Abstract
Background
The optimal strategy for adjuvant therapy after curative resection for hepatocellular carcinoma (HCC) patients with solitary tumor and microvascular invasion (MVI) is controversial. This trial evaluated the efficacy and safety of adjuvant transcatheter arterial chemoembolization (TACE) after hepatectomy versus hepatectomy alone in HCC patients with a solitary tumor ≥ 5 cm and MVI.
Methods
In this randomized, open-labeled, phase III trial, HCC patients with a solitary tumor ≥ 5 cm and MVI were randomly assigned (1:1) to receive either 1–2 cycles of adjuvant TACE after hepatectomy (Hepatectomy-TACE) or hepatectomy alone (Hepatectomy Alone). The primary endpoint was disease-free survival (DFS); the secondary endpoints included overall survival (OS) and adverse events.
Results
Between June 1, 2009, and December 31, 2012, 250 patients were enrolled and randomly assigned to the Hepatectomy-TACE group (n = 125) or the Hepatectomy Alone group (n = 125). Clinicopathological characteristics were balanced between the two groups. The median follow-up time from randomization was 37.5 months [interquartile range 18.3–48.2 months]. The median DFS was significantly longer in the Hepatectomy-TACE group than in the Hepatectomy Alone group [17.45 months (95% confidence interval [CI] 11.99–29.14) vs. 9.27 months (95% CI 6.05–13.70), hazard ratio [HR] = 0.70 (95% CI 0.52–0.95), P = 0.020], respectively. The median OS was also significantly longer in the Hepatectomy-TACE group than in the Hepatectomy Alone group [44.29 months (95% CI 25.99–62.58) vs. 22.37 months (95% CI 10.84–33.91), HR = 0.68 (95% CI 0.48–0.97), P = 0.029]. Treatment-related adverse events were more frequently observed in the Hepatectomy-TACE group, although these were generally mild and manageable. The most common grade 3 or 4 adverse events in both groups were neutropenia and liver dysfunction.
Conclusion
Hepatectomy followed by adjuvant TACE is an appropriate option after radical resection in HCC patients with solitary tumor ≥ 5 cm and MVI, with acceptable toxicity.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide [1] and the second leading cause of cancer-related death in China [2]. An estimated 466,100 new HCC cases and 422,100 deaths occurred in China in 2015 [3]. Surgical resection remains the main radical treatment for HCC, although the recurrence rate after hepatectomy is high and hampers further improvement in the prognosis of HCC patients [4, 5]. The conventional risk factors for recurrence include tumor size, multiple lesions, vascular invasion, poor differentiation, and tumor rupture [6,7,8]. Over the past decade, microvascular invasion (MVI) has been proposed as a potential risk factor for recurrence after hepatectomy [9, 10]. Recent studies have confirmed the significance of MVI in postoperative recurrence [11,12,13]. A previous study by our research group also showed that the recurrence rate was over 50% for HCC patients with solitary tumor ≥ 5 cm and MVI, where MVI was confirmed as the only independent risk factor for overall survival (OS) and disease-free survival (DFS) among that cohort [14].
Different therapeutic agents and/or approaches have been evaluated as adjuvant therapy for HCC after curative resection, including interferon [15], oral chemotherapeutic agents (1-hexylcarbamoyl-5-fluorouracil (HCFU) [16] and capecitabine [17]), hepatic arterial infusion chemotherapy [18], and targeted therapy (sorafenib) [19]. Unfortunately, it has been shown that most of these approaches did not reduce the risk of recurrence or were poorly tolerated, and, most importantly, these strategies were not associated with significant survival benefits [20]. As such, an optimal adjuvant therapy with respect to efficacy, safety, and cost-effectiveness remains to be defined. Our previous phase III randomized clinical study indicated that transcatheter arterial chemoembolization (TACE) may be an appropriate adjuvant therapy option for stage IIIA HCC patients [21]. Therefore, this present phase III clinical trial was designed to evaluate the efficacy and safety of radical hepatectomy plus adjuvant TACE (Hepatectomy-TACE), compared with radical hepatectomy alone (Hepatectomy Alone), in HCC patients with solitary tumor ≥ 5 cm and MVI after curative resection.
