Abstract
Background
This article provides an up-to-date overview of pericardial effusion in oncological practice and a guidance on its management. Furthermore, it addresses the question of when malignancy should be suspected in case of newly diagnosed pericardial effusion.
Main body
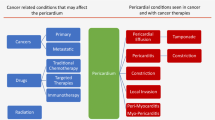
Cancer-related pericardial effusion is commonly the result of localization of lung and breast cancer, melanoma, or lymphoma to the pericardium via direct invasion, lymphatic dissemination, or hematogenous spread. Several cancer therapies may also cause pericardial effusion, most often during or shortly after administration. Pericardial effusion following radiation therapy may instead develop after years. Other diseases, such as infections, and, rarely, primary tumors of the pericardium complete the spectrum of the possible etiologies of pericardial effusion in oncological patients.
The diagnosis of cancer-related pericardial effusion is usually incidental, but cancer accounts for approximately one third of all cardiac tamponades. Drainage, which is mainly attained by pericardiocentesis, is needed when cancer or cancer treatment-related pericardial effusion leads to hemodynamic impairment. Placement of a pericardial catheter for 2-5 days is advised after pericardial fluid removal. In contrast, even a large pericardial effusion should be conservatively managed when the patient is stable, although the best frequency and timing of monitoring by echocardiography in this context are yet to be established. Pericardial effusion secondary to immune checkpoint inhibitors typically responds to corticosteroid therapy. Pericardiocentesis may also be considered to confirm the presence of neoplastic cells in the pericardial fluid, but the yield of cytological examination is low.
In case of newly found pericardial effusion in individuals without active cancer and/or recent cancer treatment, a history of malignancy, unremitting or recurrent course, large effusion or presentation with cardiac tamponade, incomplete response to empirical therapy with nonsteroidal anti-inflammatory, and hemorrhagic fluid at pericardiocentesis suggest a neoplastic etiology.
Similar content being viewed by others
Background
Pericardial effusion is quite a common finding in oncological patients. The diagnosis may be clinical, but most frequently relies on imaging techniques, especially transthoracic echocardiography (TTE) and computed tomography (CT), and treatment differs depending on the etiology and presentation.
In this narrative review article, we discuss the causes, manifestations, and management of pericardial effusion in cancer patients, with the goal of providing an up-to-date guidance for health care professionals. Furthermore, we address the question of when malignancy should be suspected in case of newly diagnosed pericardial effusion and no pre-exiting diagnosis of cancer.
Risk factors for and causes of pericardial effusion in patients with cancer
Cancer
Cancer-related pericardial effusion is usually the consequence of secondary tumor localization to the pericardium [1] (Fig. 1). Although pericardial involvement may be the presentation of cancer, it is often found at advanced stages of disease and portends a poor prognosis [2, 3]. Neoplastic cells reach the pericardium by direct invasion, lymphatic dissemination, or hematogenous spread, disrupt blood flow or infiltrate the wall of pericardial capillary and small veins, and elicit the accumulation of transudate or blood into the pericardial space [4]. Lung cancer is the most frequent cause of metastatic pericardial effusion, also defined as malignant pericardial effusion, followed by breast cancer, esophageal cancer, pancreatic cancer, melanoma, and hematological malignancies, especially B-cell lymphoma [2, 3, 5, 6]. Less commonly, tumoral invasion of mediastinal lymph nodes results in pericardial effusion, because of obstruction of lymphatic drainage of the pericardium with formation of pericardial transudate [4].
Primary tumors of the pericardium are 100-1,000 times less frequent than secondary ones, with the prevalence ranging from 0.001 to 0.007% [7, 8]. Within this group of rare tumors, mesothelioma and lymphoma predominate. The vast majority of primary cardiac lymphomas are B-cell derived, particularly diffuse B-cell lymphoma; in immunocompromised individuals, virus-associated lymphomas can be found, such as primary effusion lymphoma, Burkitt lymphoma, and Epstein-Barr virus-related lymphoproliferative disorders [9].
Radiation therapy
Radiation therapy (RT) may cause acute pericardial effusion or lead to delayed pericardial effusion, developing months to decades following treatment, with an estimated rate of 10%, but as high as 50% in certain patient subsets [10, 11]. Late RT-related pericardial effusion is typically observed in subjects with younger age at cancer diagnosis and longer disease-specific survival. Pericardial injury occurs when RT is delivered to the chest and the heart is within the radiation field, as it can happen for mediastinal lymphoma or cancer of the breast, lung, and esophagus [11, 12]. Since breast cancer and Hodgkin lymphoma are relatively common and have better prognosis than other thoracic malignancies treated with RT, survivors from these types of tumors are at greatest risk of RT-initiated pericardial effusion [13].
