Abstract
Purpose
To describe the common risk factors, complications, and management options for anterior migration of Ozurdex implant.
Methods
A comprehensive review of the literature was performed.
Results
Amongst the most common risk factors predisposing to implant anterior migration we found a history of pseudophakia or aphakia or previous vitrectomy. The most common complication is that of corneal edema.
Conclusions
A variety of management options to treat migration of the dexamethasone implant are utilized by different specialists around the world. These depend on the doctor’s preference, presence of corneal damage and history of previous migrations after repositioning the implant. The most common approaches are operative or non-operative implant repositioning and surgical implant removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Ozurdex® is a 0.46 mm in diameter and 6 mm in length biodegradable dexamethasone implant that uses the NOVADUR® drug delivery technology, in which a biodegradable material is paired with an active drug. The implant is injected within the vitreous where it slowly releases 0.7 mg of dexamethasone. It is being used to treat macular edema secondary to pathologies such as branch or central retinal vein occlusion (BRVO/CRVO), diabetic macular edema and non-infectious posterior uveitis [1,2,3].
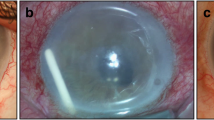
An uncommon but significant complication of Ozurdex® use is its migration to the anterior chamber of the eye. The risk factors of this complication include previous complicated intraocular lens (IOL) implantation, previous vitrectomy, iris reconstruction, aphakic or pseudophakic eyes, zonular dehiscence and open or defective lens capsule [3,4,5,6,7,8,9]. If the implant is not repositioned or removed in due course, this anterior migration can lead to increased intraocular pressure and corneal edema. In case corneal edema does not resolve the only management option to restore vision can often be a keratoplasty [2, 10, 11].
Methods
A comprehensive literature search was conducted in November 2023 using PubMed, Google Scholar, ScienceDirect and was limited to studies published in English and until November 2023. The search strategy used the following terms: ozurdex migration, dexamethasone implant migration, anterior displacement of ozurdex. We found 38 relevant papers (case-reports, research articles and case-series) published in peered reviewed journals.
Results
Out of the 59 patients reviewed, 60% were male, and 40% were female. The average age of the patients was 66 years, indicating that this issue can affect individuals across different age groups.
Most patients (98%) had at least one established risk factor for implant migration in the eye, with a history of pseudophakia and/or vitrectomy being the most common. Other factors included posterior vitrectomy, iris claw lens, iris sutured IOL, and various complications associated with prior ocular procedures.
A significant majority (83%) of the patients experienced serious complications following anterior migration of the Ozurdex implant. These complications primarily included corneal edema, which can severely impact visual acuity and patient comfort. The remaining 17% showed either no complications or only a mild rise in intraocular pressure (IOP).
Various management options were employed to address the complications resulting from anterior migration. The most common approach was surgical removal, chosen in 54% of the cases. Surgical repositioning, whether through surgical techniques (27%) or non-surgical means (14%) like postural maneuvering and ocular massage, was utilized in 41% of the cases. Only 5% of the patients followed a “watch and wait” approach, with the implant eventually dissolving on its own.
Table 1 below shows the findings of the literature review regarding the ophthalmic history of the affected eyes, complications after anterior migration and final management.
Discussion
The biodegradable dexamethasone implant Ozurdex® has been designed for use in patients suffering from macular edema secondary to CRVO, BRVO, diabetes, and non-infectious uveitis.
Jirarattanasopa et al. [41] demonstrated the effectiveness of Ozurdex in improving the best corrected visual acuity and the central retinal thickness in patients suffering from macular edema secondary to retinal vein occlusion (RVO) or diabetic retinopathy (DR). The greatest improvement was found in RVO patients and in treatment naïve eyes than in previously treated eyes. Scarammuzi et al. [42] also noted that the use of Ozurdex in diabetic macular edema (DME) can result in long term meaningful benefits while avoiding the significant side effects expected after intraocular corticosteroid injections. Likewise, Massa et al. [43] showed that Ozurdex use in macular edema secondary to non-infectious uveitis provides promising results both in terms of improving visual acuity as well as macular anatomy.
Furthermore, recent studies have emerged, arguing that Ozurdex can also be used for other pathologies such as macular edema arising after vitrectomy or cataract surgery [44, 45] or even exudative AMD [46].
A rare complication of Ozurdex implant is anterior migration from the vitreous cavity to the aqueous chamber. Several large scale studies conducted have shown the overall prevalence of this complication varying from 0.63% to 1.60%, with the incidence being significantly higher in eyes affected by established risk factors [5, 6]. The common risk factors for implant migration include aphakic or pseudophakic eyes, history of vitrectomy, reconstructed iris, zonular dehiscence and open or defective lens capsule [3,4,5, 7,8,9, 11].
