e174519.https://doi.org/10.1001/jamaoncol.2017.4519. Epub 2018 Mar 8. PMID: 29285538; PMCID: PMC5885859.
McMullen JR, Boey EJ, Ooi JY, Seymour JF, Keating MJ, Tam CS. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124(25):3829–30. https://doi.org/10.1182/blood-2014-10-604272. (PMID: 25498454).
Shafaattalab S, Lin E, Christidi E, Huang H, Nartiss Y, Garcia A, Lee J, Protze S, Keller G, Brunham L, Tibbits GF, Laksman Z. Ibrutinib displays atrial-specific toxicity in human stem cell-derived cardiomyocytes. Stem Cell Reports. 2019 May 14;12(5):996–1006. https://doi.org/10.1016/j.stemcr.2019.03.011. Epub 2019 Apr 25. PMID: 31031187; PMCID: PMC6524928.
Jiang L, Li L, Ruan Y, Zuo S, Wu X, Zhao Q, Xing Y, Zhao X, Xia S, Bai R, Du X, Liu N, Ma CS. Ibrutinib promotes atrial fibrillation by inducing structural remodeling and calcium dysregulation in the atrium. Heart Rhythm. 2019;16(9):1374–82. https://doi.org/10.1016/j.hrthm.2019.04.008. (Epub 2019 Apr 5 PMID: 30959203).
Chuang SY, Hsu PF, Lin FJ, Huang YW, Wang GZ, Chang WC, Tsai HJ. Association between nonsteroidal anti-inflammatory drugs and atrial fibrillation among a middle-aged population: a nationwide population-based cohort. Br J Clin Pharmacol. 2018 Jun;84(6):1290–1300.https://doi.org/10.1111/bcp.13558. Epub 2018 Apr 2. PMID: 29560612; PMCID: PMC5980562.
Lee CW, Muo CH, Liang JA, Lin MC, Kao CH. Atrial fibrillation is associated with morphine treatment in female breast cancer patients: a retrospective population-based time-dependent cohort study. Medicine (Baltimore). 2016;95(11): e3102. https://doi.org/10.1097/MD.0000000000003102.Erratum.In:Medicine(Baltimore).2016May13;95(19):e00c3.PMID:26986153;PMCID:PMC4839934 .
Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124(20):2264–74. https://doi.org/10.1161/CIRCULATIONAHA.111.019893. (PMID: 22083148).
Yoon YE, Kim HJ, Kim SA, Kim SH, Park JH, Park KH, Choi S, Kim MK, Kim HS, Cho GY. Left atrial mechanical function and stiffness in patients with paroxysmal atrial fibrillation. J Cardiovasc Ultrasound. 2012 Sep;20(3):140–5. https://doi.org/10.4250/jcu.2012.20.3.140. Epub 2012 Sep 21. PMID: 23185657; PMCID: PMC3498311.
Shaikh AY, Maan A, Khan UA, Aurigemma GP, Hill JC, Kane JL, Tighe DA, Mick E, McManus DD. Speckle echocardiographic left atrial strain and stiffness index as predictors of maintenance of sinus rhythm after cardioversion for atrial fibrillation: a prospective study. Cardiovasc Ultrasound. 2012;3(10):48. https://doi.org/10.1186/1476-7120-10-48. PMID:23199055;PMCID:PMC3583741.
Motoki H, Negishi K, Kusunose K, Popović ZB, Bhargava M, Wazni OM, Saliba WI, Chung MK, Marwick TH, Klein AL. Global left atrial strain in the prediction of sinus rhythm maintenance after catheter ablation for atrial fibrillation. J Am Soc Echocardiogr. 2014 Nov;27(11):1184–92. https://doi.org/10.1016/j.echo.2014.08.017. Epub 2014 Sep 23. PMID: 25260436; PMCID: PMC4425733.
Patel NR, Chyu CK, Satou GM, Halnon NJ, Nguyen KL. Left atrial function in children and young adult cancer survivors treated with anthracyclines. Echocardiography. 2018;35(10):1649–56. https://doi.org/10.1111/echo.14100. (Epub 2018 Jul 27 PMID: 30053329).
