Abstract
Background
Prader–Willi syndrome (PWS) is a genetic disease caused by loss of expression of the paternally inherited copy of several genes on the long arm of chromosome 15. Ophthalmic manifestations of PWS include strabismus, amblyopia, nystagmus, hypopigmentation of the iris and choroid, diabetic retinopathy, cataract and congenital ectropion uvea. An overlap between PWS and oculocutaneous albinism (OCA) has long been recognized and attributed to deletion of OCA2 gene located in PWS critical region (PWCR).
Case report
A 30-year-old male patient with PWS presented with vision loss in his left eye. His right eye had normal visual acuity. Multimodal imaging revealed absence of a foveal depression and extremely reduced diameter of the foveal avascular zone in the right eye and an inactive type 2 macular neovascular lesion in the left eye.
Conclusions
We report a presumed association of fovea plana and choroidal neovascularization with PWS. The use of multimodal imaging revealed novel findings in a PWS patient that might enrich our current understanding of the overlap between PWS and OCA.
Similar content being viewed by others
Background
Prader–Willi syndrome (PWS) is a complex genetic disorder caused by lack of expression of the paternally-inherited PWS critical region (PWCR) of chromosome 15q11.2-q13. It affects 1:10,000–1:30,000 live births. The clinical spectrum of PWS varies widely among different patients and evolves throughout their development [1]. Diagnostic hallmarks include infantile hypotonia and global developmental delay. Excessive eating during childhood usually results in central obesity which, if uncontrolled, predisposes 25% of patients to develop type 2 diabetes mellitus (DM) later in life. Mild intellectual impairment, hypogonadism and sleep apnea have been also associated with PWS. Patients often exhibit characteristic facial features such as almond-shaped up-slanted palpebral fissures, thin upper lip and small mouth with down-turned corners [2].
Ocular abnormalities reported in the context of PWS include strabismus, refractive errors, abnormal stereopsis, amblyopia and nystagmus [1]. Hypopigmentation of the iris and choroid, diabetic retinopathy, cataract, congenital ocular fibrosis syndrome and congenital ectropion uvea have also been reported [3]. Table 1 summarizes intraocular findings reported by previous publications in the context of PWS [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Herein, we report novel ocular findings in a case of PWS using multimodal imaging.
Case report
A 30-year old male patient was referred to our tertiary retina clinic at the University of California Irvine for evaluation of a macular lesion and long-standing vision drop in his left eye. He had a diagnosis of PWS. He was on insulin therapy for type 2 diabetes mellitus, amlodipine for hypertension and testosterone replacement therapy for hypogonadism. He had a history of strabismus surgery in his left eye at the age of 2 years.
His best-corrected visual acuity (BCVA) was 20/20 in his right eye and 20/150 in his left eye. Intraocular pressure was 16 mmHg in both eyes. He had full visual fields on confrontation. His eyes were orthophoric with full ocular motility in all cardinal directions and no nystagmus. Anterior segment examination was unremarkable. No iris transillumination was noted.
Fundus examination of both eyes revealed mild hypertensive retinopathy and mild nonproliferative diabetic retinopathy (NPDR). His left fundus showed a subfoveal disciform scar surrounded by a large area of pigmentary disturbance and mottling.
Green (532 nm) fundus autofluorescence (FAF) imaging revealed normal FAF in the right eye, while the left eye showed an area of central decreased FAF surrounded by a ring of increased FAF, which was, in turn, surrounded by an area of decreased FAF. This triple zone corresponds to the disciform scar and surrounding areas of retinal pigment epithelial disturbance and atrophy. There was a large area of mildly increased FAF surrounding the triple zone and occupying almost the entire macula. The latter area most likely corresponded to diseased retinal pigment epithelium (RPE) and is suggestive of prior presence of subretinal fluid.
Fluorescein angiography (FA) of both eyes showed scattered microaneurysms. The left eye showed staining of the disciform scar, but no leakage was detected.
Spectral-domain OCT (SD-OCT) of the right eye showed a shallow rudimentary foveal depression, incursion of inner retinal layers, widening of outer nuclear layer (ONL) and lengthening of outer segments; consistent with grade 1 foveal hypoplasia (fovea plana). The left eye had dome-shaped subretinal hyperreflective material (SHRM) beneath the fovea, causing disorganization of the overlying retinal layers. A few tiny cysts were noted overlying the SHRM.