The vitreous chamber which under normal circumstances would “hold” the implant in place is filled with aqueous humor if an eye is vitrectomized, allowing the implant to move. This state allows the implant to migrate anteriorly unopposed through the pupil especially in aphakic eyes. Even in pseudophakic eyes the implant can circumvent the pupil and reach the aqueous chamber if there is zonular weakness, disrupted posterior lens capsule or pass through an iridotomy if one is present [6, 20].
The most common and serious complication due to this migration is endothelial decompensation and corneal edema, followed by rise in IOP. The underlying mechanism of corneal edema and endothelial decompensation formation is proposed to be associated either to the chemical toxicity of the components of the implant (Dexamethasone, glycolic acid, lactic acid) or due to direct mechanical trauma to the cornea. The risk of occurrence of corneal edema increases in cases of earlier migration (< 3 weeks) compared to the ones that occur later and also in cases where the corneal endothelial cell count is already reduced [30, 31].
In terms of management of the anterior migration of Ozurdex, several options exist and depend on the surgeon’s preference, presence of corneal damage and history of previous migrations after repositioning the implant [9, 19, 20].
In the cases reviewed, the option of observing with close monitoring until the implant dissolves was followed solely in circumstances were there was no corneal edema or rise in IOP [3, 8, 14, 16]. A very common management option was that of implant repositioning to the vitreous chamber, achieved either conservatively or surgically. This option however, carries the risk of the implant remigrating back to the AC [9]. The non-operative technique involves application of a mydriatic agent followed by posturing and positioning the patient’s globe in such a way that the implant becomes dislodged from the anterior chamber and with the help of gravity re-migrates back into the vitreous chamber. This technique may be aided with corneal manipulation using a cotton tip applicator or ocular massage [11, 15, 19, 21, 25]. On the other hand, the operative methods to reposition the implant can vary. In many cases, a limbal incision was made and after insertion of viscoelastic material to protect the anterior chamber structures, the surgeon using a 30-gauge needle injected balanced salt solution to obtain implant repositioning to the vitreous cavity. Other surgeons chose to dislodge the implant from the anterior chamber using a paracentesis then using a Sinskey hook position it near the pupil margin and then through the ruptured posterior capsule back behind the IOL. A common step amongst various techniques was the use of miotic agents such as Miochol after the repositioning to help prevent recurrence of anterior migration [11, 17, 20, 29].
Finally, the course of action selected can be that of surgical removal of the implant, which is particularly common, in cases of severe corneal edema, rise in IOP or multiple failed repositioning attempts. Various techniques for removal have been described. The use of a needle attached to a syringe to aspirate the implant is the most commonly used. During this technique a paracentesis is made and a needle (usually 23-Gauge) is advanced into the anterior chamber. The dexamethasone implant is then aligned with the axis of the needle and as suction is applied, the implant is effectively grasped and eventually pulled out of the eye [1, 11, 24, 26]. Other surgical techniques include use of vitrectomy instruments and forceps [47], use of a lens injector [28] or an IV cannula [22] all of which aim to capture the implant and advance it out of the anterior chamber paracentesis. However some other approaches prioritize minimization of contact between instruments and the implant in order to reduce the chance of breaking it into fragments [12]. These include use of BSS to direct flow through a corneal incision and out of the AC [4, 10] and similarly a no-touch technique using viscoelastic to allow implant egression. In this no-touch technique, a corneal incision is made and the implant is positioned parallel to the incision’s axis. A viscoelastic cannula is advanced distal to the implant and while viscoelastic is injected into the AC, the corneal incision is depressed to direct the flow of viscoelastic out of the incision and thus encouraging the implant to flow outwards with it. This has been proposed as a low risk, cost effective technique [18, 27].
Conclusion
Migration of Ozurdex in the anterior chamber although rare is a complication that can cause severe damage to the affected eye mostly due to corneal edema and decompensation with eventual need for corneal transplant. Prompt detection and management of such complication is needed. A variety of management options exist which can either involve an operation or just use of maneuvers to reposition the implant back in the vitreous cavity.
Availability of data and materials
The data used or analysed during the current study is available from the corresponding author on reasonable request.
