Abstract
Background
Patient-reported outcome measures (PROMs) are tools to screen a population, to monitor the subjective progress of a therapy, to enable patient-centred care and to evaluate the quality of care. The QUALITOUCH Activity Index (AI) is such a tool, used in physiotherapy. This study aimed to provide reference values for expected AI outcomes.
Methods
A large data set uniting clinical routine data and AI outcomes was generated; it consisted of data of 11,948 patients. For four defined diagnoses, i.e. chronic lower back pain, tibia posterior syndrome, knee joint osteoarthritis and shoulder impingement, the AI responses related to the dimensions “maximum pain level” and “household activity” were analyzed. Reference corridors for expected AI outcomes were derived as linear trend lines representing the mean, 1st and 3rd quartile.
Results
Reference corridors for expected AI outcomes are provided. For chronic lower back pain, for example, the corridor indicates that the initial average AI value related to maximum pain of 49.3 ± 23.8 points on a visual analogue scale (VAS multiplied by factor 10) should be improved by a therapeutic intervention to 36.9 ± 23.8 points on a first follow-up after four weeks.
Conclusions
For four exemplary diagnoses and two dimensions of the AI, one related to pain and one related to limitations in daily activities, reference corridors of expected therapeutic progress were established. These reference corridors can be used to compare an individual performance of a patient with the expected progress derived from a large data sample. Data-based monitoring of therapeutic success can assist in different aspects of planning and managing a therapy.
Similar content being viewed by others

Background
There are different ways to evaluate the success of a therapeutic intervention, to monitor the progress of a therapy and to gather expectations of patients, respectively. Those commonly used are clinical-based outcome measures, performance-based outcome measures, and patient-reported outcome measures (PROMs). In addition, there are patient-reported experience measures (PREMs) and patient-defined desired outcomes [1]. All of these measures can be regarded as indicators of different dimensions that can be used to evaluate the therapeutic outcome; Verburg et al., for example, presented a suggestion for using different dimensions to assess the outcome related to low back pain [2].
PROMs, in particular, have become a more inherent part of clinical practice as a means of incorporating patient-reported outcomes into overall therapy success [3]. PROMs are useful, for instance, for screening (e.g. to identify hidden topics), for monitoring (e.g. to evaluate intervention effectiveness and track a patient’s subjective outcome over time), for strengthening patient-centred care (e.g. to achieve better health outcomes and higher patient compliance) and for evaluating the quality of care (e.g. to assess strengths and weaknesses of certain therapies based on large data collections) [4].
Within the orthopaedics application there are condition-specific PROMs, such as WOMAC [5] and the Knee Injury and Osteoarthritis Outcome Score (KOOS) [6]. Other measures use more generic questionnaires to assess the health status or the quality of life, respectively: examples are the Short Form 36 (SF-36) [7] and the EuroQol Group 5-D Instrument (EQ-5D) [8]. The QUALITOUCH Activity Index (AI) [9] was designed as a generic, internet-based, patient-reported outcome measure to assure quality and to monitor therapy in orthopaedics and musculoskeletal diseases. It is provided by the QUALITOUCH HC Foundation (Zürich, Switzerland) [10] and consists of eight questions related to pain/symptoms, quality of sleep, limitation of daily activities, general health condition and therapy outcome (Table 1). The AI is used in different clinical settings related to musculoskeletal pathologies. Patients are provided with a link to the online questionnaire at the start of the therapy and then a follow-up is sent every four weeks until the end of the therapy. When completing the questionnaire for the first time the therapist supports the patient, whereas the follow-ups are carried out by the patient from home. The AI was originally developed in German. In contrast to other PROMs the AI does not yield a final overall score, but every single question must be evaluated on its own. Therefore, the AI captures physical limitations of the individual patient in different dimensions.
