Abstract
Background
With increasing incidence of yellow fever, mass campaign vaccinations are underway and little ophthalmological alterations have been reported in literature, specially regarding non-combined vaccines.
Case presentation
We report the case of a patient with no previous ocular or systemic diseases whom received a single dose of yellow fever vaccination and developed haematological, hepatic and renal alterations progressing with a later onset bilateral asymmetric diffuse uveitis. Ophthalmological findings included fine keratic precipitates scattered throughout the cornea and mild vitritis. Multimodal evaluation showed subtle puntiform choriocapillaris changes with decreased vascular density associated. The patient had a good visual outcome after mild oral prednisone dose, but the image findings have not presented remission.
Conclusions
Clinicians should be aware of clinical and subclinical ocular manifestations such as subtle puntiform choriocapillaris changes as possible vaccine-related adverse events with potential to impact vision.
Similar content being viewed by others
Background
Yellow fever (YF) incidence rates are increasing in many countries and in large cities in Brazil, such as São Paulo and Rio de Janeiro, a significant amount of lethal cases are occuring since 2017 [1] leading to mass vaccination campaigns. Due to emerging demand, federal government used fractional dose (one-fifth of original dose) to amplify vaccination coverage. Although reported among the safest vaccines available [2] serious adverse events such as neurological complications and also vasculopathies have been reported, mainly after combined vaccination against YF and other infectious entities [3, 4] with few information about isolated YF vaccine available. It remains unknown the interaction between the host immune system and the vaccine leading to those events [2].
Case presentation
A 50-year-old caucasian Brazilian woman with no previous ocular or systemic diseases received one dose of YF vaccine in April 2017 (Biomanguinhos/Fiocruz, lot 160VFA0433, Rio de Janeiro, Brazil). After 4 days, she presented with fever, hyporexia, and nausea. Laboratory studies showed leukopenia [3.340/mm3—normal range (NR): 3.500–11.500/mm3], thrombocytopenia (15.000/mm3—NR: 150.000–450.000/mm3), elevated C-reactive protein (33.34 mg/dL—NR: < 1.00 mg/dL), leukocyturia (80.000/mL—NR: < 30.000/mL), hematuria (29.000/mL—NR: < 12.000/mL), elevated creatinine (2.58 mg/dL—NR: 0.6–1.10 mg/dL), elevated urea (112 mg/dL—NR: 10–50 mg/dL), jaundice (bilirubin, 4.11 mg/dL—NR: 0.00–0.30 mg/dL), and moderately increased liver enzymes. The patient was admitted for intensive care with support measures and monitorization. Other infectious entities such as Cytomegalovirus, Herpes, Measles, Toxoplasmosis, Dengue Fever and Hepatitis A were discarded.
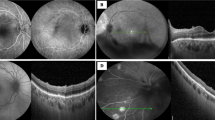
Approximately 12 days after symptoms onset, she reported blurred vision bilaterally. When ophthalmological evaluation was performed best-corrected visual acuity was 20/20 in right eye and 20/30 in left eye, and intraocular pressure was normal bilaterally. Examination showed fine keratic precipitates scattered throughout the cornea (Fig. 1) and mild vitritis bilaterally. Neither fundoscopy nor colored fundus photography have presented any relevant information but a discrete opacity of means in both eyes. Late-stage fluorescein angiography showed focal hyperfluorescence dots at the posterior pole in the left eye (Fig. 2); fundus autofluorescence disclosed widely dispersed hypoautofluorescent dots that were more prominent ipsilaterally. Cross-sectional optical coherence tomography (OCT) showed bilateral epiretinal membranes. Structural en-face OCT (Avanti, Optovue, Fremont, CA) shows diffuse hyporeflective dots at the level of the choriocapillaries with decrease vessel density at the same location with OCT angiography in the left eye (Fig. 3). Bilateral asymmetric diffuse uveitis was diagnosed and oral prednisone (40 mg/daily) was started. Patient was reevaluated after a week with same findings, and every two weeks after with progressive improvement. After 40 days inflammation signs disappeared, hence improving fundus imaging. Neither remission of the changes in the choriocapillaris nor vascular changes where seen during this period. Patient had no more visual complaints and visual acuity has improved to 20/20 OU.
Discussion and conclusions
Only in Brazil, approximately 20 million people are expected to be vaccinated against YF and many reactions can occur [5]. Likewise the recent epidemics of Zika virus and Ebola virus associated uveitis [6], YF may have a polymorphic presentation.
We present a case of a rare adverse reaction to yellow fever vaccine in which a patient developed acute panuveitis. The possible etiologies of our patient’s reaction include a type IV hypersensitivity to the vaccine or its components. The yellow fever vaccine is known to contain live attenuated virus, gelatin, and sorbitol. A review of the literature identified multiple published reports of a gelatin hypersensitivity causing symptoms of anaphylaxis, but there has never been a published case report of a type IV hypersensitivity reaction to gelatin [7]. Sorbitol has also been implicated, albeit rarely, in type I hypersensitivity [8, 9].