Yaylali YT, Saricopur A, Yurtdas M, Senol H, Gokoz-Dogu G. Atrial function in patients with breast cancer after treatment with anthracyclines. Arq Bras Cardiol. 2016 Nov;107(5):411–419. https://doi.org/10.5935/abc.20160146. Epub 2016 Oct 27. PMID: 27812678; PMCID: PMC5137385.
Fradley MG, Beckie TM, Brown SA, Cheng RK, Dent SF, Nohria A, Patton KK, Singh JP, Olshansky B. Recognition, prevention, and management of arrhythmias and autonomic disorders in cardio-oncology: a scientific statement from the american heart association. Circulation. 2021 Jul 20;144(3):e41-e55. https://doi.org/10.1161/CIR.0000000000000986. Epub 2021 Jun 17. PMID: 34134525; PMCID: PMC8992663.
Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bänsch D; Castle-af investigators. catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018 Feb 1;378(5):417–427. https://doi.org/10.1056/NEJMoa1707855. PMID: 29385358.
Sohns C, Fox H, Marrouche NF, Crijns HJGM, Costard-Jaeckle A, Bergau L, Hindricks G, Dagres N, Sossalla S, Schramm R, Fink T, El Hamriti M, Moersdorf M, Sciacca V, Konietschke F, Rudolph V, Gummert J, Tijssen JGP, Sommer P; CASTLE HTx Investigators. Catheter ablation in end-stage heart failure with atrial fibrillation. N Engl J Med. 2023 Aug 27. https://doi.org/10.1056/NEJMoa2306037. Epub ahead of print. PMID: 37634135.
Kanmanthareddy A, Vallakati A, Reddy Yeruva M, Dixit S, Biase DI, L, Mansour M, Boolani H, Gunda S, Bunch TJ, Day JD, Ruskin JN, Buddam A, Koripalli S, Bommana S, Natale A, Lakkireddy D. Pulmonary vein isolation for atrial fibrillation in the postpneumonectomy population: a feasibility, safety, and outcomes study. J Cardiovasc Electrophysiol. 2015;26(4):385–9. https://doi.org/10.1111/jce.12619. (Epub 2015 Feb 26 PMID: 25588757).
Ganatra S, Abraham S, Kumar A, Parikh R, Patel R, Khadke S, Kumar A, Liu V, Diaz ANR, Neilan TG, Martin D, Hook B, Dani SS, Asnani A, Nohria A. Efficacy and safety of catheter ablation for atrial fibrillation in patients with history of cancer. Cardiooncology. 2023;9(1):19. https://doi.org/10.1186/s40959-023-00171-4. PMID:37020260;PMCID:PMC10074889.
Boriani G, Lee G, Parrini I, Lopez-Fernandez T, Lyon AR, Suter T, Van der Meer P, Cardinale D, Lancellotti P, Zamorano JL, Bax JJ, Asteggiano R; Council of cardio-oncology of the european society of cardiology. Anticoagulation in patients with atrial fibrillation and active cancer: an international survey on patient management. Eur J Prev Cardiol. 2021 May 22;28(6):611–621. https://doi.org/10.1093/eurjpc/zwaa054. PMID: 33624005.
van Es N, Ventresca M, Di Nisio M, Zhou Q, Noble S, Crowther M, Briel M, Garcia D, Lyman GH, Macbeth F, Griffiths G, Iorio A, Mbuagbaw L, Neumann I, Brozek J, Guyatt G, Streiff MB, Baldeh T, Florez ID, Gurunlu Alma O, Agnelli G, Ageno W, Marcucci M, Bozas G, Zulian G, Maraveyas A, Lebeau B, Lecumberri R, Sideras K, Loprinzi C, McBane R, Pelzer U, Riess H, Solh Z, Perry J, Kahale LA, Bossuyt PM, Klerk C, Büller HR, Akl EA, Schünemann HJ; IPDMA Heparin use in cancer patients research group. The Khorana score for prediction of venous thromboembolism in cancer patients: An individual patient data meta-analysis. J Thromb Haemost. 2020 Aug;18(8):1940–1951. https://doi.org/10.1111/jth.14824. Epub 2020 Jul 8. PMID: 32336010.
