Abstract
Background
Cardiotoxicity is one of the most common adverse events of the chemotherapy. Physical exercise was shown to be cardioprotective. We aim to estimate the efficacy and safety of exercise in cancer patients receiving cardiotoxic chemotherapy.
Methods
We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs), which were retrieved by systematically searching PubMed, Web of Science, SCOPUS, Cochrane, Clinical Trials.gov, and MedRxiv through July 17th, 2023. We used RevMan V. 5.4 to pool dichotomous data using risk ratio (RR) and continuous data using mean difference (MD), with a 95% confidence interval (CI). PROSPERO ID: CRD42023460902.
Results
We included thirteen RCTs with a total of 952 patients. Exercise significantly increased VO2 peak (MD: 1.95 with 95% CI [0.59, 3.32], P = 0.005). However, there was no significant effect regarding left ventricular ejection fraction, global longitudinal strain, cardiac output, stroke volume, left ventricular end-diastolic volume, left ventricular end-systolic volume, E/A ratio, resting heart rate, peak heart rate, resting systolic blood pressure, and resting diastolic blood pressure. Also, there was no significant difference regarding any adverse events (AEs) (RR: 4.44 with 95% CI [0.47, 41.56], P = 0.19), AEs leading to withdrawal (RR: 2.87 with 95% CI [0.79, 10.43], P = 0.11), serious AEs (RR: 3.00 with 95% CI [0.14, 65.90], P = 0.49), or all-cause mortality (RR: 0.25 with 95% CI [0.03, 2.22], P = 0.21).
Conclusion
Exercise is associated with increased VO2 peak in cancer patients receiving cardiotoxic chemotherapy. However, there was no significant difference between exercise and usual care regarding the echocardiographic and safety outcomes.
Similar content being viewed by others
Introduction
Chemotherapy-induced cardiotoxicity (CIC) refers to the direct and indirect adverse effects of different chemotherapeutic agents on the cardiovascular system [1]. In particular, the incidence of left ventricular dysfunction among patients treated with certain anticancer drugs, such as doxorubicin at high doses (700 mg/m2), can reach 48%. In contrast, the incidence of myocardial ischemia due to 5-fluorouracil (5-FU) is reported to be as high as 10% [2, 3]. Moreover, 26–93% of patients on arsenic trioxide show prolonged QT interval, and many develop life-threatening ventricular tachyarrhythmias [4]. Besides being a not infrequently occurring event, CIC corresponds to a wide range of adverse events. According to the European Society of Cardiology’s Task Force for Cancer Treatments and Cardiovascular Toxicity, chemotherapy-related cardiovascular complications are classified as myocardial dysfunction and heart failure, coronary artery disease (CAD), arrhythmias, arterial hypertension, thromboembolic disease, peripheral vascular disease, pulmonary hypertension, and pericardial complications [2].
Consequently, different pharmacological and non-pharmacological therapies were investigated as potential preventive approaches against CIC, among them physical exercise, whose efficacy and tolerability were tested by numerous clinical trials with promising results [5, 6]. Several parameters can be used to assess the effects of exercise on cardiac function, such as left ventricular ejection fraction (LVEF), left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), and global longitudinal strain (GLS) which are all echocardiographically determined [7]. Besides this, cardiovascular fitness, i.e., peak oxygen uptake (VO2 peak) is also an interesting outcome to evaluate in this context. VO2 peak our primary outcome, is the peak value of oxygen uptake attained during exercise [8]. In a recent meta-analysis, high-intensity interval training positively affected cancer patients' functional performance [6]. Similarly, it was reported that exercise training can ameliorate cardiorespiratory fitness following chemotherapy with anthracyclines [9]. Additionally, the randomized controlled trial (RCT) known as The BREXIT Study has demonstrated that exercise can effectively prevent anthracycline-induced functional disability and cardiac impairment [10]. In contrast, another RCT has concluded the lack of feasibility of intensive aerobic training in a significant proportion of patients with metastatic breast cancer receiving chemotherapy [11].
Thus, it is not clear if the current data is sufficient to encourage the use of exercise for patients at risk of CIC, especially since exercise is not currently a part of the recommended standards of care for cancer management [12]. Furthermore, most established cardio-protective exercise abilities were observed in non-cancer populations [5]; therefore, the same effects may not necessarily be seen in cancer survivors.
This creates a solid rationale to extensively examine the findings of the current literature to provide a vigorous assessment of exercise advantages in lowering the risks of cardiovascular events following chemotherapy. Consequently, in the present systematic review and meta-analysis, we explored the quality of evidence that determines exercise's cardiac efficacy and safety in patients receiving chemotherapy. Our work may lead to insightful findings that can have key therapeutic implications.
Methodology
Protocol registration
The PRISMA statement and the Cochrane Handbook for systematic reviews and meta-analyses were followed to conduct this systematic review and meta-analysis [13, 14]. This meta-analysis process has been registered and published in PROSPERO under the following ID: CRD42023460902.
Data sources & search strategy
PubMed (MEDLINE), Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science Core Collection, EMBASE, Clinical Trials.gov, and MedRxiv were systematically searched until July 17th, 2023. We modified search terms and keywords for each database, as presented in (Table S1).
