Abstract
Background
Extraction of the mandibular third molar, the most frequent and important surgical procedure in the clinical practice of oral surgery, is associated with the risk of injury of the lingual nerve. Neuropathy of the lingual nerve poses diagnostic challenges regarding the transient or permanent nature of the injury. No consensus or criteria have been established regarding the diagnosis of lingual nerve neuropathy. We applied both Tinel’s test and clinical neurosensory testing together, which can be easily used at the bedside in the early stages of injury. Therefore, we propose a new method to differentiate between lesions with the ability to heal spontaneously and those that cannot heal without surgery.
Results
Thirty-three patients (29 women, 4 men; mean age, 35.5 years) were included in this study. For all patients, the median interval between nerve injury and initial examination was 1.6 months and that between nerve injury and the second examination before determining the need for surgical management was 4.5 months. The patients were assigned to either group A or B. The spontaneous healing group (group A, n = 10) revealed a tendency for recovery within 6 months after tooth extraction. In this group, although there were individual differences in the degree of recovery, a remarkable tendency for recovery was observed based on clinical neurosensory testing in all cases. None of the patients were diagnosed with allodynia. In seven cases, the Tinel test result was negative at the first inspection, and in three cases, the result changed to negative at the second inspection. Conversely, in group B(n = 23), no recovery trend was observed with regard to clinical neurosensory testing, and nine patients had allodynia. Further, the Tinel test result was positive for all patients in both examinations.
Conclusions
Our findings indicate that in case of transient lingual nerve paralysis, clinical neurosensory testing findings deteriorate immediately after tooth extraction and gradually recover, while Tinel’s test shows a negative result. Using Tinel’s test and clinical neurosensory testing together enabled early and easy identification of the severity of the lingual nerve disorder and of lesions that would heal spontaneously without surgical management.
Similar content being viewed by others
Background
Lingual nerve (LN) disturbance is a rare peripheral neuropathy occurring after extraction of a mandibular third molar [1,2,3,4]. Lingual neuropathy is more difficult to treat than inferior alveolar neuropathy [5, 6]. LN disturbance has several symptoms, including analgesia, with no pain even on accidentally biting the tongue; continuous tingling sensation; and allodynia, which is pain caused by a stimulus that does not normally provoke pain, for example, hot food or ice. However, diagnosing LN neuropathy can be challenging, with difficulty in determining if the disturbance is transient or permanent. Further, it is difficult to determine the need for surgical management and the postoperative prognosis. There remains a controversy regarding the appropriate time for surgical management in LN neuropathy, and no consensus has been established yet [7,8,9]. It is therefore essential to develop a diagnostic technique that can help identify the need for surgical management in the early stages of the nerve injury. Since 2000, we have diagnosed and managed more than 160 cases of LN neuropathy of varying severity developing after extraction of mandibular third molar. We have previously reported an outline of our management approach [10, 11]. The findings of our studies revealed that in case of transient paralysis, clinical neurosensory testing (CNT) results deteriorate immediately after tooth extraction and gradually recover, while Tinel’s test shows a negative result. Moreover, modern studies have proved that the persistent positive Tinel’s test may represent a poor sign of chronic peripheral neuropathy with neuroma formation. We propose a new method to differentiated between lesions with the ability to heal spontaneously and those that cannot be cured without surgery.
Methods
We aimed to evaluate the utility of combining Tinel’s test and CNT in the early stages of LN injury to predict whether the lesion would heal spontaneously or require surgical management.
Participants
This study was performed in accordance with the Declaration of Helsinki and was approved by the Wakayama Medical University Institutional Review Board (approval number 1699). Observational data were collected from patients who visited the Wakayama Medical University Hospital with unilateral LN injury after third molar extraction between May 2014 and March 2020. The included patients had no remarkable medical history, except for the LN disorder. Written informed consent was obtained from all patients before they underwent examination. For each patient, CNT and Tinel’s test were performed at every consultation. Patients were diagnosed with severe LN disturbance based on the findings of at least two detailed examinations performed at intervals. The interval between the first examination and the second examination was at least 1 month. We advised to add more examinations after the second examination if it's convenient. It is difficult to specify the indications for surgical management, but surgical management was performed if any of the following two conditions were met:
-
1.
