Abstract
Background
Early identification of cardiac dysfunction by non-invasive imaging in HER2-positive breast cancer patients treated with trastuzumab is challenging. In particular multigated acquisition (MUGA) scan, which is most widely used, is unable to detect subclinical cardiac changes. The use of N-terminal pro-brain natriuretic peptide (NT-proBNP), a serum biomarker of myocardial stress, might improve timely diagnosis.
Methods
This prospective, single-center, cohort study included patients with HER2-positive breast cancer who started trastuzumab therapy. Echocardiography was scheduled at regular intervals every 3 months during one year follow-up for cardiac function monitoring. For research purposes, NT-proBNP was determined at the same time points. Trastuzumab-induced cardiotoxicity (TIC) was the primary study endpoint, defined as a left ventricular ejection fraction (LVEF) < 45%, and/or an absolute decline in LVEF > 10% since inclusion, and/or the incidence of a clinical cardiac event.
Results
A total of 135 patients were enrolled between April 2008 and June 2016, with a median age of 54 years (IQR: 47–61). By three-dimensional echocardiography (3DE), the median LVEF at baseline was 62% (IQR: 58–65). At a median of 6 months (IQR: 5–11), 45 patients (33%) reached the study endpoint of TIC. Patients with TIC had a mean change of − 9.5% in LVEF (95% CI -7.2 to − 11.7; p = 0.001) during 1 year of trastuzumab treatment. Both NT-proBNP at baseline (HR 1.04, 95% CI 1.02–1.07; p = 0.003) and LVEF decline during anthracycline treatment prior to the start of trastuzumab (HR 1.16, 95% CI 1.07–1.25; p < 0.001) were independently associated with development of TIC. The level of NT-proBNP during follow-up was associated too with development of TIC (HR 1.06 per 10 pmol/l difference, 95% CI 1.02–1.10; p = 0.008). No steadily or sudden increase in NT-proBNP prior to TIC was observed.
Conclusions
NT-proBNP cannot be used as a surrogate monitoring tool for trastuzumab-induced cardiotoxicity in HER2-positive breast cancer patients during the first year of treatment. Patients showing an LVEF decline during anthracycline pre-treatment appeared vulnerable for trastuzumab-induced cardiotoxicity.
Similar content being viewed by others
Introduction
The identification of cardiac dysfunction in Human Epidermal growth Receptor 2 (HER2) positive breast cancer patients treated with trastuzumab is challenging, but crucial in order to prevent the development of heart failure in these patients. Trastuzumab (Herceptin®, Genetech, San Francisco CA) is a highly-effective anti-cancer drug that is widely used in patients with HER2-positive breast cancer. Addition of trastuzumab to (neo)adjuvant chemotherapy in patients with HER2-positive breast cancer improved disease-free survival (DFS) and overall survival (OS) impressively. [1,2,3] However, trastuzumab may cause cardiotoxicity, foremost an impairment of the left ventricular ejection fraction (LVEF), which may adversely affect the prognosis and limit quality of life.
In clinical practice, it is essential to identify early subclinical cardiac dysfunction in breast cancer patients treated with trastuzumab. Clinical symptomatic heart failure might than be prevented by timely prescription of cardio-protective medication, or by interruption or even discontinuation of trastuzumab treatment, because trastuzumab-induced cardiotoxicity is (partially) reversible. [4] Multigated acquisition (MUGA) scans are widely used to monitor cardiac function in this patient population, but identification of cardiotoxicity by this technique is challenging. MUGA scans not only provide LVEF assessments with high inter- and intra-observer variability [5], but also fail to detect early subclinical cardiac alterations, because of initial compensatory mechanisms of the left ventricle to prevent functional cardiac impairment. [6] In addition, cardiac monitoring by serial MUGA scans will pose a high radiation burden to the patient. [7] Echocardiography, another frequently used cardiac monitoring approach, overcomes certain limitations of MUGA scans as echocardiography evaluates the complete cardiac structure and lacks radiation exposure.
Therefore, more advanced and sensitive diagnostic strategies are needed, and cardiac biomarkers, including N-terminal pro-brain natriuretic peptide (NT-proBNP) might be useful in this respect.