Methods
Trial design
This study was an open-labeled, randomized, phase III trial conducted at the Sun Yat-sen University Cancer Center (Guangzhou, China), designed to evaluate the efficacy and safety of radical hepatectomy plus adjuvant TACE versus radical hepatectomy alone among HCC patients with solitary tumor ≥ 5 cm and MVI after curative resection. The protocol and all modifications were approved by the Institutional Review Board and Ethics Committee of our cancer center. This study complied with the Declaration of Helsinki and the Good Clinical Practice Guidelines (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), Version E6) [22]. All patients provided written informed consents. This study was registered in ClinicalTrials.gov (http://ClinicalTrials.gov, trial number NCT02788526) on March 23, 2016.
Eligibility criteria
The eligibility criteria for inclusion were as follows: 18–75 years of age; histologically confirmed HCC with MVI (MVI was defined by the presence of tumor emboli within either the central hepatic vein, the portal, or the large capsular vessels [23]); Eastern Cooperative Oncology Group performance score (ECOG PS) ≤ 2; no previous treatment for HCC; solitary tumor ≥ 5 cm before surgery confirmed by 2 radiological examinations (ultrasonography with computer tomography or magnetic resonance imaging); R0 resection; no evidence of recurrence at radiological follow-up at 3–5 weeks after surgery; adequate hematologic, hepatic, and renal functions. The exclusion criteria included histologically positive resection margin (R1 resection); evidence of recurrence at radiological follow-up 3–5 weeks after surgery; history of organ transplantation; active uncontrolled infection; allergy to any TACE agent; other malignancies over the preceding 5 years before the HCC diagnosis, except for adequately treated carcinoma in situ of the cervix and squamous or basal cell carcinoma of the skin; pregnancy, breastfeeding, or lack of use of adequate contraception among women of childbearing potential; neurological or psychiatric disorders that may affect cognitive assessment and inform consent; concomitant antitumor therapy or participation in other interventional clinical trials.
Hepatectomy
All surgical resection procedures were performed following the techniques described in our previous study [10]. Briefly, routine abdominal exploration was carefully performed to evaluate the extent of the tumor and to exclude extrahepatic metastases. After adequate mobilization of the liver, we used intraoperative ultrasound (ALOKA SSD-5500, Tokyo, Japan) to assess the number of lesions and tumor size, the presence of MVI, and the extent of resection. During tumor removal, the liver parenchyma was separated using the Cavitron Ultrasonic Surgical Aspirator (Integra LifeSciences CUSA Excel, Plainsboro, NJ, USA), and the involved vessels were ligated. The Pringle maneuver was also applied to occlude blood inflow to the liver.
Randomization
All patients were screened for enrollment at the first follow-up (3–5 weeks after hepatectomy). Full patient assessment, including demographic characteristics, medical history, physical examination, routine blood analysis (hematology and biochemistry), and radiological examinations [computed tomography (CT) or magnetic resonance imaging (MRI)], were performed within 1 week of the study enrollment. The patients with evidence of recurrence during the screening for enrollment were excluded. Then the eligible patients were randomly assigned (at a 1:1 ratio) to receive either 1–2 cycles of adjuvant TACE (Hepatectomy-TACE group) or routine follow-up without adjuvant treatment (Hepatectomy Alone group). Randomization was performed using a sealed envelope system according to a predesigned random number.
Adjuvant TACE
The patients in the Hepatectomy-TACE group underwent TACE 4–6 weeks after hepatectomy according to liver function and performance status. TACE was performed using the techniques we have described previously [24]. In brief, a catheter was placed into the proper hepatic artery through the femoral artery using the Seldinger technique, hepatic arterial angiography was performed, and 200 mg/m2 carboplatin (Carboplatin, Bristol-Myers Squibb, New York, NY, USA) and 6 mg/m2 mitomycin (Mitomycin, Hisun, Taizhou, China) were infused followed by 4–5 mL of the emulsion of iodized oil (lipiodol, Andre Guerbet, Aulnay-sous-Bois, France) and 40 mg/m2 epirubicin (Epirubicin Hydrochloride, Pfizer, New York, NY, USA). After 4–6 weeks, these patients underwent a complete assessment consisting of physical examination, routine blood analysis, and CT scan. The second cycle of TACE was performed according to the decision of investigators based on the patients’ conditions and the assessment results.