Eligibility criteria
We included randomized controlled trials (RCTs) published in English language that followed the following PICO criteria: population (P): patients diagnosed with any type of cancer receiving any cardiotoxic chemotherapeutic agent; intervention (I): any form of supervised aerobic or resistance exercise training irrespective of the exercise duration, frequency and intensity; control (C): usual care without any form of exercise training; and outcomes (O): primary outcome of this review is the VO2 peak. While our secondary outcomes include left ventricular ejection fraction (LVEF) change, change in global longitudinal strain (GLS), cardiac output (CO) (L/min) change, stroke volume (SV) (ml) change, left ventricular end-diastolic volume (LVEDV) (ml) change, left ventricular end-systolic volume (LVESV) (ml) change, E/A ratio change, respiratory exchange ratio (RER) change, resting heart rate (RHR) change, peak heart rate (PHR) change, resting systolic blood pressure (RSBP) (mmHg) change, resting diastolic blood pressure (RDBP) (mmHg) change, and safety outcomes, including the incidence of any adverse events, any serious adverse events, any adverse events leading to withdrawal, and mortality.
Study selection
To perform the review, we used the Covidence web tool. After deleting duplicates, four authors (M.T., M.I., A.N., and H.S.) independently evaluated the obtained records. Four authors (M.T., M.I., A.N., and H.S.) checked the full texts of the records that satisfied the initial eligibility criterion during the full-text screening. Any differences were settled by discussion and agreement with B.A.
Data extraction
We conducted a pilot extraction after retrieving the complete texts of relevant papers in order to prepare the data extraction sheet appropriately. The data extraction sheet, which is structured in Excel (Microsoft, USA), is divided into three sections. The first part included the summary characteristics of the included studies (name of first author, year of publication, country, exercise intensity, intervention frequency (Sessions per week), chemotherapeutic drug, exercise adherence, cancer type, cancer stage, and study design). The second part included the baseline information of the participants (sample size, age, menopausal status, body mass index (BMI), cancer stage, and comorbidities). Finally, the third part included outcomes data as previously described. Four reviewers (M.T., M.I., A.N., and H.S.) were responsible for data extraction. Any differences were settled by discussion and agreement with B.A.
Risk of bias and certainty of evidence
Using the Cochrane RoB2 tool, four reviewers (M.T., M.I., A.N., and H.S.) independently evaluated the quality of the studies [15]. They assessed five domains, including the risk of bias associated with the randomization process, deviation from the intended intervention, missing outcome data, measuring the outcome, and choosing the reported results. Any differences were settled by discussion and agreement with B.A. Two reviewers (M.A. and B.A.) followed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria [16, 17] to evaluate the certainty of evidence. Any disagreements were resolved through consensus.
Statistical analysis
The RevMan v5.3 software was used for the statistical analysis [18]. We employed the risk ratio (RR) to combine the results of dichotomous outcomes and the mean difference (MD) for continuous outcomes, both with a 95% confidence interval (CI), using the fixed-effects model. However, the random-effects model was used in case of significant heterogeneity. To assess heterogeneity, we utilized the Chi-square and I-square tests, where the Chi-square test establishes if heterogeneity exists, and the I-square test assesses the level of heterogeneity. According to the Cochrane Handbook (chapter nine) [19], we considered an alpha level of less than 0.1 for the Chi-square test to indicate significant heterogeneity, while an I-square more than 75% indicated considerable heterogeneity. When there was significant heterogeneity, sensitivity analysis was used in which we excluded one study in each scenario to detect possible heterogeneity causes.
Trial Sequential Analysis (TSA) was employed to assess the conclusiveness and reliability of the data of the pooled trials and to assess if the sample size of the current meta-analysis was adequate to make solid conclusions regarding the impact of the interventions. When the Z-line on the curve cut both the conventional and trial sequential monitoring boundary (TSMB), we assumed that the intervention's confidence level was conclusive and sufficient and that no additional studies were required. However, if the Z-line does not cut any boundaries, the evidence is insufficient, and further studies are needed [20, 21]. In this meta-analysis, we utilized an alpha error of 0.05, a beta error of 80% power, and a predicted RR reduction of 20% in dichotomous outcomes. Moreover, we made a subgroup analysis based on exercise type (aerobic exercise, restrictive exercise, and combined aerobic and restrictive exercise) and regarding whether the patients had breast cancer only or breast cancer plus other cancers throughout our primary and echocardiographic outcomes to detect possible differences between the subgroups.
Results
Search results and study selection
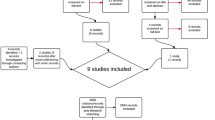
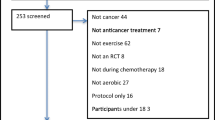
This literature search from PubMed (MEDLINE), Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science Core Collection, EMBASE, Clinical Trials.gov, and MedRxiv yielded a total of 4,446 articles. After duplication removal (n = 1371) and reviewing the title and abstract (n = 3075) for relevance, eighty-six articles were left for full-text screening. Thirteen of these studies met the inclusion criteria for our systematic review and meta-analysis. The PRISMA flow diagram displays the search results and studies selection process (Fig. 1).