NT-proBNP is a peptide stored in, and secreted predominantly from, membrane granules in the ventricles of the heart in response to increased intra cardiac pressure. [8] NT-proBNP is an established serum biomarker for the diagnosis of heart failure [9], and is associated with adverse prognosis in heart failure patients. As trastuzumab-based therapy may induce ventricle wall stress, small changes in NT-proBNP levels can potentially be detected prior to an LVEF decline.
Studies investigating the association between NT-proBNP levels and cardiotoxicity in breast cancer patients showed inconclusive results. [10,11,12,13,14,15,16,17] Two large prospective studies demonstrated increased NT-proBNP levels in breast cancer patients with cardiotoxicity [10, 11], but several others failed. [12, 13, 15,16,17] These studies are hampered by a low incidence of LVEF declines compared with population based, retrospective studies (9% vs. 19%) [11, 18, 19] or predominant focus on anthracyclines instead of trastuzumab. [10]
The current, prospective cohort study was designed to overcome these limitations. We aimed to assess the potency of a screening-strategy utilizing repeatedly measured NT-proBNP levels to detect trastuzumab-induced cardiotoxicity measured with three-dimensional echocardiography (3DE) in a representative cohort of HER2-positive breast cancer patients.
Methods
Selection and description of participants
This prospective cohort study included women with HER2-positive breast cancer, who started trastuzumab therapy between April 2008 and June 2016 in the Albert Schweitzer Hospital (ASZ), a large teaching hospital in Dordrecht, the Netherlands.
Patients were excluded from the study in case of baseline LVEF < 45%, presence of cardiac dysfunction, ischemic heart disease, valvular heart disease, severe renal dysfunction or hepatic dysfunction or known intolerability for trastuzumab therapy. This study was approved by the institutional review board of the ASZ and was conducted according to the Declaration of Helsinki. All participants provided written informed consent.
Procedures
Indications for trastuzumab therapy included (neo-) adjuvant (early-stage) and metastatic (advanced-stage) breast cancer with overexpression of the HER2-receptor. Patients were treated according to prevailing guidelines. [20] In patients with early-stage breast cancer, trastuzumab was preceded by 4 courses of doxorubicin 50–60 mg/m2 and cyclophosphamide 500–600 mg/m2 once every 3 weeks. After these 4 courses, trastuzumab 2–4 mg/kg was administered in combination with paclitaxel 80 mg/m2 weekly for 12 cycles, followed by mono-therapy trastuzumab 6 mg/kg every three weeks for one year. In patients with advanced-stage breast cancer, 6 courses of trastuzumab 2–4 mg/kg in combination with paclitaxel 80 mg/m2 were administered as initial treatment, after which trastuzumab 6 mg/kg was continued once every three weeks until relapse of breast cancer or until the development of cardiotoxicity or other reasons to stop trastuzumab. [21] In this study, patients were studied during the first year of trastuzumab treatment.
Echocardiography and laboratory assessments
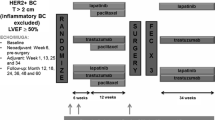
Echocardiography and laboratory assessments were scheduled during the first year of follow-up (Additional file 1: Figure S1). Within two weeks before or after blood collection, 3DE was systematically performed, with exclusion of eight days after the first trastuzumab administration (Additional file 1: Figure S1). Although the MUGA scan is currently most used in monitoring the cardiac function in these patients, ECG-gated triplane 3DE was used as routine cardiac monitoring because of the high accuracy, lack of radiation exposure, comprehensive evaluation of cardiac structure and reproducibility. [5] No MUGA scans were performed during the year of follow-up.
The 3DE images were obtained using a 1.5 to 3.6 MHz 3V probe with the Vivid 7 echo system (GE Vingmed Ultrasound, Trondheim, Norway). Gain and compression were set at 50%, and harmonic imaging was also used. Data sets were acquired from the parasternal long-axis, apical and subcostal position. Left ventricle (LV) volumes and LVEF were measured off line by means of summation of disc methodology with dedicated software (Echopac). The data set was aligned in 2 orthogonal planes along the long axis of the LV with clear depiction of the mitral valve and the LV apex. End diastolic measurements corresponded with the largest chamber size, whereas end systolic measurements corresponded with the smallest chamber size. The LVEF was determined as the difference between end diastolic volume (EDV) and end systolic volume (ESV), relative to the EDV. The echocardiograms were recorded by two independent experts at the echo core laboratory and evaluated by one cardiologist for research objectives.