The AI was developed for a broad application in orthopaedics and musculoskeletal diseases, but it can also be applied for physiotherapy/physical therapy. Several studies report the application of the AI in the case of rheumatoid arthritis patients [11], to document the progress of lower back pain [9] and to measure the quality of treatment in interventional pain therapy [12].
However, if it is to have a wider practical use, then reference values, i.e. corridors of expected patient outcomes, would be helpful. The addition of reference values could enhance the efficacy of the AI as it would allow therapists to compare the performance of an individual patient with an expected “standard performance”.
The aim of this study was to investigate whether diagnosis-specific reference values for the AI could be derived statistically. The idea was to establish a reference that would allow an assessment as to whether the physiotherapy of an individual patient was progressing as expected, when compared to a large sample. The intention is to contribute to an evidence-based assessment of the therapeutic progress, which will in turn improve quality management in physiotherapy.
Methods
The study aimed at developing statistically based reference values for the application of the AI. Four diagnoses at different body regions were chosen as clinical examples, all being of high clinical relevance, i.e. commonly seen in physiotherapy practice: chronic lower back pain (ICD-10: M54.4, M54.5, M54.8), tibia posterior syndrome (ICD-10: M77.5, M76.8, M21.4), knee joint arthrosis (ICD-10: M17.0, M17.1, M17.2, M17.4, M17.5, M17.9) and shoulder impingement (ICD-10: M75.4, M75.5).
Study design
A retrospective study design using large samples of existing, fully anonymized clinical data was implemented. Data covering the period from 2010 to 2020 were available for analysis. While the AI data were collected by the QUALITOUCH HC Foundation, the clinical data were obtained from the two medical centres of the health-care provider Spiraldynamik® in Switzerland [13]. This health-care provider specializes in non-operative orthopaedic therapy of out-patients. Treatments related to the diagnoses chosen for this study generally involve patients receiving physiotherapy.
Data sources
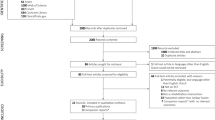
In a first step, the data received from the two sources were merged and pre-processed. Spiraldynamik® provided data referring to 21,183 individual patients. As some of the patients had visited the medical centre more than once within the 10-year period of our analysis, these 21,183 patients accounted for 54,131 medical cases. For each case there is a unique identifier in the clinical data set.
QUALITOUCH HC Foundation provided data for 12,106 patients who were treated at Spiraldynamik® and who were provided with the AI at least once. While some of these patients did not return the AI, many of them underwent therapy lasting more than one month, during which they were provided with the AI for follow-ups. This resulted in a total of 30,460 AI responses in this data set.
Data sampling
The two data sets were merged into one basic data set, from which specific data sets were generated (Fig. 1). The patient ID served as a primary key in this process. Data were only included in the basic data set if the patient had returned at least one AI and had received at least one therapeutic consultation. The basic data set comprised a total of 11,948 patients. For each case of each patient the following parameters were included:
-
• Clinical data: age, sex, body mass index (BMI), diagnosis (coded according to ICD-10 German version);
-
• AI data: responses to questions Q1 – Q8 for the initial feedback and as many follow-ups as available.
From this basic data set, four sub-sets were derived, i.e. a separate data set was created for each of the chosen diagnoses.
Data analysis
This paper focusses on question 1 (Q1) and question 4 (Q4) of the AI. These two questions are related to (maximum) pain (Q1) and to limitations to perform household activities (Q4). Note that in line with the definition of the AI in Q1 patients rate their (maximum) pain according to a visual analogue scale of 0 to 10; this value is then transferred to a point system by multiplying it with the factor 10. This results in all responses of the AI being of the same scale as, for example, Q4 is also given in values up to 100.
All data processing and all statistical analyses were conducted using the programme R-Studio (® 2009–2020 RStudio, PBC, Version 1.3.1093).