If the follow-up period after tooth extraction was less than 6 months, and no improvement, worsening of the condition, or allodynia were observed.
-
2.
If 1 year had passed since the tooth extraction and the patient condition was poor or if the patient had allodynia.
Diagnostic methods and criteria
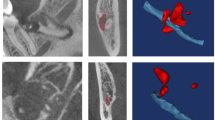
Tinel’s test involved palpation of the lingual gingiva at the extraction wound site. The earliest timing to apply the Tinel’s test is when the extracted wound has been cured after sutures removal. It is important to palpate the lesion around the lingual gingiva adjacent to the mandibular third molar extraction socket with fingertips gently and without strong pressure in all directions as indicated by the arrows in Fig. 1. In the case of positive reaction, a tingling pain can often be observed running from the tongue tip to the tongue margin, the oral mucosa and the lingual gingiva from the molar to the premolar in the affected side. In rare cases, similar pain may occur in the affected lower submandibular lesion around submandibular gland. As a control, we should similarly palpate the lingual gingiva of the unaffected contralateral mandibular third molar to remind the patient that no pain occurs on the unaffected side. This maneuver helps to elicit a distal referred tingling or hyperpathia on the side of the tongue in the region of the injury (scores: 0, no sensation was recognized; 1, some pain recognized at the target tongue region). The other hand, CNT involves a three-stage inspection method as shown in Fig. 2. The second and third levels of the CNT test are performed depending on the response to the previous level. Flow chart of CNT presented in this study suggests the rational approach of first investigating the response of the thicker nerves and later examining the response of the thinner nerves. Practically, it is often observed that the functional recovery of the nerve fibers is initiated at the thicker nerve fibers and gradually progresses to the thinner fibers. Further, CNT can be easily performed at the bedside without requiring extensive equipment. Specific evaluation criteria for each level of CNT are described below.
Level A
Static two-point discrimination (2PD): before gentle contact with caliper tips is made on the lingual mucosae, the patient is asked to indicate when contact is felt and to identify whether that contact is of one or two points, which was then expressed in millimeters. Brush stroke directional sensation with a camel hair brush (brush) was examined by applying horizontal, vertical, and rotational stimulating movements on the lingual gingiva at the extraction wound site (scores: 0, no sensation was recognized; 1, sensations were recognized in only one direction; 2, sensations were recognized in two directions; 3, sensations were recognized during all movements).
Level B
Pressure pain threshold was examined using Semmens-Weinstein monofilaments (SWM) of 20 different diameters, with “1” referring to the smallest-diameter monofilament and “20” to the largest-diameter monofilament.
Level C
The pin prick test involved pricking the lingual gingiva at the wound site with a sharp needle (scores: 0, no sensation was recognized; 1, only pressure was recognized; 2, intensive pain was recognized).
Statistical analyses
The Fisher’s exact probability test analysis was performed to identify correlations between changes in the Tinel test and CNT findings (2PD, brush, SWM, and pin prick). Two-sample Student’s t test was employed to determine the differences between the means of the variables measured within test groups. For all analyses, the statistical significance was set at P < 0.05. All data were statistically analyzed for significance using R version 4.2.1 (R foundation for Statistical Computing, Vienna, Austria).
Results
Participants
Thirty-three patients (29 women [88%], 4 men [12%]; mean age 35.5 years [range 16–67 years]) were included in this study. For all patients, the median interval between nerve injury and initial examination was 1.6 months (range 1–4 months). The median interval between nerve injury and the second examination for groups A (n = 10) and B (n = 23) before determining the need for surgical management was 4.5 months (range 2–20 months). There was no significant difference in the age distribution, sex, and timing of the first and second examinations after injury between the two groups (Table 1). The course of healing from the initial to final follow-up examination was monitored for every patient. These detailed findings are presented in Tables 2 and 3.
Tinel’s test and CNT
The patients were assigned to either group A or B. The spontaneous healing group (group A, n = 10) revealed a tendency for recovery within 6 months after tooth extraction. In seven cases, the Tinel test result was negative at the first inspection, and in three cases, the result changed to negative at the second inspection (Table 2). Further, although the CNT findings were initially deteriorated, they displayed a tendency for improvement later. None of the patients were diagnosed with allodynia. Conversely, the patients who received surgical management (group B, n = 23) underwent detailed examination at least twice within 20 months after the extraction but neither Tinel’s test nor CNT findings revealed a tendency for recovery. Further, the Tinel test result was positive for every patient in both examinations (Table 3).
