Abstract
Background
Prolonged electrocardiogram QRS durations are often present in hypertensive patients. Small increases in QRS duration serve as independent risk factors for both increased cardiovascular and all-cause mortality. Aortic stiffness is associated with increases in central aortic systolic blood pressure (CASP). However CASP and ECG QRS duration interactions have not been established in rural community populations. Our aims are to determine if QRS duration > 100 msec is associated with an elevated CASP measure in an Australian rural population.
Methods
A retrospective cross sectional population was obtained from the CSU Diabetes Screening Research Initiative data base where 68 participants had both central aortic pressure recorded and ECG derived QRS duration. Central aortic pressure was determined by directly recording radial arterial tonometry and brachial cuff pressure (HealthStats, Singapore). Resting 12-lead electrocardiograms were obtained from each subject using a Welch Allyn PC-Based ECG system.
Results
The population had a mean CASP of 137.8 mmHg, higher than previously reported in other population studies. In 8/68 subjects with a prolonged cardiac QRS duration >120 msec, CASP ranged from 129 mmHg – 182 mmHg. When subgroup analysis was stratified on the basis QRS duration <100 msec and ≥100 msec significant differences (p = 0.036) were observed for mean CASP, 130.6 mmHg ± 15.6 (SD) versus 140.6 mmHg ± 16.8 (SD), respectively.
Conclusions
Our results suggest that an arbitrary CASP reading greater than a value 140 mmHg raises suspicion of a prolonged QRS duration. QRS durations ≥100 msec in an aging rural population are associated with higher CASP measures. Our results also suggest in aging Australian rural populations CASP is likely to be elevated, possibly due to age related aortic stiffening.
Similar content being viewed by others
Background
Electrocardiogram (ECG) derived QRS durations >100 msec and >120 msec have been both associated with hypertension induced increases in left ventricular (LV) mass [1–4]. Myocardial hypertrophy can alter LV tissue repolarisation and slowing of transmural conduction velocity [5, 6]. Slowed conduction of electrical impulses through the cardiac ventricles is marked by increases in QRS duration [6]. Cardiac hypertrophy may be associated with impaired coronary vasodilator reserve, decreased capillary density, reduced connexin-43 expression, and increased individual cardiac myocyte cell diameter, all of which can result in slowed conduction velocity [7, 8].
The pathology of LVH (left ventricular hypertrophy) can be associated with increased central systolic pressure and is related to aortic stiffness [9, 10]. Aortic stiffness has been shown across various models and human populations to increase cardiac afterload and reduce coronary vasodilator reserve [11]. Persistent increases in cardiac afterload can result in cardiac hypertrophic signalling and reduced coronary perfusion. Increased cardiac mass and cardiac cellular structural and electrical remodelling as a result of increased aortic stiffening would theoretically predict an increase in QRS duration associated with developed cardiac hypertrophy [1, 12].
Aortic elastic properties are relevant to increases in central aortic systolic pressures, particularly aortic stiffening. The aorta has an elastic buffering component often referred to as a Windkessel [10]. That is the aorta can store about 50 % of the left ventricular stroke volume during systole. In diastole, the stored volume is released from the elastic forces of the aortic wall to assist peripheral circulation [10]. The elastic resistance (or stiffness), increases with aging [12, 13], with an increase in systolic central and peripheral blood pressure [10]. Chronic stiffness of large conduit vessels can lead to cardiac hypertrophy [2, 14].
On the basis of ventricular-aortic coupling, central aortic systolic pressure (CASP) has emerged as a more accurate predictor of cardiovascular structural hypertrophy than brachial pressures and pulse pressures [15]. Pressure in the brachial artery differs from pressure in the ascending aorta due to amplification as the pressure pulse transits from central to peripheral arteries [15]. Clinically, peripheral pressures do not always reflect central aortic systolic stiffening [15, 16]. Importantly, the degree of amplification is not fixed. CASP varies between individuals depending upon age, cardiovascular disease and/or drug therapy [15–17]. Central aortic pressure may be derived non-invasively by accurate registration, capture and calibration of a peripheral artery pressure waveform using an externally applied arterial sensor with or without a brachial pressure cuff reading [16]. The captured pressure waveform undergoes mathematical transformations to derive central aortic pressure, the validity of these processes having been established during direct invasive catheter measurements of the ascending aorta [17].