Characteristics of included studies
This study involves thirteen RCTs [9, 10, 22,23,24,25,26,27,28,29,30,31,32] with a total of 952 patients, diagnosed with various types of cancer undergoing treatment with cardiotoxic chemotherapeutic agents. Among them, 569 (59.77%) patients participated in supervised aerobic or resistance exercise training sessions, whereas 383 (40.23%) did not receive any type of exercise. All the RCTs included the participants with breast cancer except Tsai et al. 2019 [24], which included the patients with Sarcoma hip/thigh, Lymphoma, Multiple myeloma, Osteosarcoma, Hodgkin's disease, and Leukemias as well. Also, in all the included RCTs, participants were delivered moderate to vigorous intensity exercise; however, there were variations in the exercise character, duration, and the number of exercise sessions among the studies. The detailed summary characteristics of the included RCTs and participants’ baseline characteristics are shown in (Table 1 and 2) respectively.
Risk of bias and certainty of evidence
The risk of bias assessment for each outcome is presented in (Fig. 2). Overall, most of the included studies demonstrated a low risk of bias across all assessed domains. Specifically, four studies raised some concerns regarding the risk of bias, primarily stemming from issues related to outcome measurement. Notably, only one study was deemed to have a high risk of bias, primarily due to shortcomings in the randomization process. More details about the authors’ decision are in (Table S2). Certainty of evidence is demonstrated in a GRADE evidence profile (Table 3).
Quality assessment of the risk of bias in the included trials. The upper panel presents a schematic representation of risks (low = green, unclear = yellow, and high = red) for specific types of biases of each of the studies in the review. The lower panel presents risks (low = green, unclear = yellow, and high = red) for the subtypes of biases of the combination of studies included in this review
Primary outcome
There was a significant difference between exercise and usual care regarding VO2 peak change with (MD: 1.95 with 95% CI [0.59 -3.32], P = 0.005) (Fig. 3-A). The pooled studies were heterogeneous (I2 = 90%, P < 0.00001). Heterogeneity was not resolved by leave-one-out sensitivity analysis (Table S3). TSA showed that the available evidence crossed both the conventional boundary and TSMB, indicating robust conclusions (Fig. 3-B). The subgroup analysis showed a significant difference in exercise type subgroups (P = 0.006) with a significant increase in VO2 peak in the aerobic exercise group (MD: 1.89 with 95% CI [0.23 – 3.55], P = 0.03), and combined exercise group (MD: 2.47 with 95% CI [0.63 – 4.30], P = 0.008). However, there was no difference in the resistant exercise group (MD: 0.10 with 95% CI [-0.16 – 0.37], P = 0.44) (Figure S1). However, test for subgroup analysis was not significant regarding whether the patients had breast cancer only or breast cancer plus other cancers (P = 0.82) (Figure S2).
Secondary outcomes
Efficacy outcomes
There was no significant difference between exercise and usual care regarding LVEF change (MD: 1.18 with 95% CI [-0.45, 2.81], P = 0.16), GLS change (MD: 0.42 with 95% CI [-0.52, 1.37], P = 0.38), CO change (MD: 0.51 with 95% CI [-1.00, 2.01], P = 0.51), SV change (MD: 2.24 with 95% CI [-9.04, 13.51], P = 0.70), LVEDV change (MD: -2.47 with 95% CI [-8.13, 3.18], P = 0.39), LVESV change (MD: -1.93 with 95% CI [-4.64, 0.78], P = 0.16), E/A ratio change (MD: 0.02 with 95% CI [-0.05, 0.10], P = 0.56) (Fig. 4).
Forest plots of the secondary efficacy outcomes, (1: Left ventricular ejection fraction (LVEF) change, 2: Global longitudinal strain (GLS) change, 3: Stroke volume (SV) change, 4: Left ventricular end-diastolic volume (LVEDV) change, 5: Left ventricular end-systolic volume (LVESV) change, 6: E/A ratio change, and 7: Cardiac output (CO) change), MD: mean difference, CI: confidence interval
Moreover, there was no significant difference between exercise and usual care regarding RER change (MD: 0.02 with 95% CI [-0.02, 0.05], P = 0.31) (Figure S3), RHR change (MD: -1.63 with 95% CI [-4.64, 1.39], P = 0.29) (Figure S4), PHR change (MD: 3.45 with 95% CI [-0.35, 7.25], P = 0.08) (Figure S5), RSBP change (MD: -3.32 with 95% CI [-8.79, 2.15], P = 0.23) (Figure S6), RDBP change (MD: -2.47 with 95% CI [-6.39, 1.44], P = 0.22) (Figure S7).
The pooled studies were homogenous in LVEF change (I2 = 39%, P = 0.12), LVEDV change (I2 = 0%, P = 0.72), LVESV change (I2 = 0%, P = 0.90), E/a ratio change (I2 = 0%, P = 0.54), RER change (I2 = 0%, P = 0.75), RHR change (I2 = 0%, P = 0.78), PHR change (I2 = 0%, P = 0.97), RSBP change (I2 = 0%, P = 0.64), and RDBP change (I2 = 0%, P = 0.66). However, pooled studies were heterogeneous in GLS change (I2 = 53%, P = 0.06), CO change (I2 = 97%, P < 0.00001), and SV change (I2 = 94%, P < 0.00001). Regarding GLS change, heterogeneity was best resolved by excluding Antunes et al. 2023 and Jacquinot et al. 2022 (I2 = 19%, P = 0.29), (I2 = 0%, P = 0.44) respectively. Regarding SV change, heterogeneity was best resolved by excluding Foulkes et al. 2023 (The BREXIT) (I2 = 0%, P = 0.43). Regarding CO change, heterogeneity was best resolved by excluding Foulkes et al. 2023 (The BREXIT) (I2 = 45%, P = 0.18) (Table S3). The test of subgroup analysis regarding exercise type was insignificant in all the outcomes. The subgroup analysis can be found in (Figures S8-19). Moreover, test for subgroup analysis was not significant regarding whether the patients had breast cancer only or breast cancer plus other cancers (Figure S20-S23).