Correlation between the findings of Tinel’s test and CNT findings
Regarding the correlation between Tinel’s test and the Brush stroke and 2PD findings (CNT: level A), patients with a negative Tinel’s test in both examinations indicated a trend of improvement in CNT findings on subsequent inspection; conversely, patients with a positive Tinel’s test did not show a tendency for improvement in CNT findings. Regarding the correlation between Tinel’s test and SWM values (CNT: level B), the SWM value was normalized in cases of a negative Tinel’s test in the second examination, and conversely, patients with a positive Tinel’s test did not show a tendency for recovery. Regarding the correlation between Tinel’s test and the second pin prick reaction (CNT: level C), seven patients with a negative Tinel’s test at the first examination and three patients with a reaction in the second pin prick test with a negative Tinel’s test in the second examination showed a significant trend for improvement. Conversely, twenty-three patients with a positive Tinel’s test in both examinations could not reach score 2 (Table 4). In the second pin prick examination, eight patients perceived intensive pain and two experienced slight dull pain in the group that showed a negative Tinel’s test, whereas, in the group that showed a positive Tinel’s test, six patients experienced slight dull pain, and 17 displayed no reaction in the second pin prick test (Table 5). In the second pin prick test, in the group that showed a negative Tinel’s test, eight patients had a score of 2. Further, two patients perceived a score of 1, slight dull pain. In contrast, in the group that showed a positive Tinel’s test, 23 patients did not perceive intensive pain to pin prick test. Here, when the second Tinel’s test and CNT findings results up to level C were evaluated, patients showing a negative Tinel’s test and an improvement trend in CNT findings were consistent up to the level C stage. Considering the second pin prick reaction as the gold standard, we found that the sensitivity of Tinel’s test was 0.800, its specificity was 1.000, its positive predictive value was 0.800, and its negative predictive value was 1.000 (Table 6). As shown in Table 7, there was no correlation between the first Tinel’s test and the first CNT findings. As shown in Table 8, there was a correlation between the second Tinel’s test and second CNT findings. Furthermore, as shown in Table 9, the results of the first Tinel’s test and the second CNT findings were in good agreement, and the negative/positive results of the first Tinel’s test were reflected in the results of the second CNT findings (Table 9).
Discussion
Maxillofacial surgeons sometimes encounter patients with trigeminal nerve sensory deficits after mandibular third molar extraction. Ghali and Epker suggested practical and objective approaches to assess these patients and diagnose nerve injury, potential for recovery, and need for secondary microneurosurgical intervention; they proposed the CNT as a diagnostic method in 1989 [12]. Zuniga et al. attempted to determine the statistical efficacy of CNT and identify a correlation between the sensory impairment score obtained by preoperative testing and the degree of nerve injury. The multisite, randomized, prospective, blinded, clinical trial was conducted among 130 patients with inferior alveolar nerve and LN injuries. A statistically significant positive relationship was found between the sensory impairment score and degree of nerve injury. Moreover, the efficiency of CNT was greater for diagnosing LN injuries rather than inferior alveolar nerve injuries [13]. However, the history of the Tinel test is even older. In 1915, Tinel, a famous orthopedic surgeon, first described this test for the extremities. He reported a tingling sensation or formication produced by slight percussion of a nerve trunk following an injury. The sensation radiates into the cutaneous distribution of the specific nerve and indicates the presence of regenerating nerve fibers [14]. Miloro described Tinel’s sign in LN disturbances in his textbook of Oral Maxillofacial Surgery as follows: palpation may induce Tinel’s sign, which is a provocative test of regenerating nerve sprouts that it is performed by light palpation over the area of suspected injury. This maneuver elicits a distal referred tingling sensation at the target site. This sign is thought to indicate small-diameter fiber recovery; however, it is poorly correlated with functional recovery and is often confused with neuroma formation [6]. Gregg described in his paper as follows: Hoffman and Tinel in 1915 described the clinical phenomenon of tingling and shock-like sensation that are elicited by digital tapping over distal portions of regenerating nerves. These clinical signs were felt to represent “the presence of young axons in the process of growing”. Subsequent experiences, however, have shown that the “Tinel’s sign” may