Venous blood was systematically collected at the following time points: immediately before the start of anthracycline chemotherapy (in early-stage patients only), immediately before the start of trastuzumab, eight days after the start of trastuzumab and subsequently once every 12 weeks until 48 weeks after start of trastuzumab (Additional file 1: Figure S1). At each time point, the NT-proBNP level (Dimension Vista 500, Siemens Healthcare Diagnostics, Deerfield, Illinois) was measured according to the manufacturer’s instructions. Critical value for the NT-proBNP assay was < 10%. Treating physicians were not aware of the patients’ NT-proBNP levels.
Study endpoints
The primary endpoint was the occurrence of trastuzumab-induced cardiotoxicity (TIC), which was defined as an LVEF < 45% at any scheduled follow-up time point and/or an absolute decline in LVEF of > 10% relative to the measurement at study start – these thresholds are used by the National Cancer Research Institute as definition to interrupt trastuzumab treatment and start ACE inhibitors [22] – and/or any cardiac event for which the patient was hospitalized, including atrial fibrillation, unstable angina pectoris, acute coronary syndrome, and symptomatic heart failure. Patients who died in the year of follow-up were censored at the last available 3DE date.
Statistical analysis
Categorical baseline data are presented as numbers and percentages. Normality of continuous baseline data was evaluated by Shapiro-Wilk tests. Normal distributed data were presented as mean values ± one standard deviation (SD), and non-normal distributed data as median values and interquartile range (IQR).
Cox proportional hazard regression was used to analyze the relation between the following baseline characteristics and the development of TIC: age at diagnosis of breast cancer, LVEF at baseline, decline in LVEF during anthracycline treatment, and NT-proBNP at baseline. The decline in LVEF during anthracycline treatment was calculated as the difference between the LVEF at start trastuzumab and the LVEF at start anthracycline treatment.
Nonlinear mixed effect (NLME) models were used to study the LVEF and NT-proBNP change over time, as well as the relation between repeatedly measured NT-proBNP and LVEF. Echocardiography and blood collection was not necessarily performed at the same date. For this analysis, practically, we considered the closest NT-proBNP measurement within 30 days before or after an LVEF measurement as the corresponding value.
Joint modeling (JM) was applied to study the relation of repeatedly measured NT-proBNP with the incidence of TIC during follow-up. The JM combined an NLME model, describing the temporal evolution of NT-proBNP, with a Cox proportional hazard regression model, describing the time-to-event process.
Data analyses were performed using SPSS software, version 24.0 (SPSS, IBM, Chicago, Illinois, USA) and R statistical software (version 3.4.3), in particular the packages “nlme” and “JM”. Statistical significance of all tests was set at a two-tailed p-value of less than 0.05.
Results
Patient characteristics
Between April 2008 and June 2016, 150 patients with HER2-positive breast cancer were enrolled. A total of 15 patients were excluded from the analyses because they did not receive trastuzumab treatment (N = 4), had no echocardiography (N = 8) or NT-proBNP measurement (N = 3). Hence, a total of 135 patients were available for analyses (Fig. 1). Median (IQR) age of the patients at inclusion was 54 years (47–61; Table 1). Of all included patients, 113 patients (84%) had early-stage breast cancer, whereas the remaining patients had advanced-stage disease. Prior cardiac disease including valve insufficiency, arrhythmia and myocardial ischemia was present at start of treatment in 10 of the included patients (7%).
Trastuzumab-induced cardiotoxicity
During the 1 year of follow-up, 4 patients (3%) died. All patients died because of disease progression (Table 2). The range of the last available LVEFs of these patients was 53 to 62%.
In total, 45 patients (33%) developed TIC during treatment with trastuzumab (Table 2), of whom 44 (98%) experienced an absolute LVEF decline of > 10%. A total of 16 (36%) patients displayed an LVEF < 45% during trastuzumab treatment. We found no difference in TIC between patients with early-stage disease and advanced-stage disease (p = 0.805). The median time to TIC was 6 months (IQR 5–11 months). Two patients developed diastolic dysfunction of more than grade 2. These patients also demonstrated TIC. No other clinical cardiac events were observed.