Follow-up
All patients were followed-up at an interval of 2–3 months. To avoid the potential effect of hepatitis B virus (HBV) reactivation on recurrence, all patients with positive serum hepatitis B surface antigen (HBsAg) were administered with routine antiviral therapy with lamivudine (GlaxoSmithKline, Brentford, UK; 100 mg, once daily) or entecavir (Bristol-Myers Squibb, New York, USA; 0.5 mg, once daily). At each follow-up visit, physical examination, blood test (serum alpha-fetoprotein [AFP] and liver function), and enhanced abdominal CT or MRI scan were performed. Once suspicious recurrence/metastasis was detected, further examinations including hepatic angiography or biopsy were conducted. Recurrence/metastasis was confirmed based on the cytologic/histologic evidence or on the non-invasive diagnostic criteria for HCC by the European Association for the Study of Liver [7]. Patients with recurrence in both groups received subsequent treatment according to the decision of the multi-disciplinary team of our cancer center. Adverse events (AEs) were recorded from the day of randomization to the last day of follow-up. Toxicity was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0). The study was censored on March 31, 2016.
Statistical analyses
The primary endpoint was DFS and was defined as from the time of randomization to the diagnosis of recurrence or death from any cause. The secondary endpoints included OS, which was defined as from the time of randomization to the date of the last follow-up or death, and AEs.
Assuming an increase in median DFS of 6 months between the Hepatectomy-TACE group (18.0 months) and Hepatectomy Alone group (12.0 months) [hazard ratio (HR) 0.66], it was estimated that 176 events and a total of 210 patients (105 in each group) were required for randomization to achieve a statistical power of 85% with a significance level of 0.05 for a one-sided error. All analyses were performed according to the per-protocol principle. Survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test. The median survival with 95% confidence interval (CI) was calculated. Cox proportional analyses were performed to estimate HRs with 95% CIs. The t-test was used for group comparisons of AEs. We also performed subgroup analyses for sex (male vs. female), age (< 60 years vs. ≥ 60 years), ECOG PS (0 vs. 1–2), tumor size (5–10 cm vs. > 10 cm), cirrhosis (present vs. absent), and resection margin (< 2 cm vs. ≥ 2 cm). All statistical tests were performed with the Statistical Package for the Social Sciences (SPSS) (version 23, Chicago, IL, USA), Stata (version 13, College Station, TX, USA), and Medcalc (version 16.1, Acacialaan, Belgium) statistical software, and P values < 0.05 were considered significant.
Results
Patient characteristics and treatment administration
Between June 1, 2009, and December 31, 2012, 250 patients were enrolled and randomly assigned to receive 1–2 cycles of adjuvant TACE after radical hepatectomy (the Hepatectomy-TACE group, n = 125) or hepatectomy alone (the Hepatectomy Alone group, n = 125). In the Hepatectomy-TACE group, 2 patients withdrew consent because of the potential toxicity of TACE, 3 patients had antitumor Chinese herbal prescriptions with HCC indications, and 4 patients were lost to follow-up. These patients were therefore excluded from the analysis. In the Hepatectomy Alone group, 4 patients had antitumor Chinese herbal prescriptions, and 3 patients were lost to follow-up and were also excluded from the analysis (Fig. 1).
Baseline demographic and clinical characteristics were well balanced between the two groups (Table 1). The median follow-up time for the entire cohort was 37.5 months from randomization [interquartile range (IQR), 18.3–48.2 months]. In the Hepatectomy-TACE group, 55 patients underwent 1 cycle of TACE, and 61 patients underwent 2 cycles of TACE.
Operative variables and postoperative outcomes
The operative variables and postoperative outcomes observed from the first day after hepatectomy till the date of discharge are summarized in Table 2. Twenty-four and 23 complications occurred in the Hepatectomy-TACE and Hepatectomy Alone group, respectively, including grade 1–2 fever, ascites, transient jaundice, pleural effusion, and hypoalbuminemia. One patient in the Hepatectomy-TACE group and 2 patients in the Hepatectomy Alone group experienced grade 3 liver bleeding. No patient died of complications during hospitalization.
Efficacy of treatment
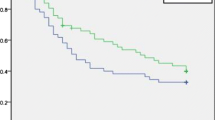
The median DFS was 17.45 months (95% CI 11.99–29.14) in the Hepatectomy-TACE group and 9.27 months (95% CI 6.05–13.70) in the Hepatectomy Alone group (HR = 0.70, 95% CI 0.52–0.95, P = 0.020; Fig. 2a). By March 31, 2016, 168 (71.8%) of the 234 patients had experienced recurrence (83 in the Hepatectomy-TACE group and 85 in the Hepatectomy Alone group). The 1-, 2-, 3-, and 5-year DFS rates for the Hepatectomy-TACE group were 58.6%, 44.7%, 38.4%, and 26.7% and were 43.5%, 30.6%, 26.5%, and 22.6% for the Hepatectomy Alone group, respectively.