Safety outcomes
There was no significant difference between exercise and usual care regarding the incidence of any adverse event (RR: 4.44 with 95% CI [0.47, 41.56], P = 0.19), any serious adverse event (RR: 3.00 with 95% CI [0.14, 65.90], P = 0.49), any adverse event leading to withdrawal (RR: 2.87 with 95% CI [0.79, 10.43], P = 0.11), and all-cause mortality (RR: 0.25 with 95% CI [0.03, 2.22], P = 0.21) (Fig. 5). Pooled studies were heterogenous in any adverse event (I2 = 74%, P = 0.02). However, the pooled studies were homogenous in any adverse event leading to withdrawal (I2 = 0%, P = 0.67) and All-cause mortality (I2 = 0%, P = 0.80). Regarding any adverse event, heterogeneity was best resolved by excluding Foulkes et al. 2023 (The BREXIT) and Kerrigan et al. 2023 (I2 = 45%, P = 0.18), (I2 = 33%, P = 0.22) respectively (Table S3).
Discussion
This meta-analysis showed that exercise is an effective enhancer of VO2 peak in chemotherapy patients. Furthermore, compared to usual care, exercise does not elicit any significant improvement in heart function-related parameters, including LVEF, GLS, CO, SV, LVEDV, LVESV, E/A ratio, RER, RHR, PHR, RSBP, and RDBP. Also, exercise-based care was a tolerable approach during chemotherapy that does not expose any additional risks for adverse events, confirming previous results from the oncology population [33,34,35].
VO2 peak refers to the limited value of oxygen uptake/consumption actually achieved during an exercise test (e.g., running on a treadmill). In other words, VO2 peak is the greatest value of the consumed oxygen by an exercising subject independently to his work rate level [36]. Notably, VO2 peak is 30% lower in cancer patients compared to age- and sex-matched healthy individuals who do not practice exercise [37]. Thus, it was shown by Jones et al. to be a strong independent predictor of survival among patients with non-small cell lung cancer. Thus, in these patients, the adjusted hazard ratio of all-cause mortality was 0.64 for a VO2 peak of 0.96–1.29 L.min − 1 and even lower, reaching 0.56 for a VO2 peak of > 1.29 L.min − 1 compared to VO2 peak < 0.96 L.min − 1 [38]. This suggests that a moderate increase in VO2 peak is beneficial to improve prognosis in the oncology population.
Our findings indicate that exercise can protect against chemotherapy-induced drop in VO2 peak, especially since cancer survivors who received neoadjuvant chemotherapy, compared to those who did not receive it, were reported to display a decreased peak VO2 per kg by 23% [39]. It is unclear how exercise would induce this effect; however, several mechanisms seem to be involved. The ability of exercise to reduce body mass index (BMI) during chemotherapy was confirmed by a recent systematic review [40]. Therefore, exercise may improve VO2 peak among chemotherapy patients by decreasing their BMI, as the latter is negatively associated with VO2 peak [41]. Exercise was also found to increase lean mass among cancer survivors, while the absence of exercise favors skeletal muscle loss within the same category [42, 43]. This can contribute to the exercise-induced improvement in cancer-related fatigue in oncology patients as lean mass increase is likely to be accompanied by a VO2 peak increase [44].In line with this, results from animal experiment have demonstrated that in rats receiving doxorubicin (a chemotherapy drug known by its toxic effects on skeletal muscle), preconditioning with exercise had enabled the prevention/minimization of skeletal muscle atrophy, contractile dysfunction, and muscular fatigue [45, 46]. Not just that but endurance exercise was shown to reverse doxorubicin-induced myotoxicity in rats [47]. All this may suggest that VO2 peak can be boosted in exercising oncology patients by a peripheral mechanism through positive effects on muscular growth, strength, metabolic function and recovery which would ultimately ameliorate oxygen uptake at the local level (muscle VO2). Especially that we found no significant benefit of exercise on central (i.e., cardiac) hemodynamics, which makes the peripheral action on skeletal muscle the more likely way to boost VO2 peak after chemotherapy. Moreover, higher systemic inflammation is correlated with lower VO2 peaks among cancer patients [48], and it is well-established that chemotherapy has pro-inflammatory effects. Therefore, exercise may also elevate VO2 peak via its potential to protect cancer survivors from systemic inflammation, particularly chemotherapy [49, 50].
Exercise failed to ameliorate the cardiovascular function of chemotherapy patients, which signifies that training therapy is potentially devoid of substantial protective effects against CIC. The absence of improvement in CO, LVEF, SV, LVEDV, LVESV, GLS, and E/A ratio indicates the inefficacy of exercise in reducing chemotherapy-induced left ventricular dysfunction and heart failure. Moreover, the fact that exercise did not show beneficial chronotropic effects (no changes in RHR and PHR) does not support the protective value of training programs against tachyarrhythmias associated with chemotherapeutic agents. Furthermore, a number of cytotoxic drugs, such as platinum components and alkylating agents, can induce secondary hypertension [51]. The insensibility of RSBP and RDBP to exercise-based therapy shows that the latter may have no notable effects on reducing the susceptibility to chemotherapy-induced hypertension.