The treating physician postponed treatment with trastuzumab in 6 patients temporarily and in 14 patients permanently because of cardiotoxicity. LVEF improved and returned to normal after discontinuation of trastuzumab treatment in all but 2 patients (10%).
Baseline factors associated with TIC
Age at diagnosis of breast cancer and baseline LVEF were not associated with TIC during 1 year of trastuzumab treatment (Table 3). However, patients who developed TIC showed an LVEF change of −6.6% during anthracycline treatment prior to the start of trastuzumab versus an LVEF change of −0.8% in patients without TIC (p = 0.033). The hazard ratio (HR) of an anthracycline-induced LVEF decline for TIC was 1.16. Although patients with TIC did not have significant higher baseline NT-proBNP than patients who remained TIC-free (median of 12 pmol/l versus 8 pmol/l, mean difference of 4 pmol/l, p = 0.229), single measurement of NT-proBNP at baseline was related with the development of TIC during follow-up (HR 1.04, 95% CI 1.02–1.07, p = 0.003).
Temporal evolution of LVEF
A total of 770 3DEs were obtained, of which 9 could not be interpreted because of poor quality, leaving 761 available for analysis, which implies a median of 6 (IQR 5–6) per patient. The median LVEF at baseline was 61% (IQR 59–65%).
In all patients together, during 1 year of trastuzumab treatment, the mean LVEF declined by 4.5% (95% CI -3.3% to − 5.8%; p < 0.001). In fact, this was mainly driven by the patients with TIC, who showed a change of − 9.5% in LVEF (95% CI -7.2% to − 11.7%; p = 0.001) as compared to a change of − 1.6% in LVEF (95% CI -0.6% to − 2.7%; p = 0.944) in their TIC-free counterparts. A post-hoc analysis of the 107 early-stage disease patients receiving anthracycline treatment demonstrated that, when comparing LVEF before anthracycline treatment with the LVEF at end of anthracycline treatment, patients with TIC experienced an LVEF decline of 0.056 per day and patients without TIC an LVEF decline of 0.002 per day. Figure 2 shows the trajectory of LVEF of patients with and without TIC in patients with and without anthracycline pretreatment.
Temporal evolution of NT-proBNP
A total of 692 NT-proBNP values were determined with a median of 6 per patient (IQR 4–7). NT-proBNP and LVEF were related, and every + 10 pmol/l difference in NT-proBNP (at any time point during follow-up) was associated with an absolute difference in LVEF of − 4.5% (95% CI -2.2% to − 6.7%; p < 0.001). Mean levels of NT-proBNP in patients with and without TIC were 16.8 and 10.1 pmol/l, respectively, which implies a mean difference of 6.7 pmol/l (p = 0.031). The HR for developing TIC was 1.06 per + 10 pmol/l difference in NT-proBNP at any time point during follow-up (95% CI 1.02–1.10, p = 0.008). NT-proBNP levels in all individual patients slightly increased from baseline (+ 2.9 pmol/l), more so in patients with TIC (+ 10.2 pmol/l) than in those without (+ 2.5 pmol/l), and this difference was statistically significant (p = 0.037). Interestingly, there was no evidence of a steadily or sudden increase in NT-proBNP prior to TIC. (Fig. 3).
Discussion
This study in HER2-positive breast cancer patients showed that serum levels of NT-proBNP increased during the first year of trastuzumab treatment. Patients who developed cardiotoxicity showed a steeper increase and had on average higher NT-proBNP levels than those in whom left ventricular function remained preserved. Still, NT-proBNP failed as a biomarker for early identification of cardiac dysfunction, as the changes were too subtle and could barely be distinguished from normal intra-subject variability. [23]
Our observation that NT-proBNP increases during trastuzumab treatment was also described by the studies of Romano et al. and Zardavas et al. [10, 11] Romano et al. also demonstrated that an increase of NT-proBNP during anthracycline is predictive for cardiotoxicity within 3 to 12 months. [10] This is in contrast with various other studies, which neither found increased NT-proBNP values during trastuzumab treatment, nor a relation between NT-proBNP and the incidence of cardiotoxicity. [12,13,14,15,16,17] The explanation of these variable results is most likely multifactorial, and related to the type of treatment, sample size, the specific NT-proBNP assay used, and, probably most relevant, the definition of cardiotoxicity (Additional file 1: Table S1). In the current study, a clinically relevant and widely accepted international definition of cardiotoxicity was used (absolute LVEF decline of > 10% and/or LVEF < 45%). We opted for this strict definition, as patients will usually only start to experience cardiac symptoms when LVEF levels below 45% are reached. In addition, an absolute LVEF decline of > 10% can suggest an increased risk of heart failure and treatment with an ACE inhibitor is advised. [22] It should also be noted that in the study of Romano et al., patients exclusively received anthracycline-based chemotherapy, whereas trastuzumab-based chemotherapy was not administered.