The AI responses were investigated with respect to their development over time. For each diagnosis the overall progress of the AI was analyzed. All available data at every point in time was used, i.e. the number of available responses differs from follow-up to follow-up. For statistical analysis it was defined that a minimum of 100 responses must be available at a point in time; follow-ups with less data were not considered. By comparing the responses of the last available follow-up to the initial AI response, the overall trend was determined for each case. By comparing all responses available at one follow-up to the initial response, the stepwise progress was analysed. To select the appropriate statistical test, QQ-plots were used to check for normal distribution. If the data were normally distributed, the t-test was used. If the data were not normally distributed, the Wilcoxon test was performed. For the statistical tests, a significance level of α = 0.05 was defined.
Finally, reference corridors were derived as a linear trend line using the results for the initial AI response and the follow-up responses. Like clinical percentile curves (e.g. [14,15,16]), the corridors are presented as scatterplots, with the mean, 1st and 3rd quartiles as linear models.
Results
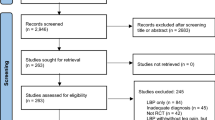
Table 2 summarizes the final data samples that were used in this study. A more detailed summary of the characteristics of the study population and the amount of data available at each follow-up is shown in the Additional file 1 (Table A1).
QQ-plots showed a normal distribution for all samples. Thus, a t-test was used for further comparisons, i.e. for analyzing the AI responses at different follow-ups in relation to the baseline value at the start of the therapy. As can be seen in Fig. 2, for all diagnoses addressed here the AI indicated a significant improvement of the maximum pain levels (Q1) from baseline to follow-up. For chronic lower back pain, for example, the corridor indicates that an initial average AI value related to maximum pain of 49.3 ± 23.8 points should be expected to improve by a therapeutic intervention to 36.9 ± 23.8 points on a first follow-up after four weeks, and then further to 35.7 ± 23.0 points on a second follow-up after eight weeks, and so on. Similarly, an initial average AI value related to pain and limitations in household activities of 37.5 ± 24.7 points should be expected to improve to 27.6 ± 22.7 points on the first follow-up, and to 26.6 ± 22.0 points on a second follow-up.
Likewise, Fig. 3 documents a significant improvement in the ability to perform household activities reported by the patients (Q4). The therapeutic success is also shown in the corresponding scatterplots (Figs. 2 and 3, right). All scatterplots indicate a decreasing mean. The scatterplots also feature a corridor that is represented by the mean and the 1st and 3rd quartiles, i.e. an AI outcome within the corridor covers 50% of the responses.
Discussion
In order to statistically derive diagnosis-specific reference values for the QUALITOUCH Activity Index (AI), two large data samples were successfully merged. The sample related to the AI was significantly smaller than the clinical data set, indicating that the AI was not issued and/or completed by all patients. Since the clinical partner also treats patients who are not the target population of the AI, this seems reasonable. Merging the data occasioned the loss of only a few entries, and these were all cases where the patient could not be identified in the clinical data set. Thus, the basic data set as derived here is as complete as possible and, due to its size, is regarded as a sound basis for further analysis, with high external validity and a strong informative value with regard to quality of care.
The patients included in the basic data set showed an average age of 50.1 years, an average BMI of 24.6 and a sex ratio of approximately one male to three females. The high proportion of women is remarkable, but age and BMI are in line with published data of the general Swiss population [17]. With respect to these overall descriptors, it can thus be assumed that the data reflect a representative population. The clearly increased age for the subsample of patients with knee osteoarthritis seems plausible given the degenerative nature of this pathology. However, additional characteristics that might have an influence on the therapeutic progress (e.g. education, occupation, comorbidities) could not be taken into account because those factors were not documented in either database.
For the statistical analysis, four different diagnoses were chosen. These cover different body regions, can be described as common fields of application in physiotherapy and require different therapeutic approaches. Therefore, it is believed that this choice can serve well as an example to demonstrate the impact of a generic PROM.
