Abstract
Background
Emerging infectious diseases in wildlife are an increasing threat to global biodiversity. White-nose syndrome (WNS) in bats is one of the most recently emerged infectious diseases in North America, causing massive declines in eastern bat populations. In the Northeast, winter behavior of bats during the hibernation period, such as flying during the day or in cold weather, has been attributed to WNS. However, winter emergence of bats in the southeastern United States, where winters are warmer, has received little attention. The goals of this study were to determine if winter emergence results from infection by Pseudogymnoascus destructans, the causative pathogen of WNS, and to investigate how pathogen load and prevalence vary by species, site, and over time.
Results
We collected epidermal swab samples from 871 active bats of 10 species captured outside of hibernacula in Tennessee during winters 2012–2013 and 2013–2014. Deoxyribonucleic acid (DNA) from P. destructans was not detected on 54% of these bats, suggesting that winter emergence occurs regardless of fungal infection. Among infected bats, Perimyotis subflavus (tri-colored bats) had the highest mean fungal load, whereas Myotis lucifugus (little brown bats) had the highest infection prevalence of all individuals captured. Less than 18% (n = 59 of 345 individuals sampled) of all M. grisescens (gray bats) captured had detectible P. destructans DNA on their forearms and muzzle. Hibernacula with large M. grisescens populations had lower fungal loads than sites used by other species; however, mean load per species did not significantly differ between M. grisescens and non-M. grisescens sites.
Conclusions
We found that pathogen load and prevalence were higher on bats captured during winter 2012–2013 than in the following winter, indicating that fungal loads on bats did not increase the longer a site was presumably contaminated. Repeated low-dose exposure, mild temperatures, and availability of prey during winter in the Southeast may provide a regional refuge for surviving bat populations.
Similar content being viewed by others
Background
Emerging infectious diseases in wildlife pose an increasing threat to global biodiversity and conservation [1, 2]. A significant proportion of these diseases are the result of “pathogen pollution”: the introduction by humans or livestock of novel pathogens into naïve wildlife populations [2, 3]. Prominent examples of pathogen pollution causing mass mortality are African rinderpest and amphibian chytridiomycosis. In the 1880’s rinderpest killed 90% of Kenya’s buffalo population, resulting in downstream effects on predator populations and ecosystem health [2]. Chytridiomycosis has infected over 50% of all amphibian species and can kill 80% of a population within 4–5 months of its introduction [4]. Such emerging infectious diseases are devastating to native species, with deleterious effects that pervade ecosystems [2, 5].
White-nose syndrome (WNS) is a recently emerged infectious disease that has rapidly spread through eastern populations of cave hibernating bats in North America. It is caused by the psychrophilic fungus Pseudogymnoascus destructans, and was first documented in North America in February 2006 at a cave in upstate New York [6, 7]. This invasive pathogen, which is hypothesized to have originated in Eurasia [8,9,10], has since spread to more than half of the United States (U.S.) and five Canadian provinces and has killed over 5.7 million bats [11]. Currently, at least six bat species are experiencing detectable population losses due to WNS, wherein once abundant species are now threatened with regional extinction [11,12,13,14]. Population declines and the loss of bat species due to WNS are likely to have major ecological and economic consequences, with expected increases in crop and forest pest populations [15, 16].
Pseudogymnoascus destructans colonizes the cutaneous membranes of the muzzle, ears, wings and tail of bats, eroding the epidermis and invading the underlying skin and connective tissue [17]. Once invasion occurs, P. destructans disrupts critical physiological functions such as cutaneous respiration, blood circulation, and water balance [18,19,20,21]. These physiological changes result in more frequent arousals from torpor and increased depletion of energy reserves needed for hibernation [21, 22]. Recent studies suggest infected individuals can elicit the initial stages of an immune response (e.g. transcription of cytokines); however, a protective response does not occur due to hibernation [23,24,25]. Bats with WNS also exhibit aberrant behavior in winter, including movement from thermally stable cave environments to locations near the cave entrance, daytime emergence, and flying in cold winter temperatures [7, 12, 26].