Considering the pathological implications for increased aortic stiffness and impaired ventricular aortic coupling, it would be logical to suggest that increased central aortic systolic pressure would be associated with increased QRS duration. However, to date there have been no studies in the literature exploring this association in a patient series using a CASP device. Our present study explores differences in ECG derived QRS duration in 68 rural subjects with and without type 2 diabetes and with and without hypertension. Interactions with age and CASP are modelled as factors that could influence prolonged QRS duration.
Methods
Human ethics clearance was obtained from Charles Stuart University (CSU) and written informed consent from the patients involved in the present study. A retrospective cross sectional population was obtained from the CSU Diabetes Screening Research Initiative (DiScRi) data base where 68 participants had both central aortic pressure recorded and ECG derived QRS duration. Subjects were excluded if pregnant, non-ambulatory, cognitively impaired, or had a history of heart failure. Two blood pressure (BP) readings at the upper arm were taken with the patient in a supine position, after resting for 30 min, and the average recorded. Central aortic pressure was determined using A-PULSE CASPro®, by directly recording radial arterial tonometry and brachial cuff pressure (HealthStats, Singapore) [15]. The A-PULSE CASPro® algorithm for determining CASP has been previous validated, for example a comparison made of CASP estimated with the B-Pro device and SphygmoCor (as the “gold standard” reference device) in 104 healthy Caucasians demonstrated an correlation coefficient (r = 0.937) [18]. There is also excellent agreement (r2 = 0.98, p < 0.001) between directly measured aortic root systolic pressures using a Millar’s catheter at cardiac catheterization versus NPMA-CASP HeathSTATS algorithm [15].
Resting 12-lead electrocardiograms were obtained from each subject using a Welch Allyn PC-Based ECG system as previously described [4]. This permitted automated QRS duration calculation from a 10 s ECG trace. Statistics were presented as means and standard deviation (SD); Pearson product–moment correlation analysis and regression analysis was used to explore age interactions with CASP and QRS duration. Statistical significance was defined as p < 0.05.
Results
The demographics for the 68 subjects are shown in Table 1. The mean age was 69.4 years and 8 subjects out of 68 subjects had QRS durations greater than 120 msec. Mean CASP levels for the study population were 138 mmHg ± 17 mmHg. The data was divided on patients self-reporting being hypertensive or not. Those diagnosed with hypertension were being treated. The consequences of being treated or not being treated with hypertension medications revealed no significant differences for mean QRS duration (104 ± 17 msec vs 107 ± 15 msec (BP treated) or mean CASP levels (134 ± 20 mmHg vs 139 ± 14 mmHg (BP treated).
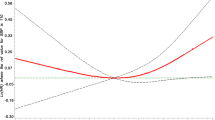
Pearson’s correlation analysis showed a modest but significant association between QRS duration and CASP (r = 0.25; p = 0.039). Both QRS duration (r = 0.25; p = 0.043) (Fig. 1) and CASP (r = 0.41; p = 0.001) (Fig. 2) had a significant but modest association with age. When we stratified subpopulations on the basis of QRS duration <100 msec and ≥100 msec significant differences (p = 0.036) were observed for mean CASP, 130.6 ± 15.6 (SD) versus 140.6 ± 16.8 (SD), respectively. The mean CASP in subjects with a QRS duration > 120 msec was 145.25 mmHg ± 14.9(SD). Of the subjects with a QRS duration >120 msec only 1 subject had a QRS duration less than 130 msec, and 4/8 subjects had a QRS duration >140 msec. Current guidelines have defined a suspicion for left bundle branch block (LBBB) using a QRS duration of ≥130 msec in women and a QRS duration of ≥ 140 msec in men [19]. Four subjects met the definition for suspicion of LBBB. The average CASP for these 4 cases was 148 ± 14 mmHg. When these 4 suspected LBBB cases were removed, the statistical difference in mean CASP stratified on the basis of QRS duration <100 msec (130.6 ± 15.6 mmHg) and ≥100 msec (140 ± 14 mmHg) was not significant at p = 0.051.