In contrast to other studies [18, 19], only one PROM was evaluated here, but it was evaluated with respect to several different diagnoses. The generic nature of the AI allowed this comparative analysis. Considering that physiotherapy is dealing with a variety of diagnoses in clinical practice, it seems reasonable and practical to use a single generic PROM instead of several diagnosis-specific ones. At the same time, this can be a limitation as analyses of diagnosis-specific aspects become more challenging, if not impossible. For this evaluation two dimensions of the AI were chosen, i.e. two questions. Both Q1 and Q4 are relevant for all patients. The maximum pain (Q1) was evaluated because it seems easier and more reliable to estimate than average pain (Q2). Discomfort during sleep (Q3) is known to be associated with pain and therefore was not used. Complaints during leisure time (Q5) and at work (Q6) were omitted in favour of focusing on household activities (Q4), which were deemed to represent some daily activity that is similarly relevant for patients of all age groups and socio-economic backgrounds.
As expected from other studies [9, 20], the AI did highlight a decline in pain and complaints after physiotherapy. For all diagnoses the AI documented a significant improvement between the first consultation and follow-up consultations. This confirms the assumption that the AI is a suitable instrument for recognising therapeutic progress and success.
The statistical procedure to derive reference corridors for expected AI progress over time was straightforward and rather simple, using a linear approximation. This reinforces the credibility and transparency of the results. The visualization as corridors allows for an easy comparison of the response of a specific patient with the statistical expectation. Hence, the corridors can be used as a monitoring tool to support both the therapist and the patient. In this way, it can be assessed whether the course of the therapy corresponds to the norm and whether it has an effect on the patient (per the different dimensions of the AI). This tool can thus quantify the effect of the therapy on the patient and complement the hands-on experience of the therapist.
Although the amount of data available was enormous, there were a few limitations in addition to those already mentioned above. In our data sample one patient can have multiple cases, and the AI questionnaire was issued for each different case, thus all were considered in the evaluation. This means that individual patients are represented several times, which could have an influence on the AI score (e.g. if chronically ill / with multiple diagnoses). Furthermore, many patients only filled in the AI at the beginning of their therapy, with a huge drop-off in the numbers of further follow-ups. To some extent this can be explained by the fact that some patients only needed a few sessions to complete their therapy and hence they stopped returning the AI at a follow-up. Others might have had poor compliance. From the data used here, it is unknown why any given patient stopped returning the AI. From a statistical point of view, while the declining number of responses from follow-up to follow-up can be explained, it does introduce uncertainties.
Besides these limitations, the established reference corridors offer a variety of opportunities related to quality of care. The use of PROMs involves the patient and contributes to considering the patient’s needs and identifying any unmet needs. Patients who are not responding well to therapy or whose success is stagnating can be identified early and options to adjust the therapy can be considered. This might also be helpful for decision-making, e.g. when weighing up conservative therapy versus surgical intervention. Using a reference corridor to compare the individual progress against a statistical expectation might help in this respect, and also in managing patient expectations. If, for example, a patient with knee joint arthrosis shows an AI score for Q1 of 70 points at the initial consultation, the reference corridor indicates that this patient is at the upper end of the statistical expectation. With this information the therapist can ensure the patient is closely monitored and if the score is reduced below 50 points at the third follow-up, the therapist is assured that such reduction represents the norm for this patient group indicating that the therapy seems to be successful whereas other patients only start a therapy with the same score. When using a PROM that covers different dimensions, as the generic AI does, a reference corridor can also be of help in prioritizing therapeutic aims and thus personalizing the intervention based on the expected outcome.
In addition to monitoring individual progress, quality control of an entire patient cohort from, for example, one physiotherapy practice is possible, and the therapeutic success of the practice can be documented and compared to the reference cohort. This enables practices to demonstrate the quality of their therapy, e.g. for health insurers [21], which is in line with current trends moving the health-care system towards a pay-for-performance system.