Kaplan-Meier estimates illustrating the differences in a disease-free survival (DFS) and b overall survival (OS) of the enrolled patients who underwent radical hepatectomy alone against those who had radical hepatectomy and adjuvant TACE. TACE: transcatheter arterial chemoembolization; HR: hazard ratio; CI: confidence interval
The median OS for the Hepatectomy-TACE group was 44.29 months (95% CI 25.99–62.58) and was 22.37 months (95% CI 10.84–33.91) in the Hepatectomy Alone group (HR = 0.68, 95% CI 0.48–0.97, P = 0.029; Fig. 2b). By March 31, 2016, 128 (54.7%) of the 234 enrolled patients had died (62 in the Hepatectomy-TACE group and 66 in the Hepatectomy Alone group). The 1-, 2-, 3-, and 5-year OS rates for the Hepatectomy-TACE group were 87.8%, 64.3%, 53.5%, and 40.2% and were 67.2%, 49.8%, 43.6%, and 28.8% for the Hepatectomy Alone group, respectively.
The results of subgroup analyses were generally consistent with those of the primary analyses. It indicated that male patients, age < 60 years, presence of cirrhosis, tumor > 10 cm, and resection margin < 2 cm were associated with a greater DFS (Fig. 3a) and OS benefits (Fig. 3b) from adjuvant TACE.
The subgroup analysis of the a disease-free survival (DFS) and b overall survival (OS) of enrolled patients who underwent radical hepatectomy only compared to those who had radical hepatectomy and adjuvant TACE. HR: hazard ratio; CI: confidence interval; ECOG PS: Eastern Cooperative Oncology Group performance score. Survival data are presented as median with 95% CI in parentheses
After recurrence, 56 patients (67.5%) in the Hepatectomy-TACE group and 46 patients (54.1%) in the Hepatectomy Alone group underwent subsequent antitumor therapies, including locoregional ablation, hepatectomy, systemic chemotherapy, sorafenib, and TACE (summarized in Table 3).
Safety of treatment
Grade 3–4 AEs from the time of randomization to the last day of follow-up were reported in 25 patients (21.6%) from the Hepatectomy-TACE group and 10 patients (8.5%) in the Hepatectomy Alone group (Table 4). Fever, nausea/vomiting, and liver dysfunction were the most common AEs in the Hepatectomy-TACE group. The most common grade 3 or 4 adverse events in both groups were neutropenia and liver dysfunction. However, most AEs were mild and manageable and no toxicity-associated deaths occurred in this study.
Discussion
In this open-labeled, randomized, phase III trial, we evaluated the efficacy and safety of adjuvant TACE versus hepatectomy alone among HCC patients with solitary tumor ≥ 5 cm and MVI after curative resection. The results showed that, compared with the Hepatectomy Alone group, the Hepatectomy-TACE group had significantly both prolonged median DFS (17.45 vs. 9.27 months, HR = 0.70, P = 0.020) and OS (44.29 vs. 22.37 months, HR = 0.68, P = 0.029) from randomization. In subgroup analyses, we found that male patients, age < 60 years, presence of cirrhosis, tumor > 10 cm, and resection margin < 2 cm may derive a greater survival benefit from adjuvant TACE and that these factors should be considered in the selection process for future clinical trials.
MVI is a recognized risk factor for recurrence after hepatectomy in HCC patients. The presence of MVI is associated with multiple factors including tumor size. In an international multicenter study which enrolled 1073 HCC patients, Pawlik et al. [25] reported that the rate of MVI increased with tumor size (≤ 3.0 cm, 25%; 3.1–5.0 cm, 40%; 5.1–6.5 cm, 55%; and > 6.5 cm, 63%) (P < 0.005). Among patients with solitary tumor only, MVI occurred more frequently with tumors measuring 5.1–6.5 cm (41%) than for those with tumors measuring ≤ 5.0 cm (27%) (P < 0.003). Although wide resection margins may decrease the postoperative recurrence rate and improve survival outcomes [10], however, adequate resection margins were often unachievable due to the cumbersome tumor location and concomitant cirrhosis. Also, MVI beyond the resection margin may become the origin of recurrence. Also, in this study, we excluded patients with solitary tumors < 5 cm because they had a relatively low risk of recurrence due to the low rate of MVI and the high achievability of wide resection margins.