Likewise our patient, Biancardi and Moraes [10] reported ocular inflammation cases after YF fractional dose vaccination but presenting as anterior or intermediate uveitis. Stangos et al. [11] as well as Escott et al. reported cases of posterior uveitis but secondary to combined vaccination for yellow fever and hepatitis A. All cases, similarly to our patient, had good outcomes with inflammation remission overtime.
It is interesting to note that En face OCT, OCT angiography and autofluorescence findings correlate well and are more severe in the left eye. Although the presence of an epiretinal membrane, a frequent finding in patients with posterior uveitis [12] and the fact that many studies have described FAF findings in uveitis involving the outer retina and choroid a predictor of poor central visual acuity, it was not what we found in our case.
We also agree with other authors [10] that it’s not possible to establish direct causal correlation between vaccination and uveitis, only presumable. But the increasing amount of reports with similar temporal correlations should draw our attention to a new spectrum of adverse reactions, especially in association to isolated YF vaccines.
Clinicians should be aware of clinical and particularly subclinical ocular manifestations such as subtle puntiform choriocapillaris changes as possible vaccine-related adverse events with potential to impact vision.
Availability of data and materials
Data is contained within the patient’s medical record and will not be distributed.
Abbreviations
- NR:
-
normal range
- OCT:
-
optical coherence tomography
- YF:
-
yellow fever
References
SÃO PAULO, Secretaria de Estado da Saúde. Coordenadoria de Controle de Doenças, Centro de Vigilância Epidemiológica Prof. Alexandre Vranjac. Boletim Epidemiológico Febre Amarela, São Paulo, 29 de janeiro de 2018. Disponível em. http://www.saude.sp.gov.br/resources/cve-centro-de-vigilancia-epidemiologica/areas-de-vigilancia/doencas-de-transmissao-por-vetores-e-zoonoses/doc/famarela/fa18_boletim_epid_2901.pdf. Acesso em: 30 jan. 2018.
Martins RDM, Leal MDLF, Homma A. Serious adverse events associated with yellow fever vaccine. Hum Vaccin Immunother. 2015;11(9):2183–7. https://doi.org/10.1080/21645515.2015.1022700.
Moysidis SN, Koulisis N, Patel VR, et al. The second blind spot. Retinal Cases Brief Rep. 2017;11:S18. https://doi.org/10.1097/icb.0000000000000391.
Escott S, Tarabishy AB, Davidorf Fh. Multifocal choroiditis following simultaneous hepatitis A, typhoid, and yellow fever vaccination. Clin Ophthalmol. 2013;7:363–5. https://doi.org/10.2147/opth.s37443.
Ministério da Saúde alinha estratégia de campanha para a febre amarela. Ministério da Saúde. 2018; published online Jan 24. http://portalms.saude.gov.br/noticias/agencia-saude/42369-ministerio-da-saude-alinha-estrategia-de-campanha-para-a-febre-amarela. Accessed Jan 30 2018.
Connors DB, Shantha JG, Yeh S. Emerging causes of viral-associated uveitis. Int Ophthalmol Clin. 2015;55(2):103–13. https://doi.org/10.1097/iio.0000000000000068.
Sakaguchi M, Nakayama T, Inouye S. Food allergy to gelatin in children with systemic immediate-type reactions, including anaphylaxis, to vaccines. Pt 1. J Allergy Clin Immunol. 1996;98:1058–61.
Hino H, Kasai S, Hattori N, Kenjo K. A case of allergic urticaria caused by erythritol. J Dermatol. 2000;27:163–5. https://doi.org/10.1111/j.1346-8138.2000.tb02143.x.
Shirao K, Inoue M, Tokuda R, Nagao M, Yamaguchi M, Okahata H, Fujisawa T. “Bitter sweet”: a child case of erythritol-induced anaphylaxis. Allergol Int. 2013;62:269–71. https://doi.org/10.2332/allergolint.12-le-0517.
Biancardi AL, Moraes HV. Anterior and intermediate uveitis following yellow fever vaccination with fractional dose: case reports. Ocul Immunol Inflamm. 2018. https://doi.org/10.1080/09273948.2018.1510529.
Stangos A, Zaninetti M, Petropoulos I, Baglivo E, Pournaras C. Multiple evanescent white dot syndrome following simultaneous hepatitis-A and yellow fever vaccination. Ocul Immunol Inflamm. 2006;14(5):301–4.
Nicholson BP, Zhou M, Rostamizadeh M, et al. Epidemiology of epiretinal membrane in a large cohort of patients with uveitis. Ophthalmology. 2014;121:2393–8.
Acknowledgements
Not applicable.
Funding
Not Applicable
Author information
Authors and Affiliations
Contributions
PMM: data collection, manuscript preparation; all other authors: critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Patient has provided consent for all the data obtained.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Marinho, P.M., Nascimento, H., Romano, A. et al. Diffuse uveitis and chorioretinal changes after yellow fever vaccination: a re-emerging epidemic. Int J Retin Vitr 5, 30 (2019). https://doi.org/10.1186/s40942-019-0180-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40942-019-0180-0