Future research should complement AI corridors for other diagnoses and provide corridors for further PROMs. Likewise, a predictive model in the form of a factor analysis could be a possibility to investigate in greater detail the predictive power of different influencing factors on such reference corridors. Table 2 already indicates several factors, such as age or BMI, that should be included in such a factor analysis. Further dimensions of the AI and medical aspects, such as comorbidity, should then also be included. Likewise, lifestyle related factors can be integrated to specify different peer groups to whom the reference corridors can be applied. The implementation of reference corridors in clinical practice and an evaluation of its impact can further contribute to discussion about evidence-based quality management in physiotherapy.
Conclusions
Based on the evaluation of clinical data for a period of 11 years, this study demonstrated that PROMs have the potential to provide a basis for monitoring therapeutic progress. This evidence-based approach contributes to quality management in physiotherapy as it complements the hands-on experience of the therapist. A statistical approach using four exemplary diagnoses and two dimensions of the generic QUALITOUCH Activity Index – one related to pain and one related to limitations in daily activities daily activities – allowed us to establish reference corridors of expected progress. These reference corridors can be used to compare the individual performance of a patient to the expected progress based on a large sample of self-reported data. A data-based monitoring of the therapeutic success can assist in different aspects of planning and managing a therapy. It can, for instance, be consulted to manage patient expectations and to check for unmet needs, to identify and document more complex cases, to personalize the intervention to ensure it is centred on the patient’s needs, to address specific aspects in which an underperformance was recognized, or to help in deciding whether to continue a conservative path or consider surgery. Furthermore, such reference corridors can be useful for more general discussions in health care, for example, matters relating to compensation or the impact of novel therapeutic approaches.
Availability of data and materials
The data sets generated and/or analyzed during the current study are not publicly available but can be made available from the corresponding author on reasonable request and if agreed by the clinical partners.
Abbreviations
- AI:
-
QUALITOUCH Activity Index, the AI is a questionnaire consisting of eight items
- BMI:
-
Body mass index
- PREM:
-
Patient-reported experience measures
- PROM:
-
Patient-reported outcome measures
References
Zeppieri G, George SZ. Patient-defined desired outcome, success criteria, and expectation in outpatient physical therapy: A longitudinal assessment. Health Qual Life Outcomes. 2017;15(1):1–11.
Verburg AC, van Dulmen SA, Kiers H, Nijhuis-van der Sanden MWG, van der Wees PJ. Development of a standard set of outcome measures for non-specific low back pain in Dutch primary care physiotherapy practices: a Delphi study. Eur Spine J. 2019;28(7):1550–64. https://doi.org/10.1007/s00586-019-05962-x (Available from).
Siljander MP, McQuivey KS, Fahs AM, Galasso LA, Serdahely KJ, Karadsheh MS. Current trends in patient-reported outcome measures in total joint arthroplasty: a study of 4 major orthopaedic journals. J Arthroplasty. 2018;33(11):3416–21.
Aaronson N, Elliott T, Greenhalgh J, Halyard M, Hess R, Miller D. et al. User’s guide to implementing patient-reported outcomes assessment in clinical practice. 2015. https://www.isoqol.org/wp-content/uploads/2019/09/2015UsersGuide-Version2.pdf. Accessed 3 Oct 2022.
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40.
Collins NJ, Prinsen CAC, Christensen R, Bartels EM, Terwee CB, Roos EM. Knee injury and osteoarthritis outcome score (KOOS): systematic review and meta-analysis of measurement properties. Osteoarthr Cartil. 2016;24(8):1317–29.
Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160–4.
Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72.
Roth P, Gengenbacher M, Theiler R. Activity-index, ein internetbasierender Patientenfragbogen zur Verlaufsdokumentation von Rückenschmerzen (Low Back Pain) – Vergleich des „Activity Index“ und des SF-12 in einer Pilotstudie TT - Activity Index – an Internet-Based Patient Questionnaire fo. Phys Medizin, Rehabil Kurortmedizin. 2012;22(03):138–41.