Mendonça JC, Martins J, Fernandes C, Carvalho C, Coutinho C, Cotter J. Cancer risk after a venous thrombotic event - RIETE score. Thromb Res. 2021;202:43–4. https://doi.org/10.1016/j.thromres.2021.02.036. (Epub 2021 Mar 2 PMID: 33721801).
Pabinger I, van Es N, Heinze G, Posch F, Riedl J, Reitter EM, Di Nisio M, Cesarman-Maus G, Kraaijpoel N, Zielinski CC, Büller HR, Ay C. A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol. 2018 Jul;5(7):e289-e298. https://doi.org/10.1016/S2352-3026(18)30063-2. Epub 2018 Jun 7. Erratum in: Lancet Haematol. 2018 Jun 15;: PMID: 29885940; PMCID: PMC7338218.
Verso M, Agnelli G, Barni S, Gasparini G, LaBianca R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern Emerg Med. 2012;7(3):291–2. https://doi.org/10.1007/s11739-012-0784-y. (Epub 2012 May 1 PMID: 22547369).
Pelzer U, Opitz B, Deutschinoff G, Stauch M, Reitzig PC, Hahnfeld S, Müller L, Grunewald M, Stieler JM, Sinn M, Denecke T, Bischoff S, Oettle H, Dörken B, Riess H. Efficacy of prophylactic low-molecular weight heparin for ambulatory patients with advanced pancreatic cancer: outcomes from the CONKO-004 trial. J Clin Oncol. 2015;33(18):2028–34. https://doi.org/10.1200/JCO.2014.55.1481. (Epub 2015 May 18 PMID: 25987694).
Raposeiras-Roubin S, Abu-Assi E, Marchán A, Fernández-Sanz T, Barreiro-Pardal C, Pousa IM, Erquicia PD, Ledo-Piñeiro A, González-Bermúdez I, Viu MM, Íñiguez-Romo A. Validation of embolic and bleeding risk scores in patients with atrial fibrillation and cancer. Am J Cardiol. 2022;1(180):44–51. https://doi.org/10.1016/j.amjcard.2022.06.044. (Epub 2022 Jul 30 PMID: 35914971).
Malavasi VL, Fantecchi E, Gianolio L, Pesce F, Longo G, Marietta M, Cascinu S, Lip GYH, Boriani G. Atrial fibrillation in patients with active malignancy and use of anticoagulants: under-prescription but no adverse impact on all-cause mortality. Eur J Intern Med. 2019;59:27–33. https://doi.org/10.1016/j.ejim.2018.10.012. (Epub 2018 Oct 29 PMID: 30385084).
Ueki Y, Bär S, Losdat S, Otsuka T, Zanchin C, Zanchin T, Gragnano F, et al. Validation of the academic research consortium for high bleeding risk (arc-hbr) criteria in patients undergoing percutaneous coronary intervention and comparison with contemporary bleeding risk scores. EuroIntervention. 2020;16(5):371–9. https://doi.org/10.4244/EIJ-D20-00052. (PMID: 32065586).
Raposeiras-Roubín S, Faxén J, Íñiguez-Romo A, Henriques JPS, D’Ascenzo F, Saucedo J, et al. Development and external validation of a post-discharge bleeding risk score in patients with acute coronary syndrome: The BleeMACS score. Int J Cardiol. 2018;1(254):10–5. https://doi.org/10.1016/j.ijcard.2017.10.103. (Epub 2018 Jan 28 PMID: 29407077).
Hijazi Z, Oldgren J, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JW, Ezekowitz MD, Held C, Hylek EM, Lopes RD, Siegbahn A, Yusuf S, Granger CB, Wallentin L; ARISTOTLE and RE-LY Investigators. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet. 2016 Jun 4;387(10035):2302–2311. https://doi.org/10.1016/S0140-6736(16)00741-8. Epub 2016 Apr 4. PMID: 27056738.
Caldeira D, Costa J, Fernandes RM, Pinto FJ, Ferreira JJ. Performance of the HAS-BLED high bleeding-risk category, compared to ATRIA and HEMORR2HAGES in patients with atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2014;40(3):277–84. https://doi.org/10.1007/s10840-014-9930-y. (Epub 2014 Jul 11 PMID: 25012972).