SD-OCT angiography (OCTA) imaging of the right eye demonstrated a very small foveal avascular zone (FAZ) area and the presence of macular foveal capillaries (MFC) crossing the FAZ on the superficial capillary plexus (SCP) slab, and a slightly wider, but still reduced, FAZ on the deep capillary plexus (DCP) slab. OCTA of the left eye showed a type 2 macular neovascular lesion.
Figures 1 and 2 show multimodal imaging findings in the right and left eyes, respectively.
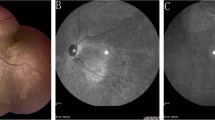
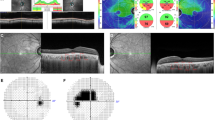
Multimodal imaging of right eye. a Color fundus photography. Normal fundus pigmentation. There is arteriolar tortuosity, opacification (copper or silver wiring) of the arteriolar wall and mild AV nicking consistent with mild hypertensive retinopathy. There are scattered microaneurysms consistent with mild nonproliferative diabetic retinopathy (NPDR). b Green fundus autofluorescence. Normal FAF. c Fluorescein angiography, mid arteriovenous phase. Microaneurysms appear as hyperfluorescent dots. d SD-OCT (horizontal scan). The shallow rudimentary foveal depression, incursion of inner retinal layers, widening of outer nuclear layer (ONL) and lengthening of outer segments are consistent with grade 1 foveal hypoplasia (fovea plana). e En-face OCTA slabs of superficial capillary plexus, deep capillary plexus, outer retina and choriocapillaris (from left to right). The SCP slab shows a very small FAZ area and the presence of macular foveal capillaries crossing the FAZ. The DCP slab shows reduced FAZ area, but wider than SCP FAZ. Outer retina and choriocapillaris slabs are normal and show no neovascularization. f B-scan structural OCT with angiographic overlay shows fovea plana and the presence of flow signals (red) in the foveal region at the levels of both SCP and DCP
Multimodal imaging of left eye. a Color fundus photography. Normal fundus pigmentation. There are similar hypertensive and diabetic changes to the right eye. There is a subfoveal disciform scar surrounded by an area of pigmentary disturbance occupying almost the entire macula. b Green fundus autofluorescence. The fovea shows a central zone of decreased FAF surrounded by a ring of increased FAF, which is surrounded by an area of decreased FAF. This triple zone corresponds to the disciform scar and surrounding areas of RPE disturbance and atrophy. The triple zone is surrounded by a larger area of mildly increased FAF occupying almost the entire macula, corresponding to diseased RPE and suggestive of prior presence of subretinal fluid. c Fluorescein angiography, mid arteriovenous phase. Microaneurysms appear as hyperfluorescent dots. There is a hyperfluorescent lesion at the fovea that shows intense staining, but not leakage, consistent with scar tissue. The lesion is surrounded by a hypofluorescent rim due to blockage by pigment, which is surrounded by a large area of mild hyperfluorescence representing a window defect. d SD-OCT (horizontal scan). There is subretinal hyperreflective material, part of which occupies almost the entire thickness of the foveal region. There are tiny cysts present mainly in the inner nuclear layer and 1 cyst in the ganglion cell layer. e En-face OCTA slabs of superficial capillary plexus, deep capillary plexus, outer retina and choriocapillaris (from left to right). There is a large type 2 macular neovascular complex originating deep in the choroid and extending into the innermost retinal layers. The lesion is noted mainly on the outer retina and choriocapillaris slabs, and the top part of the lesion appears as a small neovascular tuft on the SCP and DCP slabs. f B-scan structural OCT with angiographic overlay shows flow signals (red) within the subretinal hyperreflective material consistent with type 2 neovascularization
Discussion and conclusions
PWS results from the loss of expression of the paternally inherited copy of several contiguous genes within the PWCR on the long arm of chromosome 15. Three molecular mechanisms are implicated in the pathogenesis of PWS: Paternal deletion, maternal uniparental disomy (UPD) 15 and imprinting defect (ID). Based on clinical suspicion, diagnosis is typically confirmed by DNA methylation analysis which has a 99% sensitivity for detecting PWS caused by all 3 mechanisms, although it cannot differentiate between them. Further genetic testing is required to detect the underlying molecular mechanism and provide appropriate genetic counseling [30,31,32].