Species-specific behaviors during hibernation, such as microclimate preference, may also play a role in disease susceptibility and survival [14, 27, 28]. In North America, small bodied bats have been known to hibernate at microclimate temperatures ranging from 0 to 10 °C [20] and relative humidity as high as 90–100%, which fall within the optimal growing conditions for P. destructans. Whereas larger bodied species, like E. fuscus (mean = 12 g) and M. grisescens (mean = 10 g), often roost in colder, drier sites in a hibernacula [29]. European bats, such as M. myotis, a 30 g species, have been found to hibernate at microclimate temperatures ranging from −4 to 12 °C [30], suggesting that there is no optimal microclimate temperature for hibernating bat species, with individual-specific microclimate preferences within a species ranging widely [31, 32]. Myotis myotis is the most frequent bat in Europe documented with P. destructans and ulcerations leading to the manifestation of WNS [27, 33,34,35]. Naturally occurring P. destructans infections on M. myotis have been found to be quite extensive, yet have not lead to widespread mortality of the species [33]. Overall, the most affected bat species in Europe are larger bodied species, whereas in North America, small bodied individuals have experienced the largest population declines due to WNS [13, 14, 36].
In northeastern North America, where winters are severe and prey is limited, bats flying outside during the hibernation period are likely suffering the effects of WNS. However, bats in the southeastern U.S. are known to leave hibernacula to feed on warm winter nights [Bernard et al. unpublished], suggesting that winter activity in the South may not be a consequence of disease [37]. As an example, minimum night time temperatures throughout January in Tennessee over the last four years ranged from −17 °C to −6 °C, whereas external cave temperatures in Vermont ranged from −27 °C to −17 °C, consistently 10 °C colder [38]. As WNS has now spread throughout much of the southeastern U.S. [39] the possible effects of winter activity on the epizootiology of WNS remain unknown. Winter foraging on insects may provide bats hibernating in southern latitudes with energy not available to bats in the North. Further, arousing from torpor to engage in episodic feeding during winter will raise body temperature, which should activate the immune system and possibly bolster immunological defenses against P. destructans. Evidence from rabies in bats [40], as well as other host-pathogen systems [41, 42], demonstrates that host immunity can result from repeated low-level exposure to pathogens. Behaviorally and physiologically, bats in the South may be different from northern bats in ways that enable them to survive WNS. To investigate possible effects of winter activity on P. destructans infections on bats in southern latitudes, we examined fungal load and prevalence on bats captured outside of hibernacula during winter.
In this study, we assessed prevalence and fungal load of P. destructans and identified lesions and ulcerations caused by penetration of P. destructans into wing and tail membranes for ten species of bats captured while active outside of hibernacula during two winters in Tennessee. Our goals were to determine if emergence during winter is caused by the presence of P. destructans and to examine if there are relationships between winter activity, fungal load and prevalence, and bat species. To address these goals, we tested the following hypotheses: 1) active bats leaving caves during winter in the Tennessee will show signs of WNS as demonstrated by fungal load or ultraviolet fluorescence; 2) fungal load and prevalence will be higher on small-bodied cave hibernating species, such as M. lucifugus (little brown bats), M. septentrionalis (northern-long eared bats) and Perimyotis subflavus (tri-colored bats), than larger bodied species, such as Eptesicus fuscus (big brown bats) and M. grisescens (gray bats).
Methods
We conducted our study at the entrances of five hibernacula in Tennessee from October to April 2012–2013 and 2013–2014 (Fig. 1). Prior to the emergence of WNS, Blount Cave was the largest known endangered M. sodalis (Indiana bat) hibernaculum in the state of Tennessee, with an estimated population of 9500 individuals in February 2013 [43]. Small numbers of M. lucifugus and P. subflavus also occur in the cave. Hawkins and Warren Caves are two of the largest hibernacula for endangered M. grisescens in the state, with estimated populations of 150,000 and 400,000 M. grisescens, respectively. Both caves also contain a small population of M. sodalis during winter [44]. Campbell and White Caves contain populations of M. leibii (eastern small-footed bats), M. lucifugus, M. septentrionalis, and M. sodalis, with fewer than 1000 individuals in each cave [45]. Bats in Blount and Hawkins Caves were confirmed positive for P. destructans in the winters of 2009–2010 and 2010–2011, respectively, with all other sites confirmed by winter 2012–2013 [44, 46].