be easily misinterpreted, and, rather than a positive sign of spontaneous neural regeneration, it may also represent a poor sign of chronic peripheral neuropathy with neuroma formation. Modern studies have verified that hyperpathic clinical signs can represent sites of sensitized neuroma-in-continuity, as well as chronically sensitized amputation-type neuromas [15]. Suhaym and Miloro reported that the odd improvement was 2.28 (95% confidence interval 1.05–4.98) in the 6-month breakpoint studies according to the meta-analysis analysis results of the early treatment timing of the LN [8]. Among the more than 160 cases of LN neuropathy that we have experienced in the past, in the case of negative Tinel’s test, majority of these cases indicated a negative reaction at the first examination. There were no cases in which the transfiguration from negative to positive Tinel’s test changed in more than 6 months later after LN injury as shown in Table 2. Therefore, 6 months after LN injury would be an appropriate period to pursue Tinel’s test response. Hillerup and Stoltze followed up 46 patients with LN injury who showed spontaneous healing, and found that the highest frequency of recovery was 6 months after LN injury. They concluded that patients should be monitored repeatedly for at least 3 months and not operated on until neurosensory function no longer improved [16]. They observed that patients with a positive Tinel’s test at the initial examination also indicated a positive reaction at the final examination and that their neurological dysfunction was severe. On the contrary, patients with a negative Tinel’s test displayed a good recovery tendency of the LN function. Our findings are consistent with those of Hillerup and Stoltz, and we identified some patients with remarkable spontaneous healing ability within 6 months after LN injury. Patients with a negative Tinel’s test at the initial examination showed a good tendency for improvement of their nerve function, which is in line with the findings of Hillerup and Stoltz.
In the study by Hillerup and Stoltz, the tendency of recovery was evaluated based on the total CNT score, including scores for the perception of tactile and thermal stimuli, localization of the stimulus, and 2PD. The relationship between negative Tinel’s test and changing CNT findings over time had not yet been investigated. Our findings confirm the meaningful tendency among patients with negative Tinel’s test toward resilience and recovery from neuropathy. This may be attributable to improvement in the thick sensory nerves assessed in level A followed by gradual restoration of the normal function of the finer sensory nerves. We carefully performed both Tinel’s test and CNT at least twice at different time points. A positive Tinel’s test and poor CNT findings indicates severe LN disorder. In contrast, a negative Tinel’s test and mild symptomatic improvement with each performance of CNT findings may indicate spontaneous healing of the injured LN. Therefore, this is a meaningful diagnostic tool for oral surgeons who experience the difficulty in determining the need and appropriate timing of surgical management. Both Tinel’s test and CNT can be easily performed together, and their routine use by oral and maxillofacial surgeons should be encouraged.
Conclusion
CNT is an established and rational diagnostic criterion for determining the degree of trigeminal neuropathy. It may be supplemented with the Tinel test to identify LN injuries with the ability to heal spontaneously and distinguish them from those that require surgical management.
Availability of data and materials
The data is available from the corresponding author upon reasonable request.
Abbreviations
- LN:
-
Lingual nerve
- 2PD:
-
Two-point discrimination
- SWM:
-
Semmens Weinstein Monofilament
- CNT:
-
Clinical neurosensory testing
References
Kipp DP, Goldstein BH, Weiss WW (1980) Dysesthesia after mandibular third molar surgery: a retrospective study and analysis of 1,377 surgical procedures. J Am Dent Assoc 100:185–192. https://doi.org/10.14219/jada.archive.1980.0074
Mason DA (1988) Lingual nerve damage following lower third molar surgery. Int J Oral Maxillofac Surg 17:290–294. https://doi.org/10.1016/s0901-5027(88)80005-5
Robert RC, Bacchett PB, Pogrel MA (2005) Frequency of trigeminal nerve injuries following third molar removal. J Oral Maxillofac Surg 63:732–735. https://doi.org/10.1016/j.joms.2005.02.006
Kushnerev E, Yates JM (2015) Evidence-based outcomes following inferior alveolar and lingual nerve injury and repair: a systematic review. J Oral Rehabil 42:786–802. https://doi.org/10.1111/joor.12313. (Epub 2015 Jun 7)
Ziccardi VB, Rivera L, Gomes J (2009) Comparison of lingual and inferior alveolar nerve microsurgery outcomes. Quintessence Int 40:295–301
Miloro M (2004) Microneurosurgery. In:Miloro M(ed).Peterson’s principles of oral and maxillofacial surgery. 2nd edn.BC Decker Inc, Ontario, 2:819–837.