It is necessary to determine the safety profile of any intervention among chemotherapy patients due to their vulnerability and frequent comorbidity. Notably, we confirmed in this study that exercise is a tolerable non-pharmacological option during chemotherapy treatment. This is consistent with the findings of a recent meta-analysis, which reported the absence of any harmful effects of exercise on cancer patients undergoing systemic treatment [33]. Another meta-analysis concluded exercise safety and feasibility among colorectal cancer patients [35]. This indicates that chemotherapy survivors may receive exercise-based care without any concerns of harm to reduce the impact of cancer on quality of life (tertiary prevention) and, at the same time, decrease the cardiovascular and metabolic risk in this vulnerable population.
Strengths and limitations
Few previous meta-analyses have addressed exercise's efficacy and safety profile in preventing CIC [52,53,54]. However, they either focused on one specific oncology population (i.e., breast cancer patients), one particular chemotherapy agent, or on safety outcomes only. Whereas our study provided a more robust examination of both possible cardiac benefits and harms of training among all oncology chemotherapy survivors. We thoroughly analyzed the available evidence using data from 952 participants and generated important findings about the benefit of exercise on cardiac function and aerobic fitness among cancer survivors managed with chemotherapy.
Nevertheless, our study was prone to considerable limitations as the available data from RCT was incomplete, and the involved studies presented significant heterogeneities and risk of bias concerns that could distort the final interpretations. Additionally, we did not provide a subgroup analysis of different chemotherapeutic agents. Finally, we did not assess the contribution of exercise in altering the susceptibility to develop or exacerbate myocardial ischemia, peripheral artery disease, thromboembolic disease, and myocarditis/pericarditis among chemotherapy patients as the evaluation of these outcomes would require other biomarkers (troponin elevation, ECG changes, INR drop for patients taking anticoagulants, vascular imaging, etc.), which are not included in our study.
Implications and future perspectives
The cardiovascular complications of cytotoxic molecules regroup a large spectrum of diseases [2]. Our results demonstrated a very modest benefit of exercise on the cardiac function of patients receiving chemotherapeutic agents, thereby, its low suitability to counteract chemotherapy-induced heart dysfunction. However, there is a potential for other cardioprotective effects not evaluated in our study, such as anti-ischemic, anti-thrombotic, and anti-inflammatory effects on chemotherapy-exposed cardiovascular tissue. Hence, future research should analyze the preventive abilities of physical activity against CIC events that may not necessarily lead to altered cardiac function, such as ischemic heart disease, peripheral artery disease, venous thromboembolism, and inflammatory reactions of the heart layers (myocarditis, pericarditis). On the other hand, the findings of our study suggest that there is a need for effective pharmacological and non-pharmacological strategies to prevent the decline in cardiac function secondary to chemotherapy. The only medication approved by the United States Food and Drug Administration (FDA) and European Medicines Agency (EMA) to prevent anthracycline-related cardiomyopathy is dexrazoxane [55]. However, other treatments were also found to be effective in preventing CIC, such as statins, beta-blockers, angiotensin-converting enzyme inhibitors, and aldosterone receptor antagonists, particularly spironolactone [56]. Therefore, the effectiveness of such therapies should be further investigated, and once confirmed, they may be approved for clinical use. The good tolerability of physical training programs by chemotherapy patients should motivate more investigation about the other possible benefits of this type of care apart from enhancing cardiovascular function and preventing CIC.
Conclusion
Exercise has limited beneficial effects on cardiac function among chemotherapy patients, manifesting mainly as a relative boosting of aerobic fitness. Nevertheless, it is a safe and tolerable strategy that may hold other interesting advantages to cancer survivors worthy of investigation. Moreover, the fact that exercise did not show beneficial chronotropic effects (no changes in RHR and PHR) does not support the protective value of training programs against tachyarrhythmias associated with chemotherapeutic agents. The absence of improvement in CO, LVEF, SV, LVEDV, LVESV, GLS, and E/A ratio indicates the inefficacy of exercise in reducing chemotherapy-induced left ventricular dysfunction and heart failure. Despite the shown lack of proof of effectiveness, future studies should still search for any possible cardioprotective potentials of physical training during chemotherapy. Parallel to this, it is also necessary to identify pharmacological or non-pharmacological strategies other than exercise to antagonize the cardiovascular harms of different chemotherapeutic drugs effectively.
Availability of data and materials
Not applicable.
Abbreviations
- CIC:
-
Chemotherapy-induced cardiotoxicity
- CAD:
-
Coronary artery disease
- ROS:
-
Reactive oxygen species
- TSMB:
-
Trial sequential monitoring boundary
- TSA:
-
Trial Sequential Analysis
- GRADE:
-
Grading of Recommendations Assessment, Development, and Evaluation criteria
- LVEF:
-
Left ventricular ejection fraction
- GLS:
-
Global longitudinal strain
- LVEDV:
-
Left ventricular end-diastolic volume
- LVESV:
-
Left ventricular end-systolic volume
- 5-FU:
-
5-Fluorouracil
- CO:
-
Cardiac output
- SV:
-
Stroke volume
- RER:
-
Respiratory exchange ratio
- RHR:
-
Resting heart rate
- PHR:
-
Peak heart rate
- RDBP:
-
Resting diastolic blood pressure
- RSBP:
-
Resting systolic blood pressure
References
Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010;102(1):14–25.
Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for practice guidelines. Eur Heart J. 2016;37(36):2768–801.
Frickhofen N, Beck FJ, Jung B, Fuhr HG, Andrasch H, Sigmund M. Capecitabine can induce acute coronary syndrome similar to 5-fluorouracil. Ann Oncol [Internet]. 2002;13(5):797–801. https://doi.org/10.1093/annonc/mdf035.
Lenihan DJ, Kowey PR. Overview and management of cardiac adverse events associated with tyrosine kinase inhibitors. Oncologist. 2013;18(8):900–8.
Scott JM, Nilsen TS, Gupta D, Jones LW. Exercise therapy and cardiovascular toxicity in cancer. Circulation. 2018;137(11):1176–91.
Neuendorf T, Haase R, Schroeder S, Schumann M, Nitzsche N. Effects of high-intensity interval training on functional performance and maximal oxygen uptake in comparison with moderate intensity continuous training in cancer patients: a systematic review and meta-analysis. Supp Care Cancer Off J Multinatl Assoc Supp Care Cancer. 2023;31(12):643.
Chengode S. Left ventricular global systolic function assessment by echocardiography. Ann Card Anaesth. 2016;19(Supplement):S26-34.
Hill AV, Long CNH, Lupton H. Muscular exercise, lactic acid and the supply and utilisation of oxygen.— Parts VII–VIII. Proc R Soc London Ser B Contain Pap a Biol Character. 1997;97(682):155–76. https://doi.org/10.1098/rspb.1924.0048.
Antunes P, Joaquim A, Sampaio F, Nunes C, Ascensão A, Vilela E, et al. Effects of exercise training on cardiac toxicity markers in women with breast cancer undergoing chemotherapy with anthracyclines: a randomized controlled trial. Eur J Prev Cardiol. 2023;30(9):844–55.
Foulkes SJ, Howden EJ, Haykowsky MJ, Antill Y, Salim A, Nightingale SS, et al. Exercise for the prevention of anthracycline-induced functional disability and cardiac dysfunction: the BREXIT study. Circulation. 2023;147(7):532–45.
Scott JM, Iyengar NM, Nilsen TS, Michalski M, Thomas SM, Herndon J 2nd, et al. Feasibility, safety, and efficacy of aerobic training in pretreated patients with metastatic breast cancer: a randomized controlled trial. Cancer. 2018;124(12):2552–60.
Lakoski SG, Eves ND, Douglas PS, Jones LW. Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol. 2012;9(5):288–96.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:372.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ et al. Cochrane handbook for systematic reviews of interventions. Cochrane Handb Syst Rev Interv. 2019;1–694.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:1–8.
Guyatt GH OAKRVGFYYSHGWG. Guyatt2008. Bmj [Internet]. 2008;336(may):995–8. Available from: https://www.bmj.com/content/336/7651/995.long%0AGuyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ; GRADE Working Group. What is %22quality of evidence%22 and why is it important to clinicians? BMJ. 2008;336(7651):995-8. doi: 10.1136
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Chinese J Evid-Based Med. 2009;9(1):8–11.
RevMan | Cochrane Training [Internet]. [cited 2021 Aug 3]. Available from https://www.training.cochrane.org/online-learning/core-software-cochrane-reviews/revman.
Cochrane Handbook for Systematic Reviews Intervention. 2022;
Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. 2008;61(8):763–9. Available from:https://www.sciencedirect.com/science/article/pii/S0895435607003691.
Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17(1):1–18.
Lee K, Kang I, Mack WJ, Mortimer J, Sattler F, Salem G, et al. Feasibility of high intensity interval training in patients with breast cancer undergoing anthracycline chemotherapy: a randomized pilot trial. BMC Cancer. 2019;19(1):653.
Sturgeon KM, Smith AM, Federici EH, Kodali N, Kessler R, Wyluda E, et al. Feasibility of a tailored home-based exercise intervention during neoadjuvant chemotherapy in breast cancer patients. BMC Sport Sci Med Rehabil. 2022;14(1):31.
Tsai E, Mouhayar E, Lenihan D, Song J, Durand JB, Fadol A, et al. Feasibility and outcomes of an exercise intervention for chemotherapy-induced heart failure. J Cardiopulm Rehabil Prev. 2019;39(3):199–203.
Bolam KA, Mijwel S, Rundqvist H, Wengström Y. Two-year follow-up of the OptiTrain randomised controlled exercise trial. Breast Cancer Res Treat. 2019;175(3):637–48.
Chung WP, Yang HL, Hsu YT, Hung CH, Liu PY, Liu YW, et al. Real-time exercise reduces impaired cardiac function in breast cancer patients undergoing chemotherapy: a randomized controlled trial. Ann Phys Rehabil Med. 2022;65(2):101485.
Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(28):4396–404.