We captured bats at each site once a month using mist-nets (Avinet Inc., Dryden, NY; mesh diameter: 75/2, 2.6 m high, 4 shelves, 6–12 m wide). Site-specific single-, double- and triple-high nets were deployed 30 min before civil sunset at cave entrances and along corridors within 100 m of the cave. We kept the nets open for 5 h or until we captured 30 bats and closed them when temperatures dropped below 0 °C. After capture, individual bats were placed in paper bags and held for 30 to 60 min in an insulated box with four hand-warmers (HotHands®, Dalton, GA). Myotis grisescens and M. sodalis were held for a maximum of 30 min. We recorded species, reproductive condition, forearm length (mm), weight (g), mite load [47] and wing-damage index (WDI, [48]), and collected epidermal swab samples from each bat following established protocols (see below). During the winter of 2013–2014, we examined bats for the presence of WNS-related fluorescence by transilluminating the wings with ultraviolet (UV) light (wavelengths 385–390 nm, [49, 50]). If P. destructans has penetrated the skin, lesions fluoresce yellow-orange under UV illumination [49]. Fungal samples for each individual were collected using a sterile epidermal swab dipped in sterile deionized water and rubbed on the bat’s forearm and muzzle five times each [51]. Swabs were placed in RNAlater® Tissue Stabilization Solution (Life Technologies, Grand Island, NY) and stored at 4 °C. All cave-roosting species were banded with either 2.4 mm or 2.9 mm numbered, lipped alloy forearm bands (Porzana, Ltd., Icklesham, East Sussex, UK) and released at the site of capture. Due to the distance between sites and the lack of evidence to suggest movement occurs between caves during winter in the region, we assumed each cave was a closed population.
We extracted fungal DNA from each swab sample using DNeasy 96 Blood & Tissue kits (Qiagen Inc., Valencia, CA; [52]). All samples, as well as negative control wells distributed across each polymerase chain reaction (PCR) plate, were tested for the presence of P. destructans DNA using a Real-Time PCR assay targeting the intergenic spacer (IGS) region of the ribosomal ribonucleic acid (rRNA) gene complex [53]. All plates were run in duplicate with a quantified standard of isolate P. destructans 20,631–21. Any reaction that crossed the threshold baseline in fewer than 40 cycles on either plate was considered positive for P. destructans DNA and, when relevant, the average P. destructans load, hereafter referred to as fungal load, in nanograms (ng) was calculated in each sample based on the cycle threshold (Ct) value and a generated standard curve based on serial dilutions ([34]; nanograms P. destructans = 10–3.348xCt+22.049). Fungal load values of P. destructans were averaged across both runs.
We followed field decontamination protocols in accordance with the United States Fish and Wildlife Service WNS Decontamination Guidelines and recommendations by the state of Tennessee [54]. All capture and handling techniques were approved by the University of Tennessee Institute of Animal Care and Use Committee (IACUC 2026–0514) and were consistent with the guidelines issued by the American Society of Mammalogists [55]. We obtained both federal (USFWS TE-71613A; GRSM-2013-SCI-1053; GRSM-2014-SCI-1053) and state (TWRA 3716; TDEC 2011–031) permits to capture and handle bats at winter hibernacula for this study.
Statistical analysis
Fungal load data were log transformed prior to analyses to meet assumptions of normality and homogeneity of variance. We used separate generalized linear models (function glm in package lme4 [56] in Program R v 3.1.2 [57]) to compare changes in load and prevalence of P. destructans for each species over time. Models were run as either binomial (prevalence) or Gaussian (fungal loads) distributions, and were tested for significance using likelihood ratio tests. To determine the change in P. destructans load and prevalence over time within each model, we used a modified time axis similar to Langwig et al. 2015 [36] where time-0 represented the start of hibernation (October 1). Infection prevalence was calculated by dividing the total number of infected individuals by the total number of individuals captured during the same time period. All means are reported ± standard error. The results presented herein represent bats captured outside of each site, not of the hibernating population as a whole.
Results
We captured and swabbed 871 bats of 10 species (593 males, 276 females, 2 unknowns due to escape; Table 1). Of these, 408 individuals were positive for the presence of P. destructans DNA (Pd+) by Real-Time PCR analysis. At least one individual from all species captured was Pd+, including two Corynorhinus rafinesquii (Rafinesque’s big-eared bat), two Lasiurus borealis (eastern red bat) and one Lasionycteris noctivagans (silver-haired bat) ([58]; Table 1). However, these three species were excluded from the comparative analyses due to small sample sizes. Capture rates of M. septentrionalis, M. sodalis, and P. subflavus dramatically declined during winter 2013–2014, with M. septentrionalis rarely captured after December 2013.