No evidence was found of a steadily or sudden increase in NT-proBNP before the development of TIC. Therefore, we concluded that NT-proBNP is not suited for the early identification of TIC. Still, in the current study, NT-proBNP actually did increase during trastuzumab treatment, and this increase was indeed related with a declining LVEF. The question on the role of NT-proBNP in detecting cardiotoxicity in patients receiving trastuzumab therefore still remains. The intra- and inter-subject variability of NT-proBNP are noteworthy, and the types of NT-proBNP assays used in the studies differ. To overcome these drawbacks, further research regarding NT-proBNP should be considered in larger, international, multi-center studies.
Whether cardiac biomarkers in general are suitable for detecting cardiotoxicity in patients receiving cardio-toxic cancer treatment also remains questionable. There are multiple pathways that could be involved in the development of cardiotoxicity due to cardio-toxic cancer treatment, and therefore multiple biomarkers may be useful in detecting cardiotoxicity. The pathway of anthracycline involves inhibition of topoisomerase IIb in myocardial cells with apoptosis and radical oxygen species formation as result [24], but less is known about the specific pathway involved in trastuzumab-induced cardiotoxicity. For example, troponin is released in patients with ischemic heart disease, but until now it has not been proven efficient in detecting cardiotoxicity due to trastuzumab treatment. [15,16,17, 25, 26] More knowledge about the pathway of trastuzumab-induced cardiotoxicity could possibly identify (new) cardiac biomarkers which could be useful for detecting this cardiotoxicity. Recently, the prognostic value of the biomarker suppressor of tumorgenicity 2 (ST2) became evident for acute [27] and chronic [28] heart failure patients. However, this biomarker, alone or in combinations with other cardiac biomarkers, has not been investigated extensively in patients undergoing cardio-toxic treatment for HER2-positive breast cancer.
Three-dimensional echocardiography, not standard MUGA scan, was used in the current study for the reason that 3DE is the preferred technique for LVEF monitoring and detection of cardiotoxicity due to the high accuracy in detecting LVEF levels below the lower limit of normal, high reproducibility and low temporal variability. [5] Major limitations of measuring LVEF with MUGA scans are the questionable accuracy, the cumulative radiation exposure of serial monitoring, intra-and inter variability and its limited information on other cardiac structures. [5, 7, 29,30,31] It should be noted that the choice of imaging modality can influence the estimated incidence of cardiotoxicity, and thus the relation with NT-proBNP. The other studies investigating the relationship between NT-proBNP and cardiotoxicity used two-dimensional echocardiography (2DE) for the LVEF assessments. However, 2DE is suggested to overestimate the mean LVEF by 5%. [32] Because 3DE LVEF measurements do not systematically differ from cardiac MRI (CMR) LVEF measurements [32], the gold standard for LVEF measurements, and 3DE has a higher availability than CMR, we used 3DE in this study. However, it cannot be completely excluded that some of our results represent false-positive results.
Interestingly, our study showed that patients with a (steeper than average) decrease in LVEF after anthracycline-based chemotherapy are most likely to develop cardiotoxicity during trastuzumab-based treatment. Thus, importantly, these patients can be identified prior to the start of trastuzumab, as they can potentially benefit from strict cardiac monitoring. This is in line with the European Society for Medical Oncology (ESMO) guidelines that recommend to reassess the LVEF if during anthracycline-based chemotherapy the LVEF declines below the 50% and to consider therapy for left ventricular dysfunction if the LVEF is confirmed to be below the 50% after anthracycline-based chemotherapy. [33] More should be learned about which factors - the cardiac response to anthracycline treatment, or the cardiac response to trastuzumab treatment, or others - are most important regarding the development of cardiotoxicity during trastuzumab treatment.