Hojan K, Procyk D, Horyńska-Kęstowicz D, Leporowska E, Litwiniuk M. The preventive role of regular physical training in ventricular remodeling, serum cardiac markers, and exercise performance changes in breast cancer in women undergoing trastuzumab therapy-an REH-HER study. J Clin Med. 2020;9(5).
Hornsby WE, Douglas PS, West MJ, Kenjale AA, Lane AR, Schwitzer ER, et al. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol. 2014;53(1):65–74.
Jacquinot Q, Meneveau N, Falcoz A, Bouhaddi M, Roux P, Degano B, et al. Cardiotoxicity is mitigated after a supervised exercise program in HER2-positive breast cancer undergoing adjuvant trastuzumab. Front Cardiovasc Med. 2022;9:1000846.
Kerrigan DJ, Reddy M, Walker EM, Cook B, McCord J, Loutfi R, et al. Cardiac rehabilitation improves fitness in patients with subclinical markers of cardiotoxicity while receiving chemotherapy: a randomized controlled study. J Cardiopulm Rehabil Prev. 2023;43(2):129–34.
Kirkham AA, Eves ND, Shave RE, Bland KA, Bovard J, Gelmon KA, et al. The effect of an aerobic exercise bout 24 h prior to each doxorubicin treatment for breast cancer on markers of cardiotoxicity and treatment symptoms: a RCT. Breast Cancer Res Treat. 2018;167(3):719–29.
Thomsen SN, Lahart IM, Thomsen LM, Fridh MK, Larsen A, Mau-Sørensen M, et al. Harms of exercise training in patients with cancer undergoing systemic treatment: a systematic review and meta-analysis of published and unpublished controlled trials. eClinicalMedicine. 2023;59:101937. https://doi.org/10.1016/j.eclinm.2023.101937.
Segal R, Zwaal C, Green E, Tomasone JR, Loblaw A, Petrella T. Exercise for people with cancer: a systematic review. Curr Oncol. 2017;24(4):e290-315.
Singh B, Hayes SC, Spence RR, Steele ML, Millet GY, Gergele L. Exercise and colorectal cancer: a systematic review and meta-analysis of exercise safety, feasibility and effectiveness. Int J Behav Nutr Phys Act. 2020;17(1):122.
Whipp B. The peak versus maximum oxygen uptake issue. CpxinternationalCom [Internet]. 2010;1–10. Available from: http://www.cpxinternational.com/attachments/028_BJW-Vo2PeakvsMaxfinal2.pdf
Jones LW, Liang Y, Pituskin EN, Battaglini CL, Scott JM, Hornsby WE, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16(1):112–20.
Jones LW, Watson D, Herndon JE 2nd, Eves ND, Haithcock BE, Loewen G, et al. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer. 2010;116(20):4825–32.
Frésard I, Massé N, Bahtia C, Triponez F, Robert J, Licker M, et al. Reduction of peak oxygen consumption in patients with non-small cell lung cancer treated with neoadjuvant chemotherapy. Eur Respir J. 2013;42:28735. Available from: https://api.semanticscholar.org/CorpusID:71044224.
Li X, Wang J, Zhang J, Zhang N, Wu C, Geng Z, et al. The effect of exercise on weight and body composition of breast cancer patients undergoing chemotherapy: a systematic review. Cancer Nurs. 9900; Available from: https://journals.lww.com/cancernursingonline/fulltext/9900/the_effect_of_exercise_on_weight_and_body.93.aspx
Krachler B, Savonen K, Komulainen P, Hassinen M, Lakka TA, Rauramaa R. Cardiopulmonary fitness is a function of lean mass, not total body weight: the DRs EXTRA study. Eur J Prev Cardiol. 2015;22(9):1171–9.
Irwin ML, Alvarez-Reeves M, Cadmus L, Mierzejewski E, Mayne ST, Yu H, et al. Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity (Silver Spring). 2009;17(8):1534–41.
Stene GB, Helbostad JL, Balstad TR, Riphagen II, Kaasa S, Oldervoll LM. Effect of physical exercise on muscle mass and strength in cancer patients during treatment–a systematic review. Crit Rev Oncol Hematol. 2013;88(3):573–93.
Cicoira M, Zanolla L, Franceschini L, Rossi A, Golia G, Zamboni M, et al. Skeletal muscle mass independently predicts peak oxygen consumption and ventilatory response during exercise in noncachectic patients with chronic heart failure. J Am Coll Cardiol. 2001;37(8):2080–5.
Morton AB, Mor Huertas A, Hinkley JM, Ichinoseki-Sekine N, Christou DD, Smuder AJ. Mitochondrial accumulation of doxorubicin in cardiac and diaphragm muscle following exercise preconditioning. Mitochondrion. 2019;45:52–62.
Bredahl EC, Pfannenstiel KB, Quinn CJ, Hayward R, Hydock DS. Effects of exercise on doxorubicin-induced skeletal muscle dysfunction. Med Sci Sports Exerc. 2016;48(8):1468–73.
Kwon I. Protective effects of endurance exercise on skeletal muscle remodeling against doxorubicin-induced myotoxicity in mice. Phys Act Nutr. 2020;24(2):11–21.
Jones LW, Eves ND, Mackey JR, Peddle CJ, Haykowsky M, Joy AA, et al. Systemic inflammation, cardiorespiratory fitness, and quality of life in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2008;3(2):194–5. https://doi.org/10.1097/JTO.0b013e318160f36b.