Discussion and conclusions
In previous published population studies with age ranges 40 - 65 we have noted a median CASP of 115 mmHg [9]. We arbitrarily set a conservative CASP cut-off of 110 mm/Hg as being within normal limits for healthy aging individuals [10]. Therefore, we defined a CASP >110 mmHg as being abnormal. The mean CASP for our cohort was 137.8 mmHg – only 3/68 subjects in our cohort had a CASP less than 110 mm/Hg (Table 1). When we explored CASP readings in the 8 subjects with a QRS duration >120 msec we found that the mean CASP to be 145 mm/Hg (range 127–167 mmHg) (Table 2).
Four cases with QRS duration > 120 msec were associated with LBBB risk using the QRS duration cut-offs described in the Strauss criteria [19]. We did not have access to the original ECG traces, only the automated QRS durations for retrospective analysis, thus mid-QRS notching in the leads to confirm LBBB using the Strauss criteria were not available [19]. On the other hand we question whether LBBB was present, given that CASP was elevated for each case. For example, one case study of interment LBBB, when compared to normal conduction, LBBB was associated with a 20 mmHg decline in systolic pressures in the left ventricle, central aorta and radial artery [20].
CASP increases by approximately 3–4 mmHg per decade of life [15], while the difference between our cohort overall and those patients with a prolonged QRS was estimated to be double this value at 7 mmHg per decade of life. Our findings demonstrate that in an Australian aging rural population, mean CASP are greater (138 mmHg) than those reported in other published community studies with mixed healthy and chronic cardiovascular disease participants (115–130 mmHg) [21–23]. However, the mean CASP for our mixed population (138 mmHg) are not dissimilar to central aortic systolic pressures reported in patients undergoing cardiac catheter coronary interventions (139.6 ± 1.4 mmHg) [24]. Surprisingly, despite the mean CASP in our mixed, aging, rural population being higher than previous studies, elevated CASP in our aging cohort were rarely associated with an increase in QRS width greater than 120 msec. We observed no differences in QRS durations in those being treated for diagnosed hypertension and those not being treated (i.e. subjects without known hypertension).
Like most Australian rural populations, patients are medically undertreated for blood pressure or are non compliant with therapy. Previously, we have identified a low usage of ACE inhibitors in the rural area of Albury [25]. ACE inhibitors have been shown to reduce aortic stiffening, independent of their blood pressure lowering effects [26]. We suggest that blood pressure medication other than ACE inhibitors may have favourably influenced ventricular remodelling. For example, it has been shown that that carvedilol results in a favourable pulse pressure amplification and augmentation index by increasing arterial compliance and reducing the magnitude of wave reflection in hypertension [27]. Our cohort was older (median 70 years) than previous published data on median CASP levels and this may explain in part why our mean CASP levels were higher than in previous reports.
Additionally our aging cohort would have other inflammatory chronic conditions that would also increase vascular stiffness [28–30]. We suggest because of the likely higher burden of inflammatory conditions associated with more advanced aging, it is not surprising to find an elevated CASP in our cohort.
We acknowledge our sample size was small and therefore, the validity of the signal averaged ECG QRS duration in association with CASP needs to be verified in a larger study. Our study is cross-sectional in design and therefore we are careful not to infer causality. In summary, our results suggest that an arbitrary CASP reading greater than a value 140 mmHg raises suspicion of a prolonged QRS duration >120mesc. QRS durations ≥100 msec in an aging rural population are associated with higher CASP measures. Our results also suggest in aging Australian rural populations CASP is likely to be elevated, possibly due to age related aortic stiffening [30] and increased CASP does not always equate with a prolonged QRS duration.