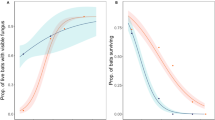
Fifty-one percent of the bats captured (n = 245/480) during winter 2012–2013 were Pd+, whereas only 41.6% of the bats (n = 163/391) were Pd + in winter 2013–2014. When pooled by season, mean fungal loads were significantly higher during the first year of sampling (likelihood ratio test: X 2 = 17.978, p < 0.0001). Excluding species with low sample size, there were significant differences in load of P. destructans per species over time (likelihood ratio test: X 2 = 278.06, p < 0.0001, Fig. 2). Fungal loads were lowest when bats entered hibernation in October (−4.99 ± 0.328 log10 ng) and peaked for most species during mid-hibernation (December–February; −2.80 ± 0.095 log10 ng; Fig. 2 and Additional file 1). Thereafter, mean fungal loads on six of the seven species remained stable through the end of the hibernation period in April. However, mean fungal load on P. subflavus, the seventh species, continued to increase through the end of hibernation, reaching levels twice as high as those recorded in December (Fig. 2). Perimyotis subflavus had the highest mean P. destructans load (−2.34 ± 0.091 log10 ng), whereas M. grisescens had the lowest mean P. destructans load of all species sampled (−4.89 ± 0.075 log10 ng). Infection prevalence varied among species, with large-bodied species, such as E. fuscus and M. grisescens, experiencing the lowest prevalence on average (Fig. 3). Fungal loads on active bats in the Southeast were also lower than on torpid bats sampled in a separate study in the northeastern U.S. (Table 2).
Mean monthly load of Pseudogymnoascus destructans per species. Mean load (± SE) of P. destructans (Pd) per month for seven species captured at five cave sites in Tennessee during October–April; 2012–2013 (closed circles) and 2013–2014 (open circles). Circles indicate months where Pd positive individuals were captured
Infection prevalence of Pseudogymnoascus destructans on active bats captured in Tennessee. Infection prevalence (# infected individuals/total # individuals sampled) of Pseudogymnoascus destructans (± SE) for seven bat species captured at five hibernacula in Tennessee during winters 2012–2013 (closed circles) and 2013–2014 (open circles)
During the second season of sampling, we examined 481 bats for WNS-related fluorescence. Ultraviolet fluorescence revealed varying degrees of damage due to the fungus, from small pin-sized lesions to large coalescing regions of fluorescence and infiltration corresponding with increased pathogen loads. Only 15 bats that fluoresced showed some signs of wing damage, varying from slight depigmentation to pin holes (WDI = 1). All bats captured during early hibernation (October and November) were negative for UV fluorescence. The highest percent of UV-infected bats were captured during mid-hibernation (December 35.9%, January 29.5%). A total of 66 bats were positive by both PCR and UV, with only two UV positives not detected as Pd + by PCR. A total of 181 individuals were Pd + from PCR but UV negative, whereas 232 bats were negative for both P. destructans and UV. Bats that were Pd + by both PCR and UV had higher fungal loads than individuals that were determined P. destructans positive only by PCR (t 200.6 = 8.83, p < 0.0001). As noted in Zukal et al. 2016 [8], we did not observe a threshold with which the presence of UV fluorescence corresponded to a minimum fungal load (UV positive: range − 4.74 – −0.22 log10 ng; UV negative: range − 5.73 – 0.36 log10 ng).