Kleckner IR, Kamen C, Cole C, Fung C, Heckler CE, Guido JJ, et al. Effects of exercise on inflammation in patients receiving chemotherapy: a nationwide NCORP randomized clinical trial. Support Care Cancer. 2019;27(12):4615–25.
Hiensch AE, Mijwel S, Bargiela D, Wengström Y, May AM, Rundqvist H. Inflammation mediates exercise effects on fatigue in patients with breast cancer. Med Sci Sports Exerc. 2021;53(3):496–504.
Cohen JB, Brown NJ, Brown SA, Dent S, Van Dorst DCH, Herrmann SM, et al. Cancer therapy-related hypertension: a scientific statement from the American Heart Association. Hypertension. 2023;80(3):E46-57.
Tsai YL, Chuang YC, Chen CP, Lee YC, Cheng YY, Ou-Yang LJ. Feasibility of aerobic exercise training to mitigate cardiotoxicity of breast cancer therapy: a systematic review and meta-analysis. Clin Breast Cancer. 2023;23(6):576–90.
Naaktgeboren WR, Binyam D, Stuiver MM, Aaronson NK, Teske AJ, van Harten WH, et al. Efficacy of physical exercise to offset anthracycline-induced cardiotoxicity: a systematic review and meta-analysis of clinical and preclinical studies. J Am Heart Assoc. 2021;10(17):e021580.
Ghignatti PV da C, Nogueira LJ, Lehnen AM, Leguisamo NM. Cardioprotective effects of exercise training on doxorubicin-induced cardiomyopathy: a systematic review with meta-analysis of preclinical studies. Sci Rep. 2021;11(1):6330.
Bansal N, Adams MJ, Ganatra S, Colan SD, Aggarwal S, Steiner R, et al. Strategies to prevent anthracycline-induced cardiotoxicity in cancer survivors. Cardio-Oncology. 2019;5(1):1–22.
Mir A, Badi Y, Bugazia S, Nourelden AZ, Fathallah AH, Ragab KM, et al. Efficacy and safety of cardioprotective drugs in chemotherapy-induced cardiotoxicity: an updated systematic review & network meta-analysis. Cardio-Oncology [Internet]. 2023;9(1):1–34. https://doi.org/10.1186/s40959-023-00159-0.
Acknowledgements
None.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). We received no funding for this study.
Author information
Authors and Affiliations
Contributions
M.A. conceived the idea. A.M.A. and M.A. designed the research workflow. A.M.A. and M.A. searched the databases. M.T., M.I., A.N., and H.S. screened the retrieved records, extracted relevant data, assessed the quality of evidence, and B.A. resolved the conflicts. A.A.I. performed the analysis. M.A., A.M.A., and Y.K. wrote the final manuscript. B.A. supervised the project. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Search strategy. Table S2. Authors' description of risk of bias assessment. Table S3. Sensitivity analysis. Figure S1. VO2 peak subgroup analysis based on exercise type. Figure S2. VO2 peak subgroup analysis based on whether the patients had breast cancer only or breast cancer plus other cancers. Figure S3. Forest plot of respiratory exchange ratio (RER) change. Figure S4. Forest plot of resting heart rate (RHR) change. Figure S5. Forest plot of peak heart rate (PHR) change. Figure S6. Forest plot of resting systolic blood pressure (RSBP) change. Figure S7. Forest plot of resting diastolic blood pressure (RDBP) change. Figure S8. Left ventricular ejection fraction (LVEF) subgroup analysis based on exercise type. Figure S9. Cardiac output (CO) subgroup analysis based on exercise type. Figure S10. E/a ratio subgroup analysis based on exercise type. Figure S11. Global longitudinal strain (GLS) subgroup analysis based on exercise type. Figure S12. Left ventricular end-systolic volume (LVESV) subgroup analysis based on exercise type. Figure S13. Left ventricular end-diastolic volume (LVEDV) subgroup analysis based on exercise type. Figure S14. Resting heart rate (RHR) subgroup analysis based on exercise type. Figure S15. Peak heart rate (PHR) subgroup analysis based on exercise type. Figure S16. Respiratory exchange ratio (RER) subgroup analysis based on exercise type. Figure S17. Resting systolic blood pressure (RSBP) subgroup analysis based on exercise type. Figure S18. Resting diastolic blood pressure (RDBP) subgroup analysis based on exercise type. Figure S19. Stroke volume (SV) subgroup analysis based on exercise type. Figure S20. Left ventricular ejection fraction (LVEF) subgroup analysis based on whether the patients had breast cancer only or breast cancer plus other cancers. Figure S21. Global longitudinal strain (GLS) subgroup analysis based on whether the patients had breast cancer only or breast cancer plus other cancers. Figure S22. Respiratory exchange ratio (RER) subgroup analysis based on whether the patients had breast cancer only or breast cancer plus other cancers. Figure S23. Peak heart rate (PHR) subgroup analysis based on whether the patients had breast cancer only or breast cancer plus other cancers.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Amin, A.M., Khlidj, Y., Abuelazm, M. et al. The efficacy and safety of exercise regimens to mitigate chemotherapy cardiotoxicity: a systematic review and meta-analysis of randomized controlled trials. Cardio-Oncology 10, 10 (2024). https://doi.org/10.1186/s40959-024-00208-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40959-024-00208-2