Pericardial effusion secondary to RT is fibrin-rich or hemorrhagic and is thought to be the result of microvascular damage from ionizing radiation, which alters venous and lymphatic drainage from the pericardium, and radiation-elicited pericardial inflammation. The risk of pericarditis rises from 5% to more than 50% with the total dose of radiation increasing from 40 to 50 Gy (Gy) [14], and if more than 30% of the cardiac area receives 50 Gy [15]. However, it must be noted that current RT schemas aim at minimizing the collateral irradiation of the heart.
Risk factors for RT-induced pericardial effusion can be categorized in RT-related and patient-related. The former are total radiation dose to the heart > 30 Gy, large-volume of irradiated pericardium, and lack of radiation shielding [11,12,13]. Patient-related factors are older age, pre-existing cardiovascular risk factors, such as diabetes and obesity, and left-sided tumor. In addition, RT enhances the cardiotoxicity of certain anticancer drugs, including anthracyclines, platinum-based agents, taxanes, gemcitabine, bevacizumab, and immune checkpoint inhibitors (ICIs). In a retrospective analysis of data from 14,358 adult survivors of various childhood and adolescent cancers, pericardial effusion was independently associated with exposure to an anthracycline dose of 250 mg/m2 or more and with exposure to any dose of cyclophosphamide [16].
The guidelines on cardio-oncology of the European Society of Cardiology (ESC) state that TTE should be performed every 5 years in cancer survivors who received a mean heart dose (MHD) of ionizing radiation > 15–25 Gy, or between 5 and 15 Gy together with doxorubicin ≥ 100 mg/m2 or equivalent. If the MHD was > 15–25 Gy, or > 5–15 Gy in combination with doxorubicin ≥ 100 mg/m2, it is recommended to perform TTE 1, 3, and 5 after completion of cardiotoxic cancer therapy and every 5 years thereafter (class of recommendation IIa) [17].
Oncological medical therapy
Anti-cancer drugs potentially causing pericardial effusion include anthracyclines, cyclophosphamide, cytarabine, busulfan, tyrosine kinase inhibitors (TKI) used for treatment of chronic myelogenous leukemia (ponatinib, dasatinib, bosutinib and, to a smaller extent, nilotinib), arsenic trioxide, all-trans retinoic acid, and interleukin-2 [18,19,20,21].
The underlying mechanisms are multiple (Fig. 1). Anthracyclines, cyclophosphamide, cytarabine, and bleomycin trigger acute pericarditis, while many of the aforementioned medications may initiate the production of oxygen free radicals with ensuing oxidative stress. Other events accounting for the pathogenesis of cancer treatment-related pericardial effusion (CTR-pericardial effusion) are pericardial and endomyocardial fibrosis (busulfan) [21] and increased endothelial permeability (interleukin-2 and dasatinib) [22, 23].
Pericardial effusion has also been increasingly reported after initiation of ICIs. In a retrospective comparison of > 2,500 consecutive patients who received ICIs with age- and cancer-type matched controls who did not receive ICIs, the risk of pericarditis or pericardial effusion was more than fourfold higher in the ICI group after adjusting for potential confounders [24]. Pericardial effusion following ICIs may be due to increase in size of pericardial micro-metastases because of T cell infiltration (so-called pseudoprogression) [25]. Indeed, pericardial effusion generally occurs soon after ICI administration, with the median time to onset being 30 days [26]. It is remarkable that pericardial effusion upon ICI administration has mainly been described in subjects with lung cancer [26]. A possible explanation is that these patients are often also treated with RT and that ionizing radiation exposes pericardial antigens, which are then recognized by T lymphocytes [27].
The anti-PD1 antibody, nivolumab, has particularly been associated with significant pericardial effusion [26,27,28], although this observation may be flawed by a reporting bias. In case studies, cytological examination of pleural fluid and biopsy specimens of pericardial leaflets revealed T-lymphocyte infiltrates, mostly CD4+, in the absence of malignant cells, supporting the hypothesis of an autoimmune process driving the development of pericardial effusion under ICIs [28, 29]. Nevertheless, malignant cells were detected in pericardial fluid from about half of patients with pericardial effusion during ICI therapy [26].