Fradley MG, Ellenberg K, Alomar M, Swanson J, Kharod A, Nguyen ATH, Khodor S, Mishra S, Duong LM, Shah N, Armanious M, Rhea IB, Schabath MB, Kip KE. Patterns of anticoagulation use in patients with cancer with atrial fibrillation and/or atrial flutter. JACC CardioOncol. 2020;2(5):747–54. https://doi.org/10.1016/j.jaccao.2020.09.008. PMID:34396290;PMCID:PMC8352174.
Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Vanassche T, Potpara T, Camm AJ, Heidbüchel H; External reviewers. 2021 european heart rhythm association practical guide on the use of Non-Vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021 Oct 9;23(10):1612–1676. https://doi.org/10.1093/europace/euab065. Erratum in: Europace. 2021 Jun 28;: PMID: 33895845.
Elias A, Morgenstern Y, Braun E, Brenner B, Tzoran I. Direct oral anticoagulants versus enoxaparin in patients with atrial fibrillation and active cancer. Eur J Intern Med. 2021;89:132–4. https://doi.org/10.1016/j.ejim.2021.04.002. (Epub 2021 May 10 PMID: 33985888).
Pastori D, Menichelli D, Bucci T, Violi F, Pignatelli P; ATHERO-AF study group. Cancer-specific ischemic complications in elderly patients with atrial fibrillation: data from the prospective ATHERO-AF study. Int J Cancer. 2020 Dec 15;147(12):3424–3430. https://doi.org/10.1002/ijc.33179. Epub 2020 Jul 11. PMID: 32588421.
Falanga A, Leader A, Ambaglio C, Bagoly Z, Castaman G, Elalamy I, Lecumberri R, Niessner A, Pabinger I, Szmit S, Trinchero A, Ten Cate H, Rocca B. EHA guidelines on management of antithrombotic treatments in thrombocytopenic patients with cancer. Hemasphere. 2022;6(8) PMID:35924068;PMCID:PMC9281983. https://doi.org/10.1097/HS9.0000000000000750
Yang P, Zhu D, Xu X, Shen W, Wang C, Jiang Y, Xu G, Wu Q. Efficacy and safety of oral anticoagulants in atrial fibrillation patients with cancer-a network meta-analysis. Heart Fail Rev. 2020;25(5):823–31. https://doi.org/10.1007/s10741-019-09844-8. (PMID: 31410758).
ROCKET AF Study Investigators. Rivaroxaban-once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation: rationale and design of the ROCKET AF study. Am Heart J. 2010;159(3):340–347.e1. https://doi.org/10.1016/j.ahj.2009.11.025. (PMID: 20211293).
Chen ST, Hellkamp AS, Becker RC, Berkowitz SD, Breithardt G, Fox KAA, Hacke W, Halperin JL, Hankey GJ, Mahaffey KW, Nessel CC, Piccini JP, Singer DE, Patel MR, Melloni C. Efficacy and safety of rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation and a history of cancer: observations from ROCKET AF. Eur Heart J Qual Care Clin Outcomes. 2019 Apr 1;5(2):145–152. https://doi.org/10.1093/ehjqcco/qcy040. PMID: 30219887.
Lopes RD, Alexander JH, Al-Khatib SM, Ansell J, Diaz R, Easton JD, Gersh BJ, Granger CB, Hanna M, Horowitz J, Hylek EM, McMurray JJ, Verheugt FW, Wallentin L; ARISTOTLE Investigators. Apixaban for reduction in stroke and other ThromboemboLic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J. 2010 Mar;159(3):331–9.https://doi.org/10.1016/j.ahj.2009.07.035. Erratum in: Am Heart J. 2010 Jun;159(6):1162. PMID: 20211292.
Melloni C, Dunning A, Granger CB, Thomas L, Khouri MG, Garcia DA, Hylek EM, Hanna M, Wallentin L, Gersh BJ, Douglas PS, Alexander JH, Lopes RD. Efficacy and safety of apixaban versus warfarin in patients with atrial fibrillation and a history of cancer: insights from the aristotle trial. Am J Med. 2017;130(12):1440–1448.e1. https://doi.org/10.1016/j.amjmed.2017.06.026. (Epub 2017 Jul 21 PMID: 28739198).