Discussion
Our study demonstrates that the emergence of bats during winter in Tennessee is not indicative of WNS, as less than half of all bats captured outside of the caves sampled during the hibernation period were positive for P. destructans. Although we can only make inference directly to bats captured outside of hibernacula in Tennessee, we find a wide range of P. destructans loads on captured bats. As we are not only sampling high-load individuals leaving the hibernacula we, therefore, can assume that our results are representative of the entire population. By capturing bats active during winter and coupling winter activity with measures of prevalence and load of P. destructans on bats, we highlight the regional differences in the responses of WNS affected species within their greater geographic range. This study demonstrates that as WNS continues to spread throughout North America, it should not be assumed that all individuals within a species will react similarly to the disease. In the Northeast, a secondary symptom of the disease is activity during winter, specifically aberrant behavior, or bats flying during cold weather or during daylight hours [12, 13, 26, 59]. Although, cases of unusual winter behavior have been reported in the Southeast [37, 46], our data highlight that nighttime emergences from hibernacula in Tennessee are not always associated with P. destructans infection. Therefore, the effects of WNS on bats in the southeastern U.S. are not directly comparable to those in the North, as regional and species-specific differences, like degree of winter activity, body condition [60] and susceptibility to disease [14, 36], likely vary significantly from those in the Northeast.
Both infection prevalence and loads varied considerably among species, with all small-bodied bats, except M. leibii, having higher fungal loads and prevalence than E. fuscus and M. grisescens. Species, such as M. septentrionalis and P. subflavus, with high rates of fungal loads and prevalence, were consistently found with the largest regions of fluorescence and wing damage, indicating high rates of tissue invasion by P. destructans [48]. Whereas, M. grisescens, which have low fungal loads and prevalence, were often found with substantial discoloration, wing damage, and tissue loss unrelated to WNS based on negative UV and PCR results, as well as WNS WDI scoring [48, 61]. According to survey records in Tennessee, M. grisescens was often observed with discolored wing membranes and significant scarring and tissue loss prior to P. destructans North American introduction (John Lamb and Troy Best, personal communication).
Although transmission of P. destructans among individuals could be associated with the accumulation of the fungus within a hibernaculum based on the time since initial introduction, number of bats, and internal cave conditions, it is important to also consider species specific biology and behaviors. When the Northeast was the leading edge of WNS infection, small, solitary bats, such as M. septentrionalis and P. subflavus, had significantly higher fungal loads than similar sized colonial species [11, 28, 41], suggesting P. destructans was spreading via density-dependent transmission in these two species as cluster size increased [14]. In contrast, our findings suggest that M. sodalis and M. lucifugus, species known to cluster in tight aggregations during hibernation, had fungal loads and prevalence similar to those of solitary species. Therefore, transmission of P. destructans among more colonial species in southern hibernacula may be a function of the frequency, or rate, of infection among individuals within the cluster, rather than the cluster size [14, 34, 62]. Interestingly, M. grisescens, the largest bat that hibernates exclusively in caves in the Midwest and southeastern U.S., had the lowest fungal loads and prevalence of P. destructans among all species sampled (Figs. 2 and 3). This finding contrasts with patterns observed in Europe, where M. myotis and other large bodied hibernating bats have the highest incidence of P. destructans [8, 35, 63]. In the Northeast, disease impacts on M. lucifugus, M. septentrionalis and P. subflavus increased with higher humidity and temperature within roosts, such that individuals sampled in the coldest and driest roosts had significantly lower fungal loads [14, 27]. Myotis grisescens, however, hibernate in aggregations of 100,000 to 1,500,000 individuals [64] in cold air traps varying from 1 to 9 °C [65], which are the lowest temperatures at which P. destructans growth occurs [66]. As of spring 2017, M. grisescens have yet to experience any WNS-related declines and their populations appear to have remained stable within Tennessee. Although some M. grisescens that we captured have been identified with secondary fungal infections, skin discoloration, and/or substantial tissue loss [Bernard unpublished data, John Lamb and Troy Best personal communication], we have yet to identify how the species is surviving WNS. Several behavioral traits, such as preferred microclimates within hibernacula, sustained activity and foraging throughout winter [37] and year-round cave use [67, 68], may enable this species to prevent or minimize the colonization of P. destructans during torpor.
When all seven species with samples sizes ≥12 individuals were combined, mean fungal load was highest during mid-hibernation, December through February, the coldest period of the year in the Southeast. Perimyotis subflavus, however, continued to experience an increase in fungal load through the end of hibernation, which could be attributed to the microclimate (11 to 23 °C; ≥ 80% Relative Humidity) used by the species during hibernation [69,70,71]. Alternatively, in vitro growth curves suggest that P. destructans may reproduce more quickly in cave environments that maintain more moderate temperatures of 10 to 15 °C in winter [66], which could result in increased growth rates of the fungus in southern hibernacula, and therefore lead to a peak in fungal load within species hibernating within that temperature range.