Other factors and comorbidities
Pericardial effusion is more frequent in males than females with the ratio being 1.3:1. It may be the consequence of heart failure, liver dysfunction with hypoalbuminemia, and renal failure, either concomitant or caused by cancer or cancer treatment [18, 30, 31]. Several other comorbidities and conditions can lead to pericardial effusion in cancer patients, such as pneumonia/empyema and connective tissue disease, as well as thoracic procedures and interventions [32]. Opportunistic infections favored by immunosuppression, such as by cytomegalovirus, tuberculosis mycobacteria, and fungi, may elicit pericarditis with pericardial effusion [33]. Finally, antithrombotic therapies can determine pericardial bleeding [34, 35].
In retrospective cohorts with severe pericardial effusion requiring drainage, the frequency of pericardial effusion not attributed to cancer or cancer treatment was 42–58% [36, 37].
Approach to the patient with cancer and pericardial effusion
The management of pericardial effusion in oncological patients varies depending on the cause and the clinical presentation [38]. The most challenging scenarios are when pericardial effusion is the reason for which the patient comes to medical attention and when pericardial effusion impairs cardiac hemodynamics (Fig. 2).
Management of large pericardial effusion in patients with cancer. BP, blood pressure; CTR, cancer treatment-related; ICI, immune checkpoint inhibitor; NSAIDs, non-steroidal anti-inflammatory drugs; PE, pericardial effusion; RT, radiation therapy; TTE, transthoracic echocardiography. The picture in the middle of the figure was obtained from Wikimedia Commons
Anytime there is a history of cancer treatment known to induce pericardial effusion, the possibility of CTR toxicity should be considered. Timing of exposure is important, since onset of CTR-pericardial effusion can be during or years after antitumor therapy, as it occurs with ICIs and RT, respectively. Some drugs also cause pleural effusion, e.g. dasatinib [39].
Fever and weight loss can be manifestations of primary cardiac lymphoma, albeit this tumor is very rare.
The mediastinal connective tissue opposes relatively low resistance to the expansion of the external (parietal) pericardium, allowing the pericardial space to contain a large amount of fluid as long as it progressively accumulates. This explains why large pericardial effusion can develop in the absence of symptoms and signs. In contrast, adaptation is not possible when fluid rapidly accumulates in the pericardial space. Consequently, the pressure in the pericardial space suddenly increases up to compressing the right chambers of the heart, which have lower pressures than the left ones. This pathophysiology (pericardial tamponade) impairs right and, thereby, left cardiac filling, leading to low cardiac output and shock. The amount necessary for compression of the cardiac chambers may be as little as 250 mL if fluid quickly occupies the pericardial space [40, 41].
Abrupt or worsening pericardial effusion is generally symptomatic for shortness of breath, cough, or chest pain and should prompt the suspicion of metastatic localization to the pericardium. If cancer has not been diagnosed yet, the malignancies most often invading the pericardium should be carefully searched: lung and breast cancer, melanoma, and lymphoma [42].
When newly found pericardial effusion is moderate to severe, it is critical to pay attention to a drop in blood pressure and to the appearance of jugular vein distention (due to congestion transmitted backwards from the compressed right atrium), as these may be signs of impending pericardial tamponade.
When pericardial effusion is very large, ECG voltages are low in approximately 50% of patients, and electrical alternans can result from swinging of the heart within the pericardium [43].
TTE is key to confirm pericardial effusion, as well as to detect cardiac tamponade by showing enhanced ventricular interdependence, right atrial systolic collapse, right ventricular early diastolic collapse, and plethora of the inferior vena cava [44]. Among other imaging techniques, chest radiography may provide nonspecific cues: enlarged cardiac silhouette in the case of tamponade and “water bottle” appearance in the presence of chronic, abundant pericardial effusion; and pleural effusion and pulmonary infiltrates as additional findings [45]. CT also demonstrates pericardial effusion and is the best imaging modality to assess pericardial thickness and calcification [46], while cardiac magnetic resonance imaging (CMR) can reveal adhesions of the thickened pericardium to the myocardium of the left and right ventricles [47, 48].
Pericardial effusion is often incidentally discovered in subjects with an established cancer diagnosis, who undergo TTE or CT imaging for other reasons. The TTE criteria to classify the severity of pericardial effusion are summarized in Table 1. TTE re-evaluation of pericardial effusion that is at least moderate at first detection is reasonable (after 7–14 days according to the ESC guidelines on cardio-oncology [17]), but the cost-effectiveness of prolonged interval monitoring remains unclear (Fig. 2).