About 65–75% of PWS cases result from an interstitial deletion on the paternal allele of 15q11.2-q13. One of the genes in this region is OCA2 (previously known as P) which is associated with type 2 (tyrosinase-positive) oculocutaneous albinism (OCA). Involvement of OCA2 by deletion has been linked to the hypopigmentation observed in 30–50% of PWS patients [33]. PWS patients with deletion are more likely to develop hypopigmentation compared to UPD patients [34]. On the other hand, 1% of type 2 OCA patients have comorbid PWS [35]. Abnormal or absent foveal reflex has been reported in PWS patients, and considered to express the overlap between PWS and OCA [4]. A case report of 1 patient with PWS utilized SD-OCT imaging, and demonstrated only slight shallowing of the foveal depression [10]. Another report by Shohat et al. found severe cone dystrophy in a girl with PWS who had rearrangement of chromosome 15 with an increase in band 15q12. This finding might point to a possible association between this area and retinal development [24].
Excavation of the foveal pit usually begins at the 25th week of gestation and terminates 15–45 months after birth. Interruption of this process results in foveal hypoplasia [36]. Foveal hypoplasia in albinism has been postulated to result from defective melanin-bearing cells in the developing retina, which, in turn, leads to abnormal regulation of the patterns of axonal growth and pathfinding during embryogenesis [4].
The term ‘fovea plana’ (FP) was coined by Marmor et al. as a mere anatomical descriptor for the lack of a foveal pit, to emphasize the fact that the absence of a foveal depression is not concomitant with poor visual acuity or lack of cone specialization. Conversely, the term ‘foveal hypoplasia’ has been traditionally linked to poor visual function in several pathologic conditions, and often implies that the absence of the foveal depression is the culprit in abnormal vision [37]. The incidence of FP in normal eyes in a pediatric population has been estimated to range from 1.7 to 3% [38].
Subsequently, Thomas and colleagues developed an OCT-based grading system for foveal hypoplasia that reflected the different stages of foveal development. They demonstrated progressively declining visual acuity in parallel to advancing structural grade, which is a surrogate for arrest of foveal development at an earlier stage. Our patient had a shallow rudimentary foveal pit, lengthening of outer segments and widening of the ONL, consistent with grade 1 foveal hypoplasia [39].
The term “macular foveal capillaries” has been used to describe the complete or partial absence of the physiologic FAZ which is crossed by intraretinal vascular nets that communicate with the surrounding retinal capillary networks. The normal FAZ measures 500–600 microns in diameter, and shows considerable variation in size and shape among the normal population [40].
Absence of the FAZ, as determined by FA, has been reported in individuals with normal visual acuity, and in a multitude of ocular diseases including albinism, aniridia, achromatopsia, nanophthalmos and retinopathy of prematurity [41,42,43]. OCTA is a relatively new, non-invasive, depth-resolved method of imaging the retinal vasculature. In their recent case series, Cicinelli and colleagues described the presence of MFC in 12 eyes of 10 patients. Associated conditions included macular pucker, post-surgical macular edema, diabetic retinopathy (DR), age-related macular degeneration (AMD), dome-shaped macula (DSM), branch retinal artery occlusion (BRAO) and chronic central serous chorioretinopathy (CSCR). Five eyes in their study had loss of foveal depression due to tractional causes, but only 4 eyes lacked a normal foveal depression on SD-OCT with good visual acuity. They considered these cases to represent FP [40]. Another study used OCTA to study foveal hypoplasia in 6 patients. Two patients had a diagnosis of OCA, while lack of a foveal depression was an incidental finding in 4 patients, all of which had a good visual acuity and an otherwise normal retina. The FAZ was completely absent in the SCP and only partially so in the DCP [44]. This is similar to the findings we observed in our patient who had almost absent FAZ in SCP and a markedly reduced FAZ in DCP, but still larger than in SCP. Other small case series and case reports have described similar findings using OCTA [45,46,47].
An inverse correlation between FAZ area and central macular thickness (CMT) and volume (CMV) has been demonstrated using OCTA. As CMT and CMV increase, foveal pit depth, volume and FAZ size decrease [48]. Another study utilizing FA and SD-OCT showed the FAZ area to inversely correlate to foveal pit depth, diameter and volume [49]. These findings emphasize the role FAZ purportedly plays in foveal pit development during primate embryogenesis.