Unfortunately, there is no universally accepted adjuvant therapy for HCC patients with MVI in which efficacy, safety, and cost-effectiveness are conclusive. Some studies have evaluated TACE as a single adjuvant approach or in combination with other therapies (including antiviral therapy and interferon-α) for HCC patients with high risks of recurrence after resection [26,27,28,29]. In addition, a recent retrospective study also showed that postoperative adjuvant TACE could prolong the recurrence-free survival (RFS) and OS among HCC patients with MVI [30]. As such, these studies provided the rational evidence to select TACE as an adjuvant therapy in this present study.
Compared with the participants in the above studies, who showed high heterogeneity in tumor stage, the participants were relatively homogeneous in our present study. Solitary HCC is considered as a curable disease, and patients usually undergo more aggressive surgical treatment, although there is no recommended adjuvant therapy in the current official guidelines for these patients with solitary HCC and MVI. To our knowledge, this is the first study to report the value of adjuvant TACE in this specific population.
Interestingly, adjuvant TACE significantly reduced early recurrence rate (within 2 years) after hepatectomy. The 1- and 2-year DFS rates were 58.6% and 44.7% for the Hepatectomy-TACE group and 43.5% and 30.6% for the Hepatectomy Alone group, respectively. However, this difference was less obvious when comparing the 5-year DFS rate between the two groups (26.7% in Hepatectomy-TACE group vs. 22.6% in Hepatectomy Alone group). MVI was found to be the only independent risk factor for early recurrence, which is consistent with our previous study [31]. With the stimulation of multiple growth factors after hepatectomy, occult tumor cells proliferate rapidly and form visible recurrences as the remnant liver regenerates. The high sensitivity of actively proliferating tumor cells to chemotherapeutic agents may be an important reason for the decreased in early recurrence rate in the Hepatectomy-TACE group. Conversely, adjuvant TACE could increase the local concentration of chemotherapeutic agents in the liver, potentially avoiding the undesirable adverse events of systemic chemotherapy.
We also analyzed the underlying reasons for the considerably prolonged OS in the Hepatectomy-TACE group. After diagnosis of tumor recurrence, only 46 patients (54.1%) in the Hepatectomy Alone group underwent subsequent antitumor therapies (such as locoregional ablation, hepatectomy, systemic chemotherapy, sorafenib, and TACE; Table 3), which were less than those in the Hepatectomy-TACE group where greater proportion of patients, 67.5% (56 patients) had antitumor therapies and therefore may have resulted in a shorter OS in the Hepatectomy Alone group. Furthermore, as shown in Table 3, a greater number of patients with recurrence in the Hepatectomy-TACE group underwent locoregional ablation and were prescribed with sorafenib as a subsequent antitumor therapy. This may reflect the fact that recurrence was often localized and controllable. Conversely, more patients with recurrence in the Hepatectomy Alone group underwent relative palliative TACE, which may be associated with more extensive recurrence, as well as unfavorable factors, such as macrovascular tumor thrombus and extrahepatic metastases. However, our results should be interpreted with caution since the impact of adjuvant TACE on recurrence patterns, together with the direct therapeutic effects of adjuvant TACE itself, might collectively contribute to the survival benefits in the Hepatectomy-TACE groups.
As an important trial, the adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM) trial did not reach its primary endpoint of prolonging RFS [19]. The negative results of the STORM trial suggested that antitumor activity against existing or advanced HCC is not necessarily associated with efficacy in the adjuvant setting against micro-metastatic disease. In the absence of established predictive biomarkers of response to sorafenib in patients with advanced HCC, a population potential benefit from adjuvant sorafenib cannot be defined [32]. Besides, the STORM trial underscored the importance of selecting appropriate candidates with a high recurrence risk in such adjuvant settings.
Despite the results of this study demonstrating the superiority of adjuvant TACE over radical hepatectomy alone, there are still some limitations worth mentioning in this study. First, this is a single-center study. To validate the significance of adjuvant TACE in this specific population, a prospective, well-designed, multicenter, and randomized trial is necessary. Second, recent studies have reported that not only the presence of MVI but also the grade of MVI can impact the recurrence and survival of HCC patients [33, 34]. However, we did not investigate the grade of MVI due to the early design of this study protocol. Third, the optimal adjuvant TACE protocol (including chemotherapeutic agents, dosage, and interval) remains to be elucidated and further studies are required.