Primary tumors of the pericardium can present with constrictive physiology. The neoplasm encasing the heart can be revealed by TTE, along with the pericardial effusion [49]. The imaging features on CT and CMR overlap with inflammatory pericarditis and include diffuse pericardial thickening and nodular enhancement.
About 30% of cardiac tamponades are due to malignancy [50], but the frequency by which, once diagnosed, cancer-related pericardial effusion progresses to tamponade appears to be low in clinical practice. In a recent investigation, 18,847 (0.1%) of 19,773,597 ≥ 18 year old patients in the US National Inpatient Sample discharged with a cancer code between January 2004 and December 2017 underwent pericardiocentesis [51]. Moreover, the prevalence of CTR-pericardial effusion requiring pericardiocentesis was reported to be 0.38%, 0.24%, 0.22%, and 0.20% in patients receiving ICIs, antimetabolites, TKIs, and monoclonal antibodies, respectively, and less than 0.14% with other therapies [26].
Cardiac tamponade is promptly resolved by percutaneous pericardiocentesis via subxiphoid or apical approach [28, 52]. The procedure can be done under the guide of TTE, fluoroscopy, or CT. TTE and fluoroscopy the choice in emergency situations. CT is a valuable alternative when the patient is stable [53]. Hemodynamically relevant pericardial effusion can also be removed by surgical pericardiotomy, but pericardiocentesis has less complications [17]. On the other hand, the need for repeat procedures may be greater if pericardial effusion is drained by pericardiocentesis [54]. The reported risk of infection with pericardiocentesis is 0.3% [55].
Pericardial drainage is usually not necessary in the absence of hemodynamic impairment [56]. Nevertheless, pericardiocentesis with cytological examination may be carried out to establish the etiology of the pericardial effusion, and to steer the diagnostic workup towards other causes if cancer cells are not found. It must be stressed that pericardiocentesis yields positive cytology in less than 50% of cancer-related pericardial effusions [57,58,59]. A frankly hemorrhagic pericardial fluid suggests that pericardial effusion is secondary to cancer metastases or primary angiosarcoma [9, 49]. Histopathologic evaluation of pericardial biopsy specimens collected by means of thoracoscopy or thoracotomy is another option to determine the etiology of pericardial effusion, but it is seldom followed [56].
A pericardial catheter may be left after pericardiocentesis for some days to promote adherence of pericardial layers, until minimal (< 30 mL/24 hours) or no liquid is drained [55, 59]. This strategy has been performed in patients with a platelet count < 50,000/µl without apparent increased risk of procedure-related bleeding and may reduce the risk of recurrence [36]. Experts advocate that a pericardial catheter is left for 2–5 days after drainage of cancer-related pericardial effusion [60]. However, no data are available about the risk of infection with prolonged pericardial catheter.
Creation of thoracoscopic or surgical pericardial window, usually into the pleural space, is another mean to prevent relapse of pericardial effusion [61, 62]. Palliation of pericardial effusion may also be achieved by intrapericardial instillation of cytotoxic/sclerosing agents or RT of radiation-sensitive tumors [52].
Pericardiectomy is the best treatment modality for primary pericardial tumors [55].
Drainage of pericardial effusion secondary to ICI therapy is rarely needed, since it normally responds to transient interruption of ICIs and corticosteroids [17, 26].
Pericardial effusion in the setting of post-actinic acute pericarditis is generally benign and does not impose to stop cancer therapy [49].
In case of clinically significant CTR-pericardial effusion, multidisciplinary discussion between the cardiologist and the oncologist/hematologist is fundamental to decide whether cancer treatment can be safely continued. Rechallenge with ICIs after pericardial effusion resolution appears to feasible [63].
Finally, pericarditis with pericardial effusion occurring in oncological patients, but unrelated to cancer or its treatment, should be managed like in the general population with colchicine and non-steroidal anti-inflammatory drugs [49]. Pericardiocentesis is indicated for inflammatory pericardial effusion that is unresponsive to treatment or symptomatic and moderate to large, and in the suspicion of bacterial or fungal infection. Pericardiectomy or a pericardial window may be considered if clinically relevant pericardial effusion recurs [49].
Approach to the general patient with pericardial effusion: can it be cancer-related?