Since the development of FAZ predates that of foveal excavation, it is plausible that the FAZ area impacts the centrifugal migration of inner retinal layers, and, hence, foveal pit morphology [50]. However, neither appears to be instrumental in determining visual function, as evidenced by the substantial overlap between foveal pit morphology in the normal population and in patients with albinism, and the lack of correlation between visual function and pit depth [49]. It is more likely that other features are more influential, such as foveal cone packing, elongation of outer segments and lengthening of Henle’s fibers. None of these features appears to be dependent on foveal pit morphology for their characterization [51].
Several causes of choroidal neovascularization (CNV) have been reported in individuals younger than 50 years. These include pathologic myopia, angioid streaks, chronic CSCR, type 2 macular telangiectasia, choroidal rupture, posterior uveitis, choroidal tumors, hereditary retinal dystrophies and optic nerve head anomalies [52]. When there is no apparent cause for neovascularization, it is termed ‘idiopathic’. Idiopathic CNV is usually unilateral and has better visual prognosis than CNV secondary to age-related macular degeneration (AMD) [53].
To the best of our knowledge, this finding has not been reported in the context of PWS. Our patient was not highly myopic. A possible explanation for CNV development is silencing of NDN gene, which is a maternally imprinted gene that maps to the PWCR and encodes for necdin. Necdin is a protein that plays a role in neuronal differentiation and development, and is thought to have tumor-suppressing and anti-angiogenic properties [54]. However, confirmation of this hypothesis requires complex genetic testing and an in-depth investigation of a cause-effect relationship. Alternatively, the CNV could be the result of unreported old trauma or posterior uveitis that resulted in chorioretinal scarring and an RPE defect. It is also possible that this is a mere coexistence of an idiopathic CNV.
Our patient displayed many characteristic features of PWS, including short stature, typical facial dysmorphism, hypogonadism and type 2 DM. He also had a history of prior strabismus surgery. He was overweight with a body mass index (BMI) of 34. There was no hypopigmentation of his skin, hair or eyes. This could be explained by the relatively old age of the patient, since patients with OCA are known to exhibit progressive darkening of skin, hair and eyes with age, and hypopigmentation in PWS patients is usually less severe than that noted with OCA patients [55].
In summary we report novel ocular findings in a PWS patient using multimodal imaging. These are fovea plana and macular foveal capillaries in 1 eye and type 2 macular neovascularization in the other eye. The patient was followed for 1 year, during which examination and multimodal imaging were repeated twice and showed the same findings each time. Based on clinical stability and absence of leakage from the neovascular lesion, the decision was made to observe the patient closely and not to administer anti-angiogenic therapy. The patient was counseled on tight control of his blood glucose level and blood pressure to prevent further progression of his fundus diabetic and hypertensive changes.
Abbreviations
- AMD:
-
age-related macular degeneration
- BCVA:
-
best-corrected visual acuity
- BMI:
-
body mass index
- BRAO:
-
branch retinal artery occlusion
- CMT:
-
central macular thickness
- CMV:
-
central macular volume
- CNV:
-
choroidal neovascularization
- CSCR:
-
central serous chorioretinopathy
- DCP:
-
deep capillary plexus
- DM:
-
diabetes mellitus
- DR:
-
diabetic retinopathy
- DSM:
-
dome-shaped macula
- FA:
-
fluorescein angiography
- FAF:
-
fundus autofluorescence
- FAZ:
-
foveal avascular zone
- FP:
-
fovea plana
- ICGA:
-
indocyanine green angiography
- ID:
-
imprinting defect
- MFC:
-
macular foveal capillaries
- NDN:
-
necdin
- nm:
-
nanometer
- NPDR:
-
nonproliferative diabetic retinopathy
- OCA:
-
oculocutaneous albinism
- OCTA:
-
optical coherence tomography angiography
- ONL:
-
outer nuclear layer
- PDR:
-
proliferative diabetic retinopathy
- PWCR:
-
Prader–Willi critical region
- PWS:
-
Prader–Willi syndrome
- RPE:
-
retinal pigment epithelium
- SCP:
-
superficial capillary plexus
- SD-OCT:
-
spectral domain optical coherence tomography
- SHRM:
-
subretinal hyperreflective material
- UPD:
-
uniparental disomy
- VEP:
-
visually-evoked potential
- VF:
-
visual field
References
Hurren BJ, Flack NA. Prader–Willi Syndrome: a spectrum of anatomical and clinical features. Clin Anat. 2016;29(5):590–605.