Abbreviations
- CRVO:
-
Central retinal vein occlusion
- BRVO:
-
Branch retinal vein occlusion
- IOL:
-
Intraocular pressure
- AC:
-
Anterior chamber
- VC:
-
Vitreous chamber
- PPV:
-
Pars plana vitrectomy
- IOL:
-
Intraocular lens
- PCIOL:
-
Posterior chamber intraocular lens
- SFIOL:
-
Scleral fixated intraocular lens
- DMEK:
-
Descemet membrane endothelial keratoplasty
- DSAEK:
-
Descemet stripping automated endothelial keratoplasty
- SFPCIOL:
-
Sutureless scleral-fixated posterior chamber intraocular lens
- ACIOL:
-
Anterior chamber intraocular lens
- PI:
-
Peripheral iridotomy
References
Stepanov A, Codenotti M, Ramoni A, et al. Anterior chamber migration of dexamethasone intravitreal implant (Ozurdex®) through basal iridectomy (Ando) in a pseudophakic patient. Eur J Ophthalmol. 2015;26(3):e52–4. https://doi.org/10.5301/ejo.5000715.
Pardo-López D, Francés-Muñoz E, Gallego-Pinazo R, Díaz-Llopis M. Anterior chamber migration of dexametasone intravitreal implant (Ozurdex®). Graefe’s Arch Clin Exp Ophthalmol. 2012;250(11):1703–4. https://doi.org/10.1007/s00417-011-1802-x.
Zafar A, Aslanides IM, Selimis V, Tsoulnaras KI, Tabibian D, Kymionis GD. Uneventful anterior migration of intravitreal ozurdex implant in a patient with iris-sutured intraocular lens and descemet stripping automated endothelial keratoplasty. Case Rep Ophthalmol. 2018;9(1):143–8. https://doi.org/10.1159/000486924.
Glidai Y, Schwartz S, Cohen E. Dexamethasone implant migration through an iris coloboma. Case Rep Ophthalmol. 2020;11(1):73–8. https://doi.org/10.1159/000505638.
Gonçalves MB, de Alves BQ, Moura R, et al. Intravitreal dexamethasone implant migration into the anterior chamber: a multicenter study from the pan-american collaborative retina study group. Retina. 2020;40(5):825–32. https://doi.org/10.1097/IAE.0000000000002475.
Röck D, Bartz-Schmidt KU, Röck T. Risk factors for and management of anterior chamber intravitreal dexamethasone implant migration. BMC Ophthalmol. 2019;19(1):1–7. https://doi.org/10.1186/s12886-019-1122-1.
Celik N, Khoramnia R, Auffarth GU, Sel S, Mayer CS. Complications of dexamethasone implants: risk factors, prevention, and clinical management. Int J Ophthalmol. 2020;13(10):1612–20. https://doi.org/10.18240/ijo.2020.10.16.
Majumder PD, Palkar AH, Pathare N, Biswas J. Anterior chamber migration of a sustained-release dexamethasone intravitreal implant: a case report and review of literature. Oman J Ophthalmol. 2019;12(2):133–7. https://doi.org/10.4103/ojo.OJO_5_2018.
Rahimy E, Khurana RN. Anterior segment migration of dexamethasone implant: Risk factors, complications, and management. Curr Opin Ophthalmol. 2017;28(3):246–51. https://doi.org/10.1097/ICU.0000000000000365.
Gullapalli VK, DiLoreto DA. Migration of intravitreal dexamethasone implant to anterior chamber. Retin Cases Br Reports. 2013;7(1):111–3. https://doi.org/10.1097/ICB.0b013e31826f08b0.
Kayıkcıoğlu Ö, Doğruya S, Sarıgül C, Mayalı H, Kurt E. Anterior chamber migration of ozurdex implants. Turkish J Ophthalmol. 2020;50(2):115–22. https://doi.org/10.4274/tjo.galenos.2019.43778.
Jamshidi F, Shah VA, Riaz KM. Migration of a dexamethasone intravitreal implant to the anterior chamber in a patient with a intra-scleral haptic fixated intraocular lens. Int Med Case Rep J. 2021;14(July):633–5. https://doi.org/10.2147/IMCRJ.S327406.
Jonas JB, Schmidbauer M. Steroid implant in anterior chamber of an aphakic vitrectomized eye. Graefe’s Arch Clin Exp Ophthalmol. 2013;251(1):385–6. https://doi.org/10.1007/s00417-011-1839-x.
Goel N, Mehta A, Batra J, Choudhry R. Anterior chamber migration of intravitreal dexamethasone implant in an eye with scleral-fixated intraocular lens. J Ophthalmic Vis Res. 2020;15(4):581–3. https://doi.org/10.18502/jovr.v15i4.7798.
Srinivasan P, Jayadev C, Shetty R. The nomadic Ozurdex®: Anterior migration of the dexamethasone implant and back! Oman J Ophthalmol. 2017;10(2):109–11. https://doi.org/10.4103/0974-620X.209110.