Although pericardial involvement is usually found in patients who have already been diagnosed with cancer, pericardial effusion can be the initial presentation of an occult malignancy, especially when it is large [32, 57, 58].
A study conducted in Denmark demonstrated that 1,550 (11%) patients out of 13,759 with pericarditis/pericardial effusion received a new cancer diagnosis during a median follow-up period of 6.4 years. Moreover, mortality was increased after cancer diagnosis [32].
Other studies also examined the risk of subsequent cancer among patients with a first-time diagnosis of pericarditis and showed that malignancy, most often pulmonary, of the breast or hematological, eventually underlied pericarditis/pericardial effusion in 12-23% of individuals [32, 64, 65].
Surprisingly, many of the clinical features believed to help to distinguish between different etiologies of pericardial effusion overlapped between cancer-related and other-etiology pericardial effusion. Fever and precordial pain, usually considered clinical markers of inflammation, were as common among patients with cancer-related pericardial effusion as among those with other causes of pericardial effusion [57].
Clinical factors that may indicate a neoplastic origin of pericardial effusion are a history of malignancy, unremitting or recurrent course, presentation with pericardial tamponade, and incomplete response to empirical therapy with nonsteroidal anti-inflammatory drugs [2, 32, 57, 58].
In the aforementioned Danish study, a history of heart failure, chronic obstructive pulmonary disease, alcohol-related diagnoses, tuberculosis, and recent pneumonia or empyema were also associated with elevated cancer standardized incidence ratios among patients with pericarditis. In contrast, the risk of cancer diagnosis was lower in subjects with than without recent thoracic surgery or myocardial infarction [32].
Normal levels of inflammatory biomarkers and hemorrhagic fluid at pericardiocentesis support the suspicion of malignant pericardial effusion [57, 64].
Cellularity and protein content of pericardial fluid were similar in pericardial effusion secondary to cancer versus heart failure or uremia. However, malignant pericardial effusion had lower glucose concentrations and higher lactate dehydrogenase concentrations [64].
We suggest starting a diagnostic work-up for malignancies in patients with cardiac tamponade, as well as moderate-to-severe pericardial effusion of unexplained origin not responding to conventional anti-inflammatory therapy.
Conclusions
Cancer and cancer treatment deserve attention as possible causes of pericardial effusion, even in the general population of patients presenting with a new pericardial effusion. Although urgent and aggressive treatment is mandatory when there is hemodynamic instability, management of cancer-related and CTR-pericardial effusion should be conservative in most cases. Given the variety of causes and therapeutic and prognostic implications, interaction between health care professionals with cardiological and oncological/hematological expertise and case-by-case discussion are key to optimize the approach to the patient with pericardial effusion and cancer.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- CMR:
-
Cardiac magnetic resonance
- CRT:
-
Cancer treatment-related
- CT:
-
Computed tomography
- ESC:
-
European Society of Cardiology
- Gy:
-
Gray
- ICIs:
-
I
mmune checkpoint inhibitors
- MHD:
-
Mean heart dose
- RT:
-
Radiation therapy
- TKI:
-
Tyrosine kinase inhibitors
- TTE:
-
Transthoracic echocardiography
References
Maggiolini S, et al. The role of early contrast-enhanced chest computed tomography in the aetiological diagnosis of patients presenting with cardiac tamponade or large pericardial effusion. Eur Heart J Cardiovasc Imaging. 2016;17(4):421–8. https://doi.org/10.1093/ehjci/jev225.
Imazio M, et al. Relation of acute pericardial disease to malignancy. Am J Cardiol. 2005,;95(11):1393–4. https://doi.org/10.1016/j.amjcard.2005.01.094.
Reuter H, Burgess LJ, Doubell AF. Epidemiology of pericardial effusions at a large academic hospital in South Africa. Epidemiol Infect. 2005;133(3):393–9. https://doi.org/10.1017/s0950268804003577.
Meng Q, Lai H, Lima J, Tong W, Qian Y, Lai S. Echocardiographic and pathologic characteristics of primary cardiac tumors: a study of 149 cases. Int J Cardiol. 2002;84(1):69–75. https://doi.org/10.1016/s0167-5273(02)00136-5.
Besnard A, et al. Current management of symptomatic pericardial effusions in cancer patients. JACC Cardio Oncol. 2019;1(1):137–40. https://doi.org/10.1016/j.jaccao.2019.07.001.