Limitations
Several limitations were inherent to the study design and limit the interpretation of our findings. First, all patients were treated and monitored in one single hospital. Although this hospital is a large, secondary, teaching-hospital in the Netherlands, it must be taken into account that external validity of the study findings had not been demonstrated. Secondly, our sample size was possibly too small in regard of the intra-subject variability of both NT-proBNP and LVEF. Although, compared with similar studies on the topic, our study had one of the highest number of included patients (Additional file 1: Table S1). Thirdly, in some cases there was a time delay between the NT-proBNP measurement and 3DE at one time point. Although we only observed a steady and slow increase in NT-proBNP coupled with a steady decrease in LVEF, the timing mismatch might have influenced our findings. At last, the echocardiograms were made by two different persons and reviewed by one cardiologist, so there may be some inter-observer variability in the results of the LVEF. However, 3DE is known to have a low inter-observer variability of 0.027% compared to 2DE. [34]
Conclusion
NT-proBNP cannot be used as a surrogate monitoring tool for trastuzumab-induced cardiotoxicity in HER2-positive breast cancer patients during the first year of treatment. Patients showing an LVEF decline during anthracycline pretreatment appeared vulnerable for trastuzumab-induced cardiotoxicity.
Abbreviations
- 2DE:
-
Two-dimensional echocardiography
- 3DE:
-
Three-dimensional echocardiography
- ACE:
-
Angiotensin-converting enzyme
- BMI:
-
Body mass index
- CABG:
-
Coronary artery bypass grafting
- CI:
-
Confidence interval
- CMR:
-
Cardiac Magnetic Resonance Imaging
- DFS:
-
Disease-free survival
- EDV:
-
End diastolic volume
- ESV:
-
End systolic volume
- HER2:
-
Human Epidermal growth factor Receptor 2
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- JM:
-
Joint model
- LV:
-
Left ventricle
- LVEF:
-
Left ventricular ejection fraction
- MI:
-
Myocardial infarction
- MUGA scan:
-
Multigated acquisition scan
- NLME model:
-
Non-linear mixed effects model
- NT-proBNP:
-
N-terminal pro-brain natriuretic peptide
- OS:
-
Overall survival
- PCI:
-
Percutaneous coronary intervention
- SD:
-
Standard deviation
- ST2:
-
Suppressor of tumorgenicity 2
- TIC:
-
Trastuzumab-induced cardiotoxicity
References
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84.
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Herceptin Adjuvant (HERA) Trial Study Team: Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92.
Ewer MS, Vooletich MT, Durand JB, Woods ML, Davis JR, Valero V, Lenihan DJ. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23(31):7820–6.
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, JM DC, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhaes A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging, 2014. Eur Heart J Cardiovasc Imaging. 15(10):1063–93.
Ewer MS, Lenihan DJ. Left ventricular ejection fraction and cardiotoxicity: is our ear really to the ground? J Clin Oncol. 2008;26(8):1201–3.
Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248(1):254–63.
Hall C. NT-ProBNP: the mechanism behind the marker. J Card Fail. 2005;11(5 Suppl):S81–3.
McCullough PA. B-type natriuretic peptides. A diagnostic breakthrough in heart failure. Minerva Cardioangiol. 2003;51(2):121–9.
Romano S, Fratini S, Ricevuto E, Procaccini V, Stifano G, Mancini M, Di Mauro M, Ficorella C, Penco M. Serial measurements of NT-proBNP are predictive of not-high-dose anthracycline cardiotoxicity in breast cancer patients. Br J Cancer. 2011;105(11):1663–8.
Zardavas D, Suter TM, Van Veldhuisen DJ, Steinseifer J, Noe J, Lauer S, Al-Sakaff N, Piccart-Gebhart MJ, de Azambuja E. Role of troponins I and T and N-terminal prohormone of brain natriuretic peptide in monitoring cardiac safety of patients with early-stage human epidermal growth factor receptor 2-positive breast Cancer receiving Trastuzumab: a Herceptin adjuvant study cardiac marker substudy. J Clin Oncol. 2017;35(8):878–84.