Kocak N, Ozturk T, Karahan E, Kaynak S. Anterior migration of dexamethasone implant in a pseudophakic patient with intact posterior capsule. Indian J Ophthalmol. 2014;62(11):1086–8. https://doi.org/10.4103/0301-4738.146763.
Pacella F, Agostinelli E, Carlesimo SC, et al. Management of anterior chamber dislocation of a dexamethasone intravitreal implant: a case report. J Med Case Rep. 2016;10(1):2–5. https://doi.org/10.1186/s13256-016-1077-2.
Stewart JM. Modified no-touch technique for removal of a dexamethasone implant that has migrated to the anterior chamber. Ocul Immunol Inflamm. 2020;28(2):238–9. https://doi.org/10.1080/09273948.2018.1552760.
Ha JY, Jang JY, Ji Y-S. Management of anterior chamber migration of dexamethasone intravitreal implant. Korean J Ophthalmol. 2017;31(6):574. https://doi.org/10.3341/kjo.2017.0100.
Kang H, Lee MW, Byeon SH, Koh HJ, Lee SC, Kim M. The clinical outcomes of surgical management of anterior chamber migration of a dexamethasone implant (Ozurdex®). Graefe’s Arch Clin Exp Ophthalmol. 2017;255(9):1819–25. https://doi.org/10.1007/s00417-017-3705-y.
Kishore SA, Schaal S. Management of anterior chamber dislocation of dexamethasone implant. Ocul Immunol Inflamm. 2013;21(1):90–1. https://doi.org/10.3109/09273948.2012.736589.
Ku JY, Mercieca K, Yau K. Removal of a migrated dexamethasone implant (Ozurdex) from the anterior chamber using an intravenous cannula. BMJ Case Rep. 2021;14(7):2020–2. https://doi.org/10.1136/bcr-2020-240504.
Lee SH, Kang H, Byeon SH, et al. Simple technique for management of anterior chamber-migrated Ozurdex® implant. Acta Ophthalmol. 2018;96(7):e893–4. https://doi.org/10.1111/aos.13584.
Nguyen T, Wolfensberger T. Management of anterior chamber dislocation of a dexamethasone implant. Klin Monbl Augenheilkd. 2019;236(04):412–4. https://doi.org/10.1055/a-0808-1847.
Rivera-Pérez de Rada P, Fernández-Avellaneda P, Barturen Herraiz LT, et al. Hole-in-one: simple non-surgical technique for the management of anterior chamber migrated Ozurdex(®) implant. GMS Ophthalmol Cases. 2020;10:Doc05. https://doi.org/10.3205/oc000132.
Depla JAM, Veckeneer M, Bleyen I. Active removal of anterior segment-migrated dexamethasone implant (Ozurdex®). GMS Ophthalmol Cases. 2022;12:Doc08. https://doi.org/10.3205/oc000195.
Rahimy E, Pitcher JD, Abbey AM, Garretson BR, Haller JA. No-touch removal of anterior segment-migrated dexamethasone implant. Retina. 2015;35(11):2414–6. https://doi.org/10.1097/IAE.0000000000000753.
Ruiz-Casas D, Gros-Otero J, Casado A. Dexamethasone implant removal from anterior chamber: surgical technique. Retin Cases Br Reports. 2016;10(4):313–5. https://doi.org/10.1097/ICB.0000000000000335.
Vela JI, Crespí J, Andreu D. Repositioning of dexamethasone intravitreal implant (Ozurdex®) migrated into the anterior chamber. Int Ophthalmol. 2012;32(6):583–4. https://doi.org/10.1007/s10792-012-9604-7.
Bansal R, Bansal P, Kulkarni P, Gupta V, Sharma A, Gupta A. Wandering Ozurdex® implant. J Ophthalmic Inflamm Infect. 2012;2(1):1–5. https://doi.org/10.1007/s12348-011-0042-x.
Khurana RN, Appa SN, McCannel CA, et al. Dexamethasone implant anterior chamber migration: risk factors, complications, and management strategies. Ophthalmology. 2014;121(1):67–71. https://doi.org/10.1016/j.ophtha.2013.06.033.
Chang SM, St. Peter DM, Im LT, Munir WM, Schocket LS. Dexamethasone implant migration in an eye with congenital glaucoma: a case report and review of the literature. Eur J Ophthalmol. 2022;32(5):NP46–50. https://doi.org/10.1177/11206721211005696.
Eadie JA, Lesser R, Capone A. Migration of Ozurdex implant into the anterior chamber. Retin Cases Br Rep. 2012;6(3):269–70. https://doi.org/10.1097/ICB.0b013e3182258b08.