Sanjeeva GN, Maganthi M, Kodishala H, Marol RKR, Kulshreshtha PS, Lorenzetto E, Kadandale JS, Hladnik U, Raghupathy P, Bhat M. Clinical and molecular characterization of Prader–Willi syndrome. Indian J Pediatr. 2017;84(11):815–21.
Fox R, Sinatra RB, Mooney MA, Feurer ID, Butler MG. Visual capacity and Prader–Willi syndrome. J Pediatr Ophthalmol Strabismus. 1999;36(6):331–6.
Apkarian P, Spekreijse H, van Swaay E, van Schooneveld M. Visual evoked potentials in Prader–Willi syndrome. Doc Ophthalmol. 1989;71(4):355–67.
Bassali R, Hoffman WH, Chen H, Tuck-Muller CM. Hyperlipidemia, insulin-dependent diabetes mellitus, and rapidly progressive diabetic retinopathy and nephropathy in Prader–Willi syndrome with del(15)(q11.2q13). Am J Med Genet. 1997;71(3):267–70.
Butler MG. Hypopigmentation: a common feature of Prader–Labhart–Willi syndrome. Am J Hum Genet. 1989;45(1):140–6.
Butler MG, Meaney FJ, Palmer CG. Clinical and cytogenetic survey of 39 individuals with Prader–Labhart–Willi syndrome. Am J Med Genet. 1986;23(3):793–809.
Creel DJ, Bendel CM, Wiesner GL, Wirtschafter JD, Arthur DC, King RA. Abnormalities of the central visual pathways in Prader–Willi syndrome associated with hypopigmentation. N Engl J Med. 1986;314(25):1606–9.
Futterweit W, Ritch R, Teekhasaenee C, Nelson ES. Coexistence of Prader–Willi syndrome, congenital ectropion uveae with glaucoma, and factor XI deficiency. JAMA. 1986;255(23):3280–2.
Gerding H, Timmermann M. Atypical RPE-pigmentation in Prader–Willi syndrome. Klin Monbl Augenheilkd. 2012;229(4):454–6.
Gillessen-Kaesbach G, Robinson W, Lohmann D, Kaya-Westerloh S, Passarge E, Horsthemke B. Genotype-phenotype correlation in a series of 167 deletion and non-deletion patients with Prader–Willi syndrome. Hum Genet. 1995;96(6):638–43.
Hattori S, Mochio S, Kageyama A, Nakajima T, Akima M, Fukunaga N. An autopsy case of Prader–Labhart–Willi syndrome. No to shinkei = Brain Nerve. 1985;37(11):1059–66.
Hayashi M, Itoh M, Kabasawa Y, Hayashi H, Satoh J, Morimatsu Y. A neuropathological study of a case of the Prader–Willi syndrome with an interstitial deletion of the proximal long arm of chromosome 15. Brain Dev. 1992;14(1):58–62.
Hered RW, Rogers S, Zang YF, Biglan AW. Ophthalmologic features of Prader–Willi syndrome. J Pediatr Ophthalmol Strabismus. 1988;25(3):145–50.
Hittner HM, King RA, Riccardi VM, Ledbetter DH, Borda RP, Ferrell RE, Kretzer FL. Oculocutaneous albinoidism as a manifestation of reduced neural crest derivatives in the Prader–Willi syndrome. Am J Ophthalmol. 1982;94(3):328–37.
Hori H, Sato Y, Nakashima M. Prader–Willi syndrome case with proliferative diabetic retinopathy in both eyes treated by early vitrectomy under local anesthesia. Nippon Ganka Gakkai zasshi. 2012;116(2):114–8.
Kalpakian B, Bateman JB, Sparkes RS, Wood GK. Congenital ocular fibrosis syndrome associated with the Prader–Willi syndrome. J Pediatr Ophthalmol Strabismus. 1986;23(4):170–3.
Lee ST, Nicholls RD, Bundey S, Laxova R, Musarella M, Spritz RA. Mutations of the P gene in oculocutaneous albinism, ocular albinism, and Prader–Willi syndrome plus albinism. N Engl J Med. 1994;330(8):529–34.
Libov AJ, Maino DM. Prader–Willi syndrome. J Am Optom Assoc. 1994;65(5):355–9.
Parcheta B, Piontek E, Zawadzki J, Ryzko J. A case of Prader–Willi syndrome with tubular acidosis and partial ocular albinism. Wiadomosci lekarskie (Warsaw, Poland: 1960). 1987;40(10):694–8.