Young JM, Goldman IR. Tumor metastasis to the heart. Circulation. 1954;9(2):220–9. https://doi.org/10.1161/01.cir.9.2.220.
e Patel J, Sheppard MN. Pathological study of primary cardiac and pericardial tumours in a specialist UK Centre: surgical and autopsy series. Cardiovasc Pathol off J Soc Cardiovasc Pathol. 2010;19(fasc 6):343–52. https://doi.org/10.1016/j.carpath.2009.07.005.
Restrepo CS, Vargas D, Ocazionez D, Martínez-Jiménez S, Betancourt Cuellar SL, Gutierrez FR. Primary pericardial tumors. Radiogr Rev Publ Radiol Soc N Am Inc. 2013;33(6):1613–30. https://doi.org/10.1148/rg.336135512.
Maleszewski JJ, Bois MC, Bois JP, Young PM, Stulak JM, Klarich KW. Neoplasia and the heart: pathological review of effects with clinical and radiological correlation. J Am Coll Cardiol. 2018;72(2):202–27. https://doi.org/10.1016/j.jacc.2018.05.026.
Madan R, Benson R, Sharma DN, Julka PK, Rath GK. Radiation induced heart disease: pathogenesis, management and review literature. J Egypt Natl Cancer Inst. 2015;27(4):187–93. https://doi.org/10.1016/j.jnci.2015.07.005.
Ning MS, et al. Incidence and predictors of pericardial effusion after chemoradiation therapy for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;99(1):70–9. https://doi.org/10.1016/j.ijrobp.2017.05.022.
Tamari K, et al. Risk factors for pericardial effusion in patients with stage I esophageal cancer treated with chemoradiotherapy. Anticancer Res. 2014;34(12):7389–93.
Marinko T. Pericardial disease after breast cancer radiotherapy. Radiol Oncol. 2018;53(1):1–5. https://doi.org/10.2478/raon-2018-0035.
Emami B, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–22. https://doi.org/10.1016/0360-3016(91)90171-y.
Wang K, et al. Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol. 2017;35(13):1387–94. https://doi.org/10.1200/JCO.2016.70.0229.
Mulrooney DA, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the childhood cancer survivor study cohort. BMJ. 2009;339:b4606. https://doi.org/10.1136/bmj.b4606.
Lyon AR, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43(41):4229–361. https://doi.org/10.1093/eurheartj/ehac244.
Ala CK, Klein AL, Moslehi JJ. Cancer treatment-associated pericardial disease: epidemiology, clinical presentation, diagnosis, and management. Curr Cardiol Rep. 2019;21(12):156. https://doi.org/10.1007/s11886-019-1225-6.
Saunders S, Anwar M. Capecitabine-induced myopericarditis - a case report and review of literature. J Oncol Pharm Pract. 2019;25(4):1006–10. https://doi.org/10.1177/1078155218774871.
Bock J, Doenitz A, Andreesen R, Reichle A, Hennemann B. Pericarditis after high-dose chemotherapy: more frequent than expected? Onkologie. 2006;29(7):321–4. https://doi.org/10.1159/000093528.
Terpstra W, de Maat CE. Pericardial fibrosis following busulfan treatment. Neth J Med. 1989;35(5–6):249–52.
Jeong GH, et al. Incidence of capillary leak syndrome as an adverse effect of drugs in cancer patients: a systematic review and meta-analysis. J Clin Med. 2019;8(2):143. https://doi.org/10.3390/jcm8020143.
Dasgupta SK, Le A, Vijayan KV, Thiagarajan P. Dasatinib inhibits actin fiber reorganization and promotes endothelial cell permeability through RhoA-ROCK pathway. Cancer Med. 2017;6(4):809–18. https://doi.org/10.1002/cam4.1019.
Gong J, et al. Pericardial disease in patients treated with immune checkpoint inhibitors. J Immunother Cancer. 2021;9(6):e002771. https://doi.org/10.1136/jitc-2021-002771.
Di Giacomo AM, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58(8):1297–306. https://doi.org/10.1007/s00262-008-0642-y.
Palaskas N, et al. Targeted cancer therapies with pericardial effusions requiring pericardiocentesis focusing on immune checkpoint inhibitors. Am J Cardiol. 2019;123(8):1351–7. https://doi.org/10.1016/j.amjcard.2019.01.013.
Salem JE, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–89. https://doi.org/10.1016/S1470-2045(18)30608-9.