Kumar D, Dhawan A, Narayanan S, Agarwal A. Anterior chamber migration of intravitreal dexamethasone implant in glued intraocular lens. Indian J Ophthalmol. 2019;67(2):268. https://doi.org/10.4103/ijo.IJO_841_18.
Kumar A, Ambiya V, Kapoor G, Arora A. Wandering Ozurdex in eyes with scleral fixated intraocular lens and its management: a report of two cases. J Curr Ophthalmol. 2019;31(3):345–8. https://doi.org/10.1016/j.joco.2018.10.007.
Madi HA, Morgan SJ, Ghosh S. Corneal graft failure due to migration of Ozurdex™ implant into the anterior chamber. Am J Ophthalmol Case Rep. 2017;8:25–7. https://doi.org/10.1016/j.ajoc.2017.08.002.
Majumdar B, Sen A, Goel N, et al. Migration of dexamethasone implant (OZURDEX®) into the anterior chamber in a pseudophakic eye with an intact capsular bag. Indian J Ophthalmol—Case Rep. 2023;3(2):357. https://doi.org/10.4103/IJO.IJO_3127_22.
Marchese V, Piscitello S, Vaccaro C, Giunchiglia G. Management of intravitreal implant migration into the anterior chamber in a patient with a posterior chamber intraocular lens. JCRS Online Case Rep. 2014;2(2):e31–4. https://doi.org/10.1016/j.jcro.2014.03.003.
Chen CT, Chao SC, Lin HY. A wandering Ozurdex in the anterior chamber. Taiwan J Ophthalmol. 2023. https://doi.org/10.4103/tjo.TJO-D-23-00013. https://journals.lww.com/tjop/abstract/9000/a_wandering_ozurdex_in_the_anterior_chamber.99927.aspx
Stavrakas P, Gartaganis P, Totou S, Chalkiadaki E, Manousakis E, Karmiris E. Anterior chamber dislocation of dexamethasone implant in the presence of carlevale sutureless scleral fixation intraocular lens. Case Rep Ophthalmol. 2023. https://doi.org/10.1159/000529790.
Jirarattanasopa P, Jiranoppasakdawong S, Ratanasukon M, Bhurayanontachai P, Dangboon W. Results of intravitreal dexamethasone implant (Ozurdex®) for retinal vascular diseases with macular edema: an observational study of real-life situations. Medicine. 2022;101(27):e29807. https://doi.org/10.1097/MD.0000000000029807.
Scaramuzzi M, Querques G, La SC, Lattanzio R, Bandello F. Repeated intravitreal dexamethasone implant (ozurdex) for diabetic macular EDEMA. Retina. 2015;35(6):1216–22. https://doi.org/10.1097/IAE.0000000000000443.
Massa H, Georgoudis P, Panos GD. Dexamethasone intravitreal implant (OZURDEX®) for macular edema secondary to noninfectious uveitis: a review of the literature. Ther Deliv. 2019;10(6):343–51. https://doi.org/10.4155/tde-2019-0024.
Furino C, Boscia F, Recchimurzo N, Sborgia C, Alessio G. Intravitreal dexamethasone implant for refractory macular edema secondary to vitrectomy for macular pucker. Retina. 2014;34(8):1612–6. https://doi.org/10.1097/IAE.0000000000000105.
Klamann A, Böttcher K, Ackermann P, Geerling G, Schargus M, Guthoff R. Intravitreal dexamethasone implant for the treatment of postoperative macular edema. Ophthalmologica. 2017;236(4):181–5. https://doi.org/10.1159/000448057.
Calvo P, Ferreras A, Al Adel F, Wang Y, Brent MH. Dexamethasone intravitreal implant as adjunct therapy for patients with wet age-related macular degeneration with incomplete response to ranibizumab. Br J Ophthalmol. 2015;99(6):723–6. https://doi.org/10.1136/bjophthalmol-2014-305684.
Stelton CR, Townsend J, Peterson LT, Khurana RN, Yeh S. Surgical management of anterior chamber migration of a dexamethasone intravitreal implant. Ophthalmic Surgery, Lasers Imaging Retin. 2015;46(7):756–9. https://doi.org/10.3928/23258160-20150730-11.
Acknowledgements
Not applicable.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
Conceptualization, PT; methodology, PT; investigation, PT; writing—original draft preparation, PT, DK; writing—review and editing, PT; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors have declared that no competing interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tsoutsanis, P., Kapantais, D. Anterior migration of Ozurdex implant: a review on risk factors, complications, and management. Int J Retin Vitr 9, 74 (2023). https://doi.org/10.1186/s40942-023-00513-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40942-023-00513-5