Qualitouch HC, Foundation for Quality Assurance in Health Care. 2022. http://qualitouch-hc.org/. Accessed 3 Oct 2022.
Ren L. Monitoring the time course of disability through a self-assessment instrument “activity index” (IA) in RA patients. J Rheum Dis Treat. 2018;4:3.
Kirrstetter AR, Brenig C, Gengenbacher M, Meier B, Ott A, Theiler R. Experience in measuring the quality of treatment in interventional pain therapy : The Activity Index on a touchscreen PC. Schmerz. 2017;31(2):131–8.
Jencik R, Binzer F, Larsen C. Spiraldynamik®, intelligent movement. 2022. https://www.spiraldynamik.com/. Accessed 3 Oct 2022.
Hemmelmann C, Brose S, Vens M, Hebebrand J, Ziegler A. Perzentilen des Body-Mass-Index auch für 18- bis 80-Jährige? Daten der Nationalen Verzehrsstudie II. Dtsch Medizinische Wochenschrift. 2010;135(17):848–52.
Jeffries LM, Laforme Fiss A, Westcott McCoy S, Bartlett D, Avery L, Hanna S. Developmental trajectories and reference percentiles for range of motion, endurance, and muscle strength of children with cerebral palsy. Phys Ther. 2019;99(3):329–38.
Vanhelst J, Ternynck C, Ovigneur H, Deschamps T. Normative health-related fitness values for French children: The diagnoform programme. Scand J Med Sci Sports. 2020;30(4):690–9. https://doi.org/10.1111/sms.13607 (Available from).
Statistical Data on Switzerland 2020 Neuchâtel: Bundesamt für Statistik (BFS); 2020. https://www.bfs.admin.ch. Accessed 3 Oct 2022.
Kyte DG, Calvert M, van der Wees PJ, ten Hove R, Tolan S, Hill JC. An introduction to patient-reported outcome measures (PROMs) in physiotherapy. Physiotherapy. 2015;101(2):119–25. Available from https://www.sciencedirect.com/science/article/pii/S0031940614001138.
Ostendorf M, van Stel HF, Buskens E, Schrijvers AJP, Marting LN, Verbout AJ, et al. Patient-reported outcome in total hip replacement. J Bone Joint Surg Br. 2004;86-B(6):801–8. https://doi.org/10.1302/0301-620X.86B6.14950 (Available from).
Sohil P, Hao PY, Mark L. Potential impact of early physiotherapy in the emergency department for non-traumatic neck and back pain. World J Emerg Med. 2017;8(2):110–5.
Westby MD, Klemm A, Li LC, Jones CA. Emerging role of quality indicators in physical therapist practice and health service Delivery. Phys Ther. 2016;96(1):90–100.
Acknowledgements
The authors are grateful to Dr Christian Larsen, Spiraldynamik®, and Prof. Dr Robert Theiler, Qualitouch HC Foundation, for providing the data for this study and to Ms. Rachel Pierce, Verba Editing House, for editing the manuscript.
Funding
This study was funded from the authors’ own internal sources.
Author information
Authors and Affiliations
Contributions
MZ prepared the data samples and performed the statistical analysis. HB and KUS contributed to the design of the study, the analysis and the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study made use of anonymized clinical routine data. All data were provided to the authors in anonymous form, i.e. the authors are unable to identify individual patients. The focus of this study is on quality, hence the study does not fall under the Swiss Human Research Act.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table A1.
Study population and number of data points considered in the evaluation. It was defined that a minimum of 100 responses was required to include the follow-up in the statistical analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zaugg, M., Baur, H. & Schmitt, KU. Applying patient-reported outcome measures (PROMs) in physiotherapy: an evaluation based on the QUALITOUCH Activity Index. Arch Physiother 12, 27 (2022). https://doi.org/10.1186/s40945-022-00152-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40945-022-00152-3