Ritch R, Forbes M, Hetherington J Jr, Harrison R, Podos SM. Congenital ectropion uveae with glaucoma. Ophthalmology. 1984;91(4):326–31.
Roy MS, Milot JA, Polomeno RC, Barsoum-Homsy M. Ocular findings and visual evoked potential response in the Prader–Willi syndrome. Can J Ophthalmol J Canadien d’ophtalmologie. 1992;27(6):307–12.
Savir A, Dickerman Z, Karp M, Laron Z. Diabetic retinopathy in an adolescent with Prader–Labhart–Willi syndrome. Arch Dis Child. 1974;49(12):963–4.
Shohat M, Shohat T, Rimoin DL, Mohandas T, Heckenlively J, Magenis RE, Davidson MB. Korenberg JR: Rearrangement of chromosome 15 in the region q11.2—q12 in an individual with obesity syndrome and her normal mother. Am J Med Genet. 1990;37(2):173–7.
Spritz RA, Bailin T, Nicholls RD, Lee ST, Park SK, Mascari MJ, Butler MG. Hypopigmentation in the Prader–Willi syndrome correlates with P gene deletion but not with haplotype of the hemizygous P allele. Am J Med Genet. 1997;71(1):57–62.
Wallis CE, Beighton PH. Synchrony of oculocutaneous albinism, the Prader–Willi syndrome, and a normal karyotype. J Med Genet. 1989;26(5):337–9.
Wang XC, Norose K, Kiyosawa K, Segawa K. Ocular findings in a patient with Prader–Willi syndrome. Jpn J Ophthalmol. 1995;39(3):284–9.
Watanabe M, Yamamoto K, Hori S. A case of Prader–Willi syndrome treated by vitrectomy. Nippon Ganka Gakkai zasshi. 2006;110(6):473–6.
Wiesner GL, Bendel CM, Olds DP, White JG, Arthur DC, Ball DW, King RA. Hypopigmentation in the Prader–Willi syndrome. Am J Hum Genet. 1987;40(5):431–42.
Kubota T, Sutcliffe JS, Aradhya S, Gillessen-Kaesbach G, Christian SL, Horsthemke B, Beaudet AL, Ledbetter DH. Validation studies of SNRPN methylation as a diagnostic test for Prader–Willi syndrome. Am J Med Genet. 1996;66(1):77–80.
Glenn CC, Saitoh S, Jong MT, Filbrandt MM, Surti U, Driscoll DJ, Nicholls RD. Gene structure, DNA methylation, and imprinted expression of the human SNRPN gene. Am J Hum Genet. 1996;58(2):335–46.
Glenn CC, Driscoll DJ, Yang TP, Nicholls RD. Genomic imprinting: potential function and mechanisms revealed by the Prader–Willi and Angelman syndromes. Mol Hum Reprod. 1997;3(4):321–32.
Angulo MA, Butler MG, Cataletto ME. Prader–Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Invest. 2015;38(12):1249–63.
Torrado M, Araoz V, Baialardo E, Abraldes K, Mazza C, Krochik G, Ozuna B, Leske V, Caino S, Fano V, et al. Clinical-etiologic correlation in children with Prader–Willi syndrome (PWS): an interdisciplinary study. Am J Med Genet Part A. 2007;143a(5):460–8.
Spritz RA. Molecular genetics of oculocutaneous albinism. Hum Mol Genet. 1994;3(Spec No):1469–75.
Yuodelis C, Hendrickson A. A qualitative and quantitative analysis of the human fovea during development. Vis Res. 1986;26(6):847–55.
Marmor MF, Choi SS, Zawadzki RJ, Werner JS. Visual insignificance of the foveal pit: reassessment of foveal hypoplasia as fovea plana. Arch Ophthalmol. 2008;126(7):907–13.
Noval S, Freedman SF, Asrani S, El-Dairi MA. Incidence of fovea plana in normal children. J AAPOS Off Publ Am Assoc Pediatric Ophthalmol Strabismus. 2014;18(5):471–5.
Thomas MG, Kumar A, Mohammad S, Proudlock FA, Engle EC, Andrews C, Chan WM, Thomas S, Gottlob I. Structural grading of foveal hypoplasia using spectral-domain optical coherence tomography a predictor of visual acuity? Ophthalmology. 2011;118(8):1653–60.