References
Brenyo A, Zareba W. Prognostic significance of QRS duration and morphology. Cardiol J. 2011;18:8–17.
Dhingra R, Ho Nam B, Benjamin EJ, Wang TJ, Larson MG, D’Agostino Sr RB, et al. Cross-sectional relations of electrocardiographic QRS duration to left ventricular dimensions: the Framingham heart study. J Am Coll Cardiol. 2005;45:685–9.
Oikarinen L, Nieminen MS, Viitasalo M, Toivonen L, Jern S, Dahlöf B, et al. QRS duration and QT interval predict mortality in hypertensive patients with left ventricular hypertrophy: the Losartan intervention for endpoint reduction in hypertension study. Hypertension. 2004;43(5):1029–34.
Leng YL, Zhou Y, Ke H, Jelinek H, McCabe J, Assareh H, et al. Electrocardiogram derived QRS duration >120 ms is associated with elevated plasma homocysteine levels in a rural Australian cross-sectional population. Medicine (Baltimore). 2015;94(27):e1080.
Hill JA. Hypertrophic reprogramming of the left ventricle: translation to the ECG. J Electrocardiol. 2012;45(6):624–9.
Bacharova L, Szathmary V, Kovalcik M, Mateasik A. Effect of changes in left ventricular anatomy and conduction velocity on the QRS voltage and morphology in left ventricular hypertrophy: a model study. J Electrocardiol. 2010;43(3):200–8.
Li J, Patel VV, Kostetskii I, Xiong Y, Chu AF, Jacobson JT, et al. Cardiac-specific loss of N-cadherin leads to alteration in connexins with conduction slowing and arrhythmogenesis. Circ Res. 2005;97(5):474–81.
Greve AM, Gerdts E, Boman K, Gohlke-Baerwolf C, Rossebø AB, Devereux RB. Impact of QRS duration and morphology on the risk of sudden cardiac death in asymptomatic patients with aortic stenosis: the SEAS (Simvastatin and Ezetimibe in Aortic Stenosis) study. J Am Coll Cardiol. 2012;59(13):1142–9.
Milan A, Tosello F, Naso D, Avenatti E, Leone D, Magnino C, et al. Ascending aortic dilatation, arterial stiffness and cardiac organ damage in essential hypertension. J Hypertens. 2013;31:109–16.
Kass DA. Ventricular arterial stiffening integrating the pathophysiology. Hypertension. 2005;46(1):185–93.
Nemes A, Csanády M, Forster T. Does increased aortic stiffness predict reduced coronary flow velocity reserve in patients with suspected coronary artery disease? Acta Physiologica Hungarica. 2012;99(3):271–8.
Cooper LL, Odening KE, Hwang MS, Chaves L, Schofield L, Taylor CA, et al. Electromechanical and structural alterations in the aging rabbit heart and aorta. Am J Physiol Heart Circ Physiol. 2012;302(8):H1625–35.
Li Y, Staessen JA, Sheng CS, Huang QF, O’Rourke M, Wang JG. Age dependency of peripheral and central systolic blood pressures: cross-sectional and longitudinal observations in a Chinese population. Hypertens Res. 2011;35(1):115–22.
DeLoach SS, Daskalakis C, Gidding S, Falkner B. Central blood pressures are associated with left ventricular mass index among African-American adolescents. Am J Hypertens. 2012;25(1):41–5.
Williams B, Lacy PS, Yan P, Hwee CN, Liang C, Ting CM. Development and validation of a novel method to derive central aortic systolic pressure from the radial pressure waveform using an N-point moving average method. J Am Coll Cardiol. 2011;57(8):951–61.
Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure validation of generalized transfer function. Circulation. 1997;95(7):1827–36.
Theilade S, Hansen TW, Joergensen C, Lajer M, Rossing P. Tonometric devices for central aortic systolic pressure measurements in patients with type 1 diabetes: comparison of the BPro and SphygmoCor devices. Blood Press Monit. 2013;18(3):156–60.