Susarla SM, Kaban LB, Donoff RB, Dodson TB (2007) Does early repair of lingual nerve injuries improve functional sensory recovery? J Oral Maxillofac Surg 65:1070–1076. https://doi.org/10.1016/j.joms.2006.10.010
Suhaym O, Miloro M (2020) Does early repair of trigeminal nerve injuries influence neurosensory recovery? A systematic review and meta-analysis. Int J Oral Maxillofac Surg 50:820–829. https://doi.org/10.1016/j.ijom.2020.10.002
Robinson PP, Loescher AR, Yates JM, Smith KG (2004) Current management of damage to the inferior alveolar and lingual nerves as a result of removal of third molars. Br J Oral Maxillofac Surg 42:285–292. https://doi.org/10.1016/j.bjoms.2004.02.024
Fujita S, Tojyo I, Yamada M, Go Y, Matsumoto T, Kiga N (2014) Outcome following lingual nerve repair with vein graft cuff: a preliminary report. J Oral Maxillofac Surg 72:1433.e1–7. https://doi.org/10.1016/j.joms.2014.03.018
Fujita S, Tojyo I, Nakanishi T, Suzuki S (2022) Comparison of prognosis in two methods for the lingual nerve repair: direct suture with vein graft cuff and collagen allograft method. Maxillofac Plast Reconstr Surg 44:6. https://doi.org/10.1186/s40902-022-00335-9
Ghali GE, Epker BN (1989) Clinical neurosensory testing: practical applications. J Oral Maxillofac Surg 47:1074–1078. https://doi.org/10.1016/0278-2391(89)90184-5
Zuniga JR, Meyer RA, Gregg JM, Miloro M, Davis LF (1998) The accuracy of clinical neurosensory testing for nerve injury diagnosis. J Oral Maxillofac Surg 56:2–8. https://doi.org/10.1016/s0278-2391(98)90904-1
Tinel J (1915) Le signe du “fourmillement” dans les lesions des nerfs peripheriques. Presse Med 23:388–389
Gregg JM (2013) Historical perspectives on trigeminal nerve injuries. In: Miloro M(ed) Trigeminal Nerve injuries, 1stedn. Springer-Verlag. Heidelberg, 1:1–16.
Hillerup S, Stoltze K (2007) Lingual nerve injury in third molar surgery 1. Observations on recovery of sensation with spontaneous healing. Int J Oral Maxillofac Surg 36:884–889. https://doi.org/10.1016/j.ijom.2007.06.004
Acknowledgements
We acknowledge the assistance provided by Professor Toshio Shimokawa of the Clinical Study Support Center, Wakayama Medical University, in performing the statistical analysis. We also thank Honyaku Center Inc. for English language editing.
Funding
The study was supported by a grant from the Grant-in-Aid for Scientific Research from Japan Society for the promotion of Science (No.18K09751).
Author information
Authors and Affiliations
Contributions
SF read and wrote the manuscript. IT and SS prepared the retrospective data. SS and FT analyzed and interpreted this data. SF designed and wrote the entire article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki for medical protocols and was approved by the Wakayama Medical University Institutional Review Board (approval number 1689). Written informed consent for the publication was obtained.
Consent for publication
Written informed consent for publication was obtained from the patients.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fujita, S., Tojyo, I., Suzuki, S. et al. Application of Tinel’s test combed with clinical neurosensory test distinguishes spontaneous healing of lingual nerve neuropathy after mandibular third molar extraction. Maxillofac Plast Reconstr Surg 45, 21 (2023). https://doi.org/10.1186/s40902-023-00389-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40902-023-00389-3