Saade A, et al. Pericardial effusion under nivolumab: case-reports and review of the literature. J Immunother Cancer. 2019;7(1):266. https://doi.org/10.1186/s40425-019-0760-4.
Zatarain-Nicolás E, et al. Cardiovascular toxicity of checkpoint inhibitors: review of associated toxicity and design of the Spanish immunotherapy registry of cardiovascular toxicity. Clin Transl Oncol. 2023;25(11):3073–85. https://doi.org/10.1007/s12094-023-03217-2.
Benjamini O, Kimhi O, Lishner M. Severe pleuropericarditis and cardiomyopathy induced by high dose interferon alpha-2b. Isr Med Assoc J. 2007;9(6):486–7.
Yang X, Liu W, Lyons A, Song Z, Zhai S, Hu K. Pericarditis associated with cytarabine therapy for acute myelocytic leukemia: a case report. Eur J Clin Pharmacol. 2018;74(2):181–2. https://doi.org/10.1007/s00228-017-2355-7.
Søgaard KK, Farkas DK, Ehrenstein V, Bhaskaran K, Bøtker HE, Sørensen HT. Pericarditis as a marker of occult cancer and a prognostic factor for cancer mortality. Circulation. 2017;136(11):996–1006. https://doi.org/10.1161/CIRCULATIONAHA.116.024041.
Katz WE, Ferson PF, Lee RE, Killinger WA, Thompson ME, Gorcsan J. Images in cardiovascular medicine. metastatic malignant melanoma to the heart. Circulation. 1996;93(5):1066. https://doi.org/10.1161/01.cir.93.5.1066.
Lancellotti P, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14(8):721–40. https://doi.org/10.1093/ehjci/jet123.
Kantarjian H, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346(9):645–52. https://doi.org/10.1056/NEJMoa011573.
Haddad DE, et al. Outcomes of cancer patients undergoing percutaneous pericardiocentesis for pericardial effusion. J Am Coll Cardiol. 2015;66(10):1119–28. https://doi.org/10.1016/j.jacc.2015.06.1332.
Pawlak Cieślik A, et al. Diagnosis of malignant pericarditis: a single centre experience. Kardiol Pol. 2012;70(11):1147–53.
Maisch B, Ristic A, Pankuweit ES. Evaluation and management of pericardial effusion in patients with neoplastic disease prog. Cardiovasc. 2010;53(2):157–63. https://doi.org/10.1016/j.pcad.2010.06.003.
Quintás-Cardama A, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol. 2007;25(25):3908–14. https://doi.org/10.1200/JCO.2007.12.0329.
Guberman BA, Fowler NO, Engel PJ, Gueron M, Allen JM. Cardiac tamponade in medical patients. Circulation. 1981;64(3):633–40. https://doi.org/10.1161/01.cir.64.3.633.
Imazio M, et al. Colchicine as first-choice therapy for recurrent pericarditis: results of the CORE (COlchicine for REcurrent pericarditis) trial. Arch Intern Med. set.2005,;165(17):1987–91. https://doi.org/10.1001/archinte.165.17.1987.
Kim S-H, et al. Clinical characteristics of malignant pericardial effusion associated with recurrence and survival. Cancer Res Treat. 2010,;42(4):210–6. https://doi.org/10.4143/crt.2010.42.4.210.
Spodick DH. Diagnostic electrocardiographic sequences in acute pericarditis. Significance of PR segment and PR vector changes. Circulation. 1973;48(3):575–80. https://doi.org/10.1161/01.cir.48.3.575.
Burazor I, Imazio M, Markel G, Adler Y. Malignant pericardial effusion. Cardiology. 2013;124(4):224–32. https://doi.org/10.1159/000348559.
Refaat MM, Katz WE. Neoplastic pericardial effusion. Clin Cardiol. 2011;34(10):593–8. https://doi.org/10.1002/clc.20936.
Meyers DG, Meyers RE, Prendergast TW. The usefulness of diagnostic tests on pericardial fluid. Chest. 1997;111(5):1213–21. https://doi.org/10.1378/chest.111.5.1213.
Aldweib N, Farah V, Biederman RWW. Clinical utility of cardiac magnetic resonance imaging in pericardial diseases. Curr Cardiol Rev. 2018;14(3):200–12. https://doi.org/10.2174/1573403X14666180619104515.
Bogaert J, Francone M. Cardiovascular magnetic resonance in pericardial diseases. J Cardiovasc Magn Reson. 2009;11(1):14. https://doi.org/10.1186/1532-429X-11-14.