Conclusions
Our findings demonstrate the survival and safety benefits of adjuvant TACE in HCC patients with solitary tumor ≥ 5 cm and MVI after curative resection. However, future prospective, multicenter, randomized clinical trials are necessary to evaluate the optimal TACE regimens (including drugs and dosages) and the feasibility of combination with other antitumor therapies.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. https://doi.org/10.3322/caac.21262.
He MK, Le Y, Li QJ, Yu ZS, Li SH, Wei W, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Cancer. 2017;36(1):83. https://doi.org/10.1186/s40880-017-0251-2.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. https://doi.org/10.3322/caac.21338.
Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48(Suppl 1):S20–37. https://doi.org/10.1016/j.jhep.2008.01.022.
de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56(Suppl 1):S75–87. https://doi.org/10.1016/S0168-8278(12)60009-9.
Bruix J, Sherman M, American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–2. https://doi.org/10.1002/hep.24199.
European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–43. https://doi.org/10.1016/j.jhep.2011.12.001.
Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38(2):200–7.
Shi M, Zhang CQ, Zhang YQ, Liang XM, Li JQ. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg. 2004;28(4):376–81. https://doi.org/10.1007/s00268-003-7308-x.
Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS, Zhang CQ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245(1):36–43. https://doi.org/10.1097/01.sla.0000231758.07868.71.
Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254(1):108–13. https://doi.org/10.1097/SLA.0b013e31821ad884.
Hung HH, Lei HJ, Chau GY, Su CW, Hsia CY, Kao WY, et al. Milan criteria, multi-nodularity, and microvascular invasion predict the recurrence patterns of hepatocellular carcinoma after resection. J Gastrointest Surg. 2013;17(4):702–11. https://doi.org/10.1007/s11605-012-2087-z.
Du M, Chen L, Zhao J, Tian F, Zeng H, Tan Y, et al. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer. 2014;14:38. https://doi.org/10.1186/1471-2407-14-38.
Li SH, Wei W, Guo RP, Shi M, Guo ZX, Chen ZY, et al. Long-term outcomes after curative resection for patients with macroscopically solitary hepatocellular carcinoma without macrovascular invasion and an analysis of prognostic factors. Med Oncol. 2013;30(4):696. https://doi.org/10.1007/s12032-013-0696-3.
Chen LT, Chen MF, Li LA, Lee PH, Jeng LB, Lin DY, et al. Long-term results of a randomized, observation-controlled, phase III trial of adjuvant interferon Alfa-2b in hepatocellular carcinoma after curative resection. Ann Surg. 2012;255(1):8–17. https://doi.org/10.1097/SLA.0b013e3182363ff9.
Yamamoto M, Arii S, Sugahara K, Tobe T. Adjuvant oral chemotherapy to prevent recurrence after curative resection for hepatocellular carcinoma. Br J Surg. 1996;83(3):336–40.
Xia Y, Qiu Y, Li J, Shi L, Wang K, Xi T, et al. Adjuvant therapy with capecitabine postpones recurrence of hepatocellular carcinoma after curative resection: a randomized controlled trial. Ann Surg Oncol. 2010;17(12):3137–44. https://doi.org/10.1245/s10434-010-1148-3.
Nitta H, Beppu T, Imai K, Hayashi H, Chikamoto A, Baba H. Adjuvant hepatic arterial infusion chemotherapy after hepatic resection of hepatocellular carcinoma with macroscopic vascular invasion. World J Surg. 2013;37(5):1034–42. https://doi.org/10.1007/s00268-013-1957-1.
Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16(13):1344–54. https://doi.org/10.1016/S1470-2045(15)00198-9.
Kuczynski EA, Kerbel RS. Implications of vessel co-option in sorafenib-resistant hepatocellular carcinoma. Chin J Cancer. 2016;35(1):97. https://doi.org/10.1186/s40880-016-0162-7.
Zhong C, Guo RP, Li JQ, Shi M, Wei W, Chen MS, et al. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for Stage III A hepatocellular carcinoma. J Cancer Res Clin Oncol. 2009;135(10):1437–45. https://doi.org/10.1007/s00432-009-0588-2.
Group ICoHEW. ICH harmonised tripartite—guideline for good clinical practice E6 (R1). Buckinghamshire: Institute of Clinical Research Marlow; 1996.
Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20(6):1527–36.