Contrary to our prediction and the findings of studies from northern hibernacula [69], both pathogen load and prevalence were lower in the second year of the study for seven of the ten bat species captured [58]. By the second survey year, all caves had been contaminated by P. destructans for at least two years. Although this could be due to the decrease in the capture of highly susceptible species (Table 1) caused by WNS related declines within each cave [72], climatic variation between years could also impact disease spread. Similar trends have been documented after the arrival of Batrachochytrium dendrobatidis, the pathogenic fungal agent of chytridiomycosis in frogs. Pathogen loads in naïve frog populations increased dramatically in the first year, causing a rapid rise in infection intensity and prevalence in densely populated habitats [73]. As the pathogen load on infected frogs increased, many populations suffered from high rates of mortality. However, the survival of infected individuals led to pathogen endemism and population persistence on the frogs. A similar dynamic may be occurring in hibernacula contaminated by P. destructans [74, 75]. Individuals with high pathogen loads perish in the first year, perhaps allowing for individuals with minor P. destructans infections to return to the hibernacula the following winter. Further, some scientists hypothesize that increased incidences of chytridiomycosis are linked to increases in global temperatures creating optimal sites for the pathogen [76, 77]. Similar responses may occur with WNS if regions of the Southeast experience more extreme winters, creating more favorable conditions for P. destructans growth or limited opportunities for bats to replenish fat stores. Repeated low-level exposure to P. destructans or endemism of the pathogen, mild winters, and episodic feeding may allow for persistence of bat populations hibernating in the Southeast.
Recent evidence from the Northeast suggests some populations of bats have the ability to persist and reproduce despite continued exposure to WNS [42, 75, 78, 79]. Comparing P. destructans loads on the same species sampled while torpid in the Northeastern and active in the Southeastern U.S., we find the average fungal loads over the season were consistently lower on active bats (Table 2). Eptesicus fuscus and M. lucifugus captured in Tennessee had lower loads than those sampled in northeastern hibernacula, whereas fungal loads on M. septentrionalis and M. sodalis were similar towards the beginning and end of hibernation. Perimyotis subflavus in the Northeast, however, had higher loads than individuals sampled in the Southeast. Meaningful direct comparisons are lacking due to insufficient numbers sampled in the Northeast. Ultimately, we are seeing that Pd + bats captured in Tennessee have similar loads to torpid individuals sampled in more northern areas of their range, indicating that activity and survival in the Southeast may be more closely linked with short, mild winters and moderate prey levels during winter.
Conclusions
The depopulation of naïve bat hosts by WNS will likely lead to chronic population depression [2] due to the long-term persistence of P. destructans within cave environments. Whereas, mortality in the Northeast can reach 90% within two years of WNS confirmation [12, 13], population declines likely attributed to WNS in the Southeast occur four to five years after confirmation and tend to be less severe in some species [26, 27, 40, 80]. Our findings support the hypothesis that emergence from caves during winter may influence the variation seen in pathogen load and infection intensity among species. By understanding the species-specific dynamics of P. destructans within active winter populations, management strategies, such as regional area closures and bio-control treatments can be implemented more effectively. In the Southeast, mitigation measures, such as cave area closures used to minimize external cave disturbances (e.g. Great Smoky Mountains National Park) or bio-control agents (e.g. Rhodococcus rhodochrous [81] and chitosan), may work best when targeting hibernacula with small-bodied bats such as M. lucifugus, M. septentrionalis, and P. subflavus; species that are being hit the hardest by WNS in the Southeast [80, 61]. Finally, our study suggests that populations of some bats are persisting regardless of repeated exposure to P. destructans. Although the region is currently experiencing WNS-related mortality within highly affected species, mild temperatures and the persistent availability of prey during winter may allow the Southeast to serve as a refuge for surviving bat populations.