Ky B, Putt M, Sawaya H, French B, Januzzi JL Jr, Sebag IA, Plana JC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63(8):809–16.
Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5(5):596–603.
Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, Gosavi S, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107(9):1375–80.
Putt M, Hahn VS, Januzzi JL, Sawaya H, Sebag IA, Plana JC, Picard MH, Carver JR, Halpern EF, Kuter I, Passeri J, Cohen V, Banchs J, Martin RP, Gerszten RE, Scherrer-Crosbie M, Ky B. Longitudinal changes in multiple biomarkers are associated with cardiotoxicity in breast Cancer patients treated with doxorubicin, Taxanes, and Trastuzumab. Clin Chem. 2015;61(9):1164–72.
Ponde N, Bradbury I, Lambertini M, Ewer M, Campbell C, Ameels H, Zardavas D, Di Cosimo S, Baselga J, Huober J, Izquierdo M, Fumagalli D, Bozovic-Spasojevic I, Maetens M, Harbeck N, Pusztai L, Berghorn M, Im YH, Borrego MR, Chen DR, Rodeheffer R, Piccart M, Suter T, de Azambuja E. Cardiac biomarkers for early detection and prediction of trastuzumab and/or lapatinib-induced cardiotoxicity in patients with HER2-positive early-stage breast cancer: a NeoALTTO sub-study (BIG 1–06). Breast Cancer Res Treat. 2018;168(3):631–8.
Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, Tian G, Kirkpatrick ID, Singal PK, Krahn M, Grenier D, Jassal DS. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57(22):2263–70.
Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60(24):2504–12.
Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, Allen LA, Nekhlyudov L, Goddard KA, Davis RL, Habel LA, Yood MU, McCarty C, Magid DJ, Wagner EH. Pharmacovigilance study team: risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104(17):1293–305.
Romond EH, Jeong JH, Rastogi P, Swain SM, Geyer CE Jr, Ewer MS, Rathi V, Fehrenbacher L, Brufsky A, Azar CA, Flynn PJ, Zapas JL, Polikoff J, Gross HM, Biggs DD, Atkins JN, Tan-Chiu E, Zheng P, Yothers G, Mamounas EP, Wolmark N. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30(31):3792–9.
Giordano SH, Temin S, Kirshner JJ, Chandarlapaty S, Crews JR, Davidson NE, Esteva FJ, Gonzalez-Angulo AM, Krop I, Levinson J, Lin NU, Modi S, Patt DA, Perez EA, Perlmutter J, Ramakrishna N, Winer EP. American Society of Clinical Oncology: systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(19):2078–99.
Jones AL, Barlow M, Barrett-Lee PJ, Canney PA, Gilmour IM, Robb SD, Plummer CJ, Wardley AM, Verrill MW. Management of cardiac health in trastuzumab-treated patients with breast cancer: updated United Kingdom National Cancer Research Institute recommendations for monitoring. Br J Cancer. 2009;100(5):684–92.
Bruins S, Fokkema MR, Romer JW, Dejongste MJ, van der Dijs FP, van den Ouweland JM, Muskiet FA. High intraindividual variation of B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with stable chronic heart failure. Clin Chem. 2004;50(11):2052–8.
Yeh ET, Chang HM. Oncocardiology-past, present, and future: a review. JAMA Cardiol. 2016;1(9):1066–72.
Onitilo AA, Engel JM, Stankowski RV, Liang H, Berg RL, Doi SA. High-sensitivity C-reactive protein (hs-CRP) as a biomarker for trastuzumab-induced cardiotoxicity in HER2-positive early-stage breast cancer: a pilot study. Breast Cancer Res Treat. 2012;134(1):291–8.
Morris PG, Chen C, Steingart R, Fleisher M, Lin N, Moy B, Come S, Sugarman S, Abbruzzi A, Lehman R, Patil S, Dickler M, McArthur HL, Winer E, Norton L, Hudis CA, Dang CT. Troponin I and C-reactive protein are commonly detected in patients with breast cancer treated with dose-dense chemotherapy incorporating trastuzumab and lapatinib. Clin Cancer Res. 2011;17(10):3490–9.
van Vark LC, Lesman-Leegte I, Baart SJ, Postmus D, Pinto YM, Orsel JG, Westenbrink BD, Brunner-la Rocca HP, van Miltenburg AJM, Boersma E, Hillege HL, Akkerhuis KM. TRIUMPH investigators: prognostic value of serial ST2 measurements in patients with acute heart failure. J Am Coll Cardiol. 2017;70(19):2378–88.