Ruff CT, Giugliano RP, Antman EM, Crugnale SE, Bocanegra T, Mercuri M, Hanyok J, Patel I, Shi M, Salazar D, McCabe CH, Braunwald E. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the Effective aNticoaGulation with factor xA next GEneration in Atrial Fibrillation-Thrombolysis In Myocardial Infarction study 48 (ENGAGE AF-TIMI 48). Am Heart J. 2010;160(4):635–41. https://doi.org/10.1016/j.ahj.2010.06.042. (PMID: 20934556).
Fanola CL, Ruff CT, Murphy SA, Jin J, Duggal A, Babilonia NA, Sritara P, Mercuri MF, Kamphuisen PW, Antman EM, Braunwald E, Giugliano RP. Efficacy and safety of edoxaban in patients with active malignancy and atrial fibrillation: analysis of the engage af - timi 48 trial. J Am Heart Assoc. 2018;7(16) PMID:30369307;PMCID:PMC6201390. https://doi.org/10.1161/JAHA.118.008987
Reilly PA, Lehr T, Haertter S, Connolly SJ, Yusuf S, Eikelboom JW, Ezekowitz MD, Nehmiz G, Wang S, Wallentin L; RE-LY Investigators. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J Am Coll Cardiol. 2014 Feb 4;63(4):321–8. https://doi.org/10.1016/j.jacc.2013.07.104. Epub 2013 Sep 27. PMID: 24076487
Deitelzweig S, Keshishian AV, Zhang Y, Kang A, Dhamane AD, Luo X, Klem C, Ferri M, Jiang J, Yuce H, Lip GYH. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients with active cancer. JACC CardioOncol. 2021;3(3):411–24. https://doi.org/10.1016/j.jaccao.2021.06.004.PMID:34604802;PMCID:PMC8463723 .
Shah S, Norby FL, Datta YH, Lutsey PL, MacLehose RF, Chen LY, Alonso A. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv. 2018;2(3):200–9. https://doi.org/10.1182/bloodadvances.2017010694. PMID:29378726;PMCID:PMC5812321.
Mariani MV, Magnocavallo M, Straito M, Piro A, Severino P, Iannucci G, Chimenti C, Mancone M, Rocca DGD, Forleo GB, Fedele F, Lavalle C. Direct oral anticoagulants versus vitamin K antagonists in patients with atrial fibrillation and cancer a meta-analysis. J Thromb Thrombolysis. 2021 Feb;51(2):419–429. https://doi.org/10.1007/s11239-020-02304-3. Epub 2020 Oct 12. PMID: 33044735; PMCID: PMC7886836.
Papanastasiou A, Morsi-Yeroyannis A, Karagiannidis E, Kartas A, Doundoulakis I, Karvounis H, Giannakoulas G. Association of anticoagulant-related bleeding events with cancer detection in atrial fibrillation: A systematic review and meta-analysis. Hellenic J Cardiol. 2021 Sep-Oct;62(5):359–365. https://doi.org/10.1016/j.hjc.2020.11.007. Epub 2020 Nov 24. PMID: 33242617.
Khorana AA, Noble S, Lee AYY, Soff G, Meyer G, O’Connell C, Carrier M. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16(9):1891–4. https://doi.org/10.1111/jth.14219. (Epub 2018 Jul 19 PMID: 30027649).
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021 Feb 1;42(5):373–498. https://doi.org/10.1093/eurheartj/ehaa612. Erratum in: Eur Heart J. 2021 Feb 1;42(5):507. Erratum in: Eur Heart J. 2021 Feb 1;42(5):546–547. Erratum in: Eur Heart J. 2021 Oct 21;42(40):4194. PMID: 32860505.
Zhan Y, Joza J, Al Rawahi M, Barbosa RS, Samuel M, Bernier M, Huynh T, Thanassoulis G, Essebag V. Assessment and management of the left atrial appendage thrombus in patients with nonvalvular atrial fibrillation. Can J Cardiol. 2018;34(3):252–61. https://doi.org/10.1016/j.cjca.2017.12.008. (Epub 2017 Dec 15 PMID: 29395705).