Abbreviations
- Ct :
-
Cycle threshold
- DNA:
-
Deoxyribonucleic acid
- IGS:
-
Intergenic spacer
- ng:
-
Nanograms
- PCR:
-
Polymerase chain reaction
- Pd-:
-
Pseudogymnoascus destructans negative
- Pd +:
-
Pseudogymnoascus destructans positive
- rRNA:
-
Ribosomal ribonucleic acid
- U.S.:
-
United States
- UV:
-
Ultraviolet light
- WDI:
-
Wing damage index
- WNS:
-
White-nose syndrome
References
Daszak P, Berger L, Cunningham AA, Hyatt AD, Green DE, Speare R. Emerging infectious diseases and amphibian population declines. Emerg Infect Dis. 1999;5:735–48. doi:10.3201/eid0506.990601.
Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science. 2000;287:443–9. doi:10.1126/science.287.5452.443.
Rachowicz LJ, Hero J-M, Alford RA, Taylor JW, Morgan JAT, Vredenburg VT, et al. The novel and endemic pathogen hypotheses: competing explanations for the origin of emerging infectious diseases of wildlife. Conserv Biol. 2005;19:1441–8. doi:10.1111/j.1523-1739.2005.00255.x.
Mendelson JR, Lips KR, Gagliardo RW, Rabb GB, Collins JP, Diffendorfer JE, et al. Confronting amphibian declines and extinctions. Science. 2006;313:48.
Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, et al. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484:186–94. doi:10.1038/nature10947.
Blehert DS, Hicks AC, Behr MJ, Meteyer CU, Berlowski-Zier BM, Buckles EL, et al. Bat White-nose syndrome: an emerging fugal pathogen? Science. 2009;323:227.
Blehert DS, Lorch JMJM, Ballmann AE, Cryan PM, Meteyer CU. Bat White-nose syndrome in North America. Fungal diseases: an emerging threat to human, animal and plant health. Washington, D.C.: The National Academies Press; 2011. p. 167–76.
Zukal J, Bandouchova H, Brichta J, Cmokova A, Jaron KS, Pikula J, et al. White-nose syndrome without borders: Pseudogymnoascus destructans infection tolerated in Europe and Palearctic Asia but not in North America. Sci Rep. 2016;6:19829. doi:10.1038/srep19829.
Hoyt JR, Sun K, Parise KL, Lu G, Langwig KE, Jiang T, et al. Widespread bat White-nose syndrome fungus, Northeastern China. Emerg Infect Dis. 2016;22:140–2.
Leopardi S, Blake D, Puechmaille SJ. White-nose syndrome fungus introduced from Europe to North America. Curr Biol. 2015;25:R217–9. doi:10.1016/j.cub.2015.01.047.
U.S. Fish and Wildlife Service. White-nose syndrome: the devastating disease of hibernating bats in North America. Hadley: Fish and Wildlife Service; 2014. p. 2.
Turner GG, Reeder DM, Coleman JTH. A five-year assessment of mortality and geographic spread of White-nose syndrome in North American bats and a look to the future. Bat Res News. 2011;52:13–27.
Frick WF, Pollock JF, Hicks AC, Langwig KE, Reynolds DS, Turner GG, et al. An emerging disease causes regional population collapse of a common North American bat species. Science. 2010;329:679–82. doi:10.1126/science.1188594.
Langwig KE, Frick WF, Bried JT, Hicks AC, Kunz TH, Marm KA. Sociality, density-dependence and microclimates determine the persistence of populations suffering from a novel fungal disease, White-nose syndrome. Ecol Lett. 2012;15:1050–7. doi:10.1111/j.1461-0248.2012.01829.x.
Maine JJ, Boyles JG. Bats initiate vital agroecological interactions in corn. Proc Natl Acad Sci. 2015:1–6. doi:10.1073/pnas.1505413112.
Kunz TH, Braun de Torrez E, Bauer D, Lobova T, Fleming TH. Ecosystem services provided by bats. Ann N Y Acad Sci. 2011;1223:1–38. doi:10.1111/j.1749-6632.2011.06004.x.
Meteyer CU, Buckles EL, Blehert DS, Hicks AC, Green DE, Shearn-Bochsler V, et al. Histopathologic criteria to confirm White-nose syndrome in bats. J Vet Diagnostic Investig. 2009;21:411–4. doi:10.1177/104063870902100401.
Warnecke L, Turner JM, Bollinger TK, Misra V, Cryan PM, Blehert DS, et al. Pathophysiology of White-nose syndrome in bats: a mechanistic model linking wing damage to mortality. Biol Lett. 2013;9:20130177.