Shi M, Chen JA, Lin XJ, Guo RP, Yuan YF, Chen MS, et al. Transarterial chemoembolization as initial treatment for unresectable hepatocellular carcinoma in southern China. World J Gastroenterol. 2010;16(2):264–9.
Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11(9):1086–92. https://doi.org/10.1002/lt.20472.
Zhu SL, Zhong JH, Ke Y, Xiao HM, Ma L, Chen J, et al. Comparative efficacy of postoperative transarterial chemoembolization with or without antiviral therapy for hepatitis B virus-related hepatocellular carcinoma. Tumour Biol. 2015;36(8):6277–84. https://doi.org/10.1007/s13277-015-3313-6.
Zuo CH, Xia M, Liu JS, Qiu XX, Lei X, Xu RC, et al. Transcatheter arterial chemoembolization combined with interferon-alpha is safe and effective for patients with hepatocellular carcinoma after curative resection. Asian Pac J Cancer Prev. 2015;16(1):245–51.
Liu C, Sun L, Xu J, Zhao Y. Clinical efficacy of postoperative adjuvant transcatheter arterial chemoembolization on hepatocellular carcinoma. World J Surg Oncol. 2016;14(1):100. https://doi.org/10.1186/s12957-016-0855-z.
Peng BG, He Q, Li JP, Zhou F. Adjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Surg. 2009;198(3):313–8. https://doi.org/10.1016/j.amjsurg.2008.09.026.
Sun JJ, Wang K, Zhang CZ, Guo WX, Shi J, Cong WM, et al. Postoperative adjuvant transcatheter arterial chemoembolization after R0 hepatectomy improves outcomes of patients who have hepatocellular carcinoma with microvascular invasion. Ann Surg Oncol. 2016;23(4):1344–51. https://doi.org/10.1245/s10434-015-5008-z.
Li SH, Guo ZX, Xiao CZ, Wei W, Shi M, Chen ZY, et al. Risk factors for early and late intrahepatic recurrence in patients with single hepatocellular carcinoma without macrovascular invasion after curative resection. Asian Pac J Cancer Prev. 2013;14(8):4759–63.
Kelley RK. Adjuvant sorafenib for liver cancer: wrong stage, wrong dose. Lancet Oncol. 2015;16(13):1279–81. https://doi.org/10.1016/S1470-2045(15)00296-X.
Iguchi T, Shirabe K, Aishima S, Wang H, Fujita N, Ninomiya M, et al. New pathologic stratification of microvascular invasion in hepatocellular carcinoma: predicting prognosis after living-donor liver transplantation. Transplantation. 2015;99(6):1236–42. https://doi.org/10.1097/TP.0000000000000489.
Sumie S, Nakashima O, Okuda K, Kuromatsu R, Kawaguchi A, Nakano M, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol. 2014;21(3):1002–9. https://doi.org/10.1245/s10434-013-3376-9.
Authors’ contributions
RPG designed and conducted this study and contributed to manuscript drafting. WW, PEJ, SHL and ZXG contributed equally to the data collection, analysis and interpretation, and manuscript drafting. YFZ, YHL, XJL, LX, MS, LZ, and MSC contributed to participant enrollment, clinical treatment, follow-up, data interpretation, and critical manuscript revision. All authors read and approved the final manuscript.
Acknowledgements
We acknowledge all the staffs of the Department of Hepatobiliary and Pancreatic Surgery in Sun Yat-sen University Cancer Center for their assistance and collaboration in this work. We would like to thank Editage (http://www.editage.com) for their assistance in the English language editing.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Key raw data were also verified and uploaded onto the Research Data Deposit public platform (http://www.researchdata.org.cn) with an approval number RDDA2017000369.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study complied with the Declaration of Helsinki and the Good Clinical Practice Guidelines (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), Version E6). The protocol and all modifications have been approved by the Institutional Review Board and Ethics Committee of Sun Yat-Sen University Cancer Center (Approval No: YB 2005-06-08). All patients provided written informed consents to participate.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81172037); Science and Technology Program of Guangdong Province, China (No. 2013B021800159); and Clinical Trials Project (308 Project) of Sun Yat-sen University Cancer Center (No. 308-2015-014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wei, W., Jian, PE., Li, SH. et al. Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: a randomized clinical trial of efficacy and safety. Cancer Commun 38, 61 (2018). https://doi.org/10.1186/s40880-018-0331-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40880-018-0331-y