Garcia-Ortiz L, Recio-Rodríguez JI, Canales-Reina JJ, Cabrejas-Sánchez A, Gomez-Arranz A, Magdalena-Belio JF, et al. Comparison of two measuring instruments, B-pro and SphygmoCor system as reference, to evaluate central systolic blood pressure and radial augmentation index. Hypertens Res. 2012;35(6):617–23.
Galeotti L, van Dam PM, Loring Z, Chan D, Strauss DG. Evaluating strict and conventional left bundle branch block criteria using electrocardiographic simulations. Europace. 2013;15(12):1816–21.
Bourassa MG, Khairy P, Roy D. An early proof-of-concept of cardiac resynchronization therapy. World J Cardiol. 2011;3(12):374–6.
McEniery CM, Yasmin, McDonnell B, Munnery M, Wallace SM, Rowe CV, et al. Central pressure: variability and impact of cardiovascular risk factors: the Anglo-Cardiff Collaborative Trial II. Hypertension. 2008;51:1476–82.
Recio-Rodriguez JI, Gomez-Marcos MA, Patino-Alonso MC, Romaguera-Bosch M, Grandes G, Menendez-Suarez M, et al. Association of television viewing time with central hemodynamic parameters and the radial augmentation index in adults. Am J Hypertens. 2013;26:488–94.
Sule AA, Hwang TH, Chin TJ. Very high central aortic systolic pressures in a young hypertensive patient on telmisartan: is central aortic systolic pressure associated with white coat hypertension? Int J Angiol. 2010;19(4):e132–4.
Shih YT, Cheng HM, Sung SH, Hu WC, Chen CH. Comparison of two generalized transfer functions for measuring central systolic blood pressure by an oscillometric blood pressure monitor. J Hum Hypertens. 2013;27:204–10.
Ng E, Lu Y, Hambly B, Jelinek HF, Yu B, Matthews S, et al. Angiotensin-converting enzyme gene DD genotype is associated with increased systolic blood pressure in an Australian rural type 2 diabetic cohort. Hypertens Res. 2013;36(4):381–2.
Asmar R. Effect of antihypertensive agents on arterial stiffness as evaluated by pulse wave velocity: clinical implications. Am J Cardiovasc Drugs. 2001;1(5):387–97.
Shah NK, Smith SM, Nichols WW, Lo MC, Ashfaq U, Satish P, et al. Carvedilol reduces aortic wave reflection and improves left ventricular/vascular coupling: a comparison with atenolol (CENTRAL study). J Clin Hypertens (Greenwich). 2011;13:917–24.
Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46(5):1118–22.
Fan F, Galvin A, Fang L, White DA, Moore XL, Sparrow M, et al. Comparison of inflammation, arterial stiffness and traditional cardiovascular risk factors between rheumatoid arthritis and inflammatory bowel disease. J Inflamm (Lond). 2014;11(1):29.
Butlin M, Avolio AP. Age-related changes in the mechanical properties of large arteries. In: Derby B, Akhtar R (eds). Mechanical properties of aging soft tissues. Springer International Publishing; 2015. pp 37-74
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YLYL – Assisted with the drafting of the manuscript and performed statistical analysis; PR performed all CASP measurements; HJ is curator of the demographic data base; YSL critically reviewed and contributed to the final manuscript; YZ – reviewed and contributed to the final manuscript; BH contributed to the final manuscript through critical review; JM contributed to the interpretation of data around QRS duration; SM reviewed and contributed to the final manuscript; HK provided assistance with the final manuscript; HA provided review and contributed to the final manuscript; CSM designed the study, introducing the concept of CASP measures and was the chief writer of initial and final versions of the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lee, Y.Y., Reidler, P., Jelinek, H. et al. Electrocardiogram derived QRS duration associations with elevated central aortic systolic pressure (CASP) in a rural Australian population. Clin Hypertens 22, 6 (2015). https://doi.org/10.1186/s40885-016-0039-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40885-016-0039-8