Cicinelli MV, Carnevali A, Rabiolo A, Querques L, Zucchiatti I, Scorcia V, Bandello F, Querques G. Clinical spectrum of macular-foveal capillaries evaluated with optical coherence tomography angiography. Retina. 2017;37(3):436–43.
Charles SJ, Green JS, Grant JW, Yates JR, Moore AT. Clinical features of affected males with X linked ocular albinism. Br J Ophthalmol. 1993;77(4):222–7.
Walsh MK, Goldberg MF. Abnormal foveal avascular zone in nanophthalmos. Am J Ophthalmol. 2007;143(6):1067–8.
Recchia FM, Recchia CC. Foveal dysplasia evident by optical coherence tomography in patients with a history of retinopathy of prematurity. Retina. 2007;27(9):1221–6.
Pakzad-Vaezi K, Keane PA, Cardoso JN, Egan C, Tufail A. Optical coherence tomography angiography of foveal hypoplasia. Br J Ophthalmol. 2017;101(7):985–8.
Bazvand F, Karkhaneh R, Roohipoor R, Rajabi MB, Ebrahimiadib N, Davoudi S, Modjtahedi BS. Optical coherence tomography angiography in foveal hypoplasia. Ophthalmic Surg Lasers Imaging Retina. 2016;47(12):1127–31.
Dolz-Marco R, Phasukkijwatana N, Sarraf D, Freund KB. Optical coherence tomography angiography in fovea plana. Ophthalmic Surg Lasers Imaging Retina. 2016;47(7):670–3.
Kaidonis G, Silva RA, Sanislo SR, Leng T. The superficial and deep retinal capillary plexus in cases of fovea plana imaged by spectral-domain optical coherence tomography angiography. Am J Ophthalmol Case Rep. 2017;6:41–4.
Samara WA, Say EA, Khoo CT, Higgins TP, Magrath G, Ferenczy S, Shields CL. Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina. 2015;35(11):2188–95.
Dubis AM, Hansen BR, Cooper RF, Beringer J, Dubra A, Carroll J. Relationship between the foveal avascular zone and foveal pit morphology. Invest Ophthalmol Vis Sci. 2012;53(3):1628–36.
Hendrickson APJ. Comparison of development of the primate fovea centralis with peripheral retina. In: Sernagor EES, Harris B, Wong R, editors. Retinal development. Cambridge: Cambridge University Press; 2006.
McAllister JT, Dubis AM, Tait DM, Ostler S, Rha J, Stepien KE, Summers CG, Carroll J. Arrested development: high-resolution imaging of foveal morphology in albinism. Vis Res. 2010;50(8):810–7.
Spaide RF. Choroidal neovascularization in younger patients. Curr Opin Ophthalmol. 1999;10(3):177–81.
Fan X, Gao N, Li J, Lei J, Kang Q. Effects of VEGF levels on anti-VEGF therapy for patients with idiopathic choroidal neovascularization. Mol Cell Biochem. 2018;441(1–2):173–9.
Chapman EJ, Knowles MA. Necdin: a multi functional protein with potential tumor suppressor role? Mol Carcinog. 2009;48(11):975–81.
Saadeh R, Lisi EC, Batista DA, McIntosh I, Hoover-Fong JE. Albinism and developmental delay: the need to test for 15q11-q13 deletion. Pediatr Neurol. 2007;37(4):299–302.
Authors’ contributions
MAH contributed in acquisition of data and writing the manuscript. MCM contributed in analysis and interpretation of data and critically reviewing the manuscript. BDK contributed to analysis and interpretation of data and critically reviewing the manuscript. All authors read approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and material
All the data generated or analyzed during this study are included in this manuscript and attached figures.
Consent for publication
No consent for publication was obtained from the patient as all the images used in this case report are entirely unidentifiable and there are no personal data included in the manuscript that can be traced to an individual patient.
Ethics approval and consent to participate
The University of California Irvine (UCI) does not require an Institutional Review Board (IRB) for case reports that do not involve any kind of medical or surgical intervention as is the case for our report.
Funding
No funding sources.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hamid, M.A., Mehta, M.C. & Kuppermann, B.D. Multimodal imaging in a patient with Prader–Willi syndrome. Int J Retin Vitr 4, 45 (2018). https://doi.org/10.1186/s40942-018-0147-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40942-018-0147-6