Aimo A, Vergaro G, Passino C, Ripoli A, Ky B, Miller WL, Bayes-Genis A, Anand I, Januzzi JL, Emdin M. Prognostic value of soluble suppression of Tumorigenicity-2 in chronic heart failure: a meta-analysis. JACC Heart Fail. 2017;5(4):280–6.
Skrypniuk JV, Bailey D, Cosgriff PS, Fleming JS, Houston AS, Jarritt PH, Whalley DR. UK audit of left ventricular ejection fraction estimation from equilibrium ECG gated blood pool images. Nucl Med Commun. 2005;26(3):205–15.
Bailey EA, Bailey DL. Results from an Australian and New Zealand audit of left ventricular ejection fraction from gated heart pool scan analysis. Nucl Med Commun. 2012;33(1):102–11.
Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, Pennell DJ. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21(16):1387–96.
Armstrong GT, Plana JC, Zhang N, Srivastava D, Green DM, Ness KK, Daniel Donovan F, Metzger ML, Arevalo A, Durand JB, Joshi V, Hudson MM, Robison LL, Flamm SD. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30(23):2876–84.
Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, Criscitiello C, Goldhirsch A, Cipolla C, Roila F. ESMO Guidelines Working Group: Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23(Suppl 7):vii155–66.
Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61(1):77–84.
Acknowledgements
This study was funded by the Stichting Vriendenfonds of the Albert Schweitzer hospital, Dordrecht, The Netherlands. In addition, we thank Astrid Gundlach and Lianne Stomp – van de Burgt for obtaining the echocardiograms of all patients included in this study.
Funding
The study was funded by Stichting Vriendenfonds of the Albert Schweitzer hospital in Dordrecht, The Netherlands. The funding source had no involvement in the study design, data collection, analysis and interpretation of data, in the writing of the manuscript, and in the decision to submit the article for publication.
Availability of data and materials
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
NB contributed to the conception and design of the manuscript; and data collection; and analysis and interpretation of data; and manuscript writing; and approved the submitted version and agreed to be personally accountable for the contributions. CL contributed to the conception and design of the manuscript; and data collection; and manuscript writing; and approved the submitted version and agreed to be personally accountable for the contributions. MK contributed to the conception and design of the manuscript; and interpretation of data; and manuscript writing; and approved the submitted version and agreed to be personally accountable for the contributions. SS contributed to the data collection; and approved the submitted version and agreed to be personally accountable for the contributions. JB contributed to the conception and design of the manuscript; and approved the submitted version and agreed to be personally accountable for the contributions. JK contributed to the conception and design of the manuscript; and approved the submitted version and agreed to be personally accountable for the contributions. MF contributed to the conception and design of the manuscript; and approved the submitted version and agreed to be personally accountable for the contributions. ML contributed to the conception and design of the manuscript; and data collection; and analysis and interpretation of data; and manuscript writing; and approved the submitted version and agreed to be personally accountable for the contributions. EB contributed to the conception and design of the manuscript; and data collection; and analysis and interpretation of data; and manuscript writing; and approved the submitted version and agreed to be personally accountable for the contributions. ICMJE criteria for authorship have been met and no person or persons other than the authors listed have contributed to its preparation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the institutional review board of the Albert Schweitzer hospital (WOAC) and was conducted according to the Declaration of Helsinki. All participants provided written informed consent.
Consent for publication
The content of the manuscript has been read and approved for submission and publication by all authors.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Figure S1. Study procedures during 1 year follow-up. Table S1. Overview of studies investigating the relation of NT-proBNP and cardiotoxicity. (DOCX 63 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bouwer, N.I., Liesting, C., Kofflard, M.J.M. et al. NT-proBNP correlates with LVEF decline in HER2-positive breast cancer patients treated with trastuzumab. Cardio-Oncology 5, 4 (2019). https://doi.org/10.1186/s40959-019-0039-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40959-019-0039-4