Tyebally S, et al. Cardiac tumors: JACC cardiooncology state-of-the-art review. JACC CardioOncol. 2020;2(2):293–311. https://doi.org/10.1016/j.jaccao.2020.05.009.
Sánchez-Enrique C, et al. Cause and long-term outcome of cardiac tamponade. Am J Cardiol. 2016;117(4):664–9. https://doi.org/10.1016/j.amjcard.2015.11.023.
Matetic A, et al. Prevalence, characteristics and mortality of cancer patients undergoing pericardiocentesis in the United States between 2004 and 2017. Cancer Med. 2023;12(5):5471–84. https://doi.org/10.1002/cam4.5373.
Fowler NO, Harbin AD. Recurrent acute pericarditis: follow-up study of 31 patients. J Am Coll Cardiol. 1986;7(2):300–5. https://doi.org/10.1016/s0735-1097(86)80495-8.
Ingber RB, Lodhi U, Mootz J, Siegel A, Al-Roubaie M, Greben C. Comparing outcomes of CT-guided percutaneous pericardial drainage with surgical pericardial window in patients with symptomatic pericardial effusions. Acad Radiol. 2023;30(11):2533–40. https://doi.org/10.1016/j.acra.2023.02.014.
Saltzman AJ, et al. Comparison of surgical pericardial drainage with percutaneous catheter drainage for pericardial effusion. J Invasive Cardiol. 2012;24(11):590–3.
Imazio M, Adler Y. Management of pericardial effusion. Eur Heart J. 2013;34(16):1186–97. https://doi.org/10.1093/eurheartj/ehs372.
Burke A, Jeudy J, Virmani R. Cardiac tumours: an update: cardiac tumours. Heart Br Card Soc. 2008;94(1):117–23. https://doi.org/10.1136/hrt.2005.078576.
Ben-Horin S, Bank I, Guetta V, Livneh A. Large symptomatic pericardial effusion as the presentation of unrecognized cancer: a study in 173 consecutive patients undergoing pericardiocentesis. Medicine (Baltimore). 2006;85(1):49–53. https://doi.org/10.1097/01.md.0000199556.69588.8e.
Sagristà-Sauleda J, Mercé J, Permanyer-Miralda G, Soler-Soler J. Clinical clues to the causes of large pericardial effusions. Am J Med. 2000;109(2):95–101. https://doi.org/10.1016/s0002-9343(00)00459-9.
Lekhakul A, et al. Safety and outcome of percutaneous drainage of pericardial effusions in patients with cancer. Am J Cardiol. 2018;122(6):1091–4. https://doi.org/10.1016/j.amjcard.2018.06.002.
Eh D, et al. Outcomes of cancer patients undergoing percutaneous pericardiocentesis for pericardial effusion. J Am Coll Cardiol. 2015;66(10):ago. https://doi.org/10.1016/j.jacc.2015.06.1332.
Imazio M. Indicators of poor prognosis of acute pericarditis. Circulation. 2007;115(21):2739–44. https://doi.org/10.1161/CIRCULATIONAHA.106.662114.
Imazio M, et al. Medical therapy of pericardial diseases: part II: noninfectious pericarditis, pericardial effusion and constrictive pericarditis. J Cardiovasc Med Hagerstown Md. 2010;11(11):785–94.
Inno A, Maurea N, Metro G, Carbone A, Russo A, Gori S. Immune checkpoint inhibitors-associated pericardial disease: a systematic review of case reports. Cancer Immunol Immunother. 2021;70(10):3041–53. https://doi.org/10.1007/s00262-021-02938-z.
Cheong XP, et al. Causes and prognosis of symptomatic pericardial effusions treated by pericardiocentesis in an Asian academic medical centre». Singapore Med J. 2020;61(3):137–41. https://doi.org/10.11622/smedj.20190.
Strobbe A. Etiology and long-term outcome of patients undergoing pericardiocentesis. J Am Heart Assoc. 2017;6(12):e007598. https://doi.org/10.1161/JAHA.117.007598.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
S.M., M.B., L.G., S.S. and P.A. contributed to the conception of the work and drafted the manuscript, and C.C., P.S. and I.P. critically revised it.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mori, S., Bertamino, M., Guerisoli, L. et al. Pericardial effusion in oncological patients: current knowledge and principles of management. Cardio-Oncology 10, 8 (2024). https://doi.org/10.1186/s40959-024-00207-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40959-024-00207-3