Willis CKR, Menzies AK, Boyles JG, Wojciechowski MS. Evaporative water loss is a plausible explanation for mortality of bats from White-nose syndrome. Integr Comp Biol. 2011;51:364–73. doi:10.1093/icb/icr076.
Cryan PM, Meteyer CU, Boyles JG, Blehert DS. Wing pathology of White-nose syndrome in bats suggests life-threatening disruption of physiology. BMC Biol. 2010;8:135. doi:10.1186/1741-7007-8-135.
Verant ML, Meteyer CU, Speakman JR, Cryan PM, Lorch JM, Blehert DS. White-nose syndrome initiates a cascade of physiologic disturbances in the hibernating bat host. BMC Physiol. 2014;14:10. doi:10.1186/s12899-014-0010-4.
Warnecke L, Turner JM, Bollinger TK, Lorch JM, Misra V, Cryan PM, et al. Inoculation of bats with European Geomyces destructans supports the novel pathogen hypothesis for the origin of White-nose syndrome. Proc Natl Acad Sci. 2012;109:6999–7003. doi:10.1073/pnas.1200374109.
Field KA, Johnson JS, Lilley TM, Reeder SM, Rogers J, Behr MJ, et al. The White-nose syndrome transcriptome: activation of anti-fungal host responses in wing tissue of hibernating little brown Myotis. PLoS Pathog. 2015;11:e1005168. doi:10.1371/journal.ppat.1005168.
Bouma HR, Carey HV, Kroese FGM. Hibernation: the immune system at rest? J Leukoc Biol. 2010;88:619–24. doi:10.1189/jlb.0310174.
Lilley TM, Prokkola JM, Johnson JS, Rogers EJ, Gronsky S, Kurta A, et al. Immune responses in hibernating little brown myotis (Myotis lucifugus) with White-nose syndrome. Proc R Soc B. 2017;284:20162232.
Foley J, Clifford D, Castle K, Cryan PM, Ostfeld RS. Investigating and managing the rapid emergence of White-nose syndrome, a novel, fatal, infectious disease of hibernating bats. Conserv Biol. 2011;25:223–31. doi:10.1111/j.1523-1739.2010.01638.x.
Hayman DTS, Pulliam JRC, Marshall JC, Cryan PM, Webb CT. Environment, host, and fungal traits predict continental-scale White-nose syndrome in bats. Sci Adv. 2016;2:1–13. doi:10.1126/sciadv.1500831.
Frank CL, Michalski A, Mcdonough AA, Rahimian M. The resistance of a North American bat species (Eptesicus fuscus) to White-nose syndrome (WNS). PLoS One. 2014;9:e113958. doi:10.1371/journal.pone.0113958.
Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol. 2004;66:239–74. doi:10.1146/annurev.physiol.66.032102.115105.
Wojciechowski MS, Jefimow M, Tegowska E. Environmental conditions, rather than season, determine torpor use and temperature selection in large mouse-eared bats (Myotis myotis). Comp Biochem Physiol Part A Mol Integr Physiol. 2007;147:828–40. doi:10.1016/j.cbpa.2006.06.039.
Boyles JG, Boyles E, Dunlap RK, Johnson SA, Brack V. Long-term microclimate measurements add further evidence there is no “optimal” temperature for bat hibernation. Mamm Biol. 2017; doi:10.1016/j.mambio.2017.03.003.
Webb PI, Speakman JR, Racey PA. How hot is a hibernaculum? A review of the temperatures at which bats hibernate. Can J Zool. 1996;74:761–5. doi:10.1139/z96-087.
Bandouchova H, Bartonicka T, Berkova H, Brichta J, Cerny J, Kovacova V, et al. Pseudogymnoascus destructans: evidence of virulent skin invasion for bats under natural conditions, Europe. Transbound Emerg Dis. 2006;2014:1–5. doi:10.1111/tbed.12282.
Zukal J, Bandouchova H, Bartonička T, Berkova H, Brack V, Brichta J, et al. White-nose syndrome fungus: a generalist pathogen of hibernating bats. PLoS One. 2014;9:e97224.
Martínková N, Bačkor P, Bartonička T, Blažková P, Cervený J, Falteisek L, et al. Increasing incidence of Geomyces destructans fungus in bats from the Czech Republic and Slovakia. PLoS One. 2010;5:e