Beigel R, Wunderlich NC, Ho SY, Arsanjani R, Siegel RJ. The left atrial appendage: anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging. 2014;7(12):1251–65. https://doi.org/10.1016/j.jcmg.2014.08.009. PMID:25496544.5Echocardiography.2008Sep;25(8):918-24.
Hilberath JN, Oakes DA, Shernan SK, Bulwer BE, D’Ambra MN, Eltzschig HK. Safety of transesophageal echocardiography. J Am Soc Echocardiogr. 2010;23(11):1115–27.
Lacomis JM, Goitein O, Deible C, Moran PL, Mamone G, Madan S, Schwartzman D. Dynamic multidimensional imaging of the human left atrial appendage. Europace. 2007;9(12):1134–40. https://doi.org/10.1093/europace/eum227. (Epub 2007 Oct 17 PMID: 17942583).
Romero J, Husain SA, Kelesidis I, Sanz J, Medina HM, Garcia MJ. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circ Cardiovasc Imaging. 2013;6(2):185–94. https://doi.org/10.1161/CIRCIMAGING.112.000153. (Epub 2013 Feb 13 PMID: 23406625).
Mohrs OK, Nowak B, Petersen SE, Welsner M, Rubel C, Magedanz A, Kauczor HU, Voigtlaender T. Thrombus detection in the left atrial appendage using contrast-enhanced MRI: a pilot study. AJR Am J Roentgenol. 2006;186(1):198–205. https://doi.org/10.2214/AJR.04.1504. (PMID: 16357402).
Shabtaie SA, Tan NY, Ward RC, Lewis BR, Yang EH, Holmes DR Jr, Herrmann J. Left atrial appendage occlusion in patients with atrial fibrillation and cancer. JACC CardioOncol. 2023;5(2):203–12. https://doi.org/10.1016/j.jaccao.2022.10.016.PMID:37144110;PMCID:PMC10152198 .
Kumar S, Yoon S, Milioglou I, Tashtish N, Farmakis I, Dallan LAP, Mogalapalli A, Arruda M, Filby SJ. Left atrial appendage closure outcomes in patients with cancer at a single tertiary center. Am J Cardiol. 2023 Jul 11;202:176–181. https://doi.org/10.1016/j.amjcard.2023.06.068. Epub ahead of print. PMID: 37441832.
Isogai T, Saad AM, Abushouk AI, Shekhar S, Kuroda S, Gad MM, Wazni OM, Krishnaswamy A, Kapadia SR. Procedural and short-term outcomes of percutaneous left atrial appendage closure in patients with cancer. Am J Cardiol. 2021;15(141):154–7. https://doi.org/10.1016/j.amjcard.2020.12.003. (Epub 2020 Dec 3 PMID: 33279485).
Sequeira AR, Bhandari A, Kilpatrick B, Fradley MG, Mohanty BD. Managing thromboembolic risk from atrial fibrillation in patients with cancer: a role for nonpharmacologic approaches. Future Cardiol. 2020;16(6):687–93.
Joosten LPT, van Doorn S, van de Ven PM, Köhlen BTG, Nierman MC, Koek HL, Hemels MEW, Huisman MV, Kruip M, Faber LM, Wiersma NM, Buding WF, Fijnheer R, Adriaansen HJ, Roes KC, Hoes AW, Rutten FH, Geersing GJ. Safety of switching from a Vitamin K antagonist to a Non-Vitamin K antagonist oral anticoagulant in frail older patients with atrial fibrillation: results of the frail-af randomized controlled trial. Circulation. 2023 Aug 27.https://doi.org/10.1161/CIRCULATIONAHA.123.066485. Epub ahead of print. PMID: 37634130.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nardi, E., Santoro, C., Prastaro, M. et al. Crosslink between atrial fibrillation and cancer: a therapeutic conundrum. Cardio-Oncology 10, 48 (2024). https://doi.org/10.1186/s40959-024-00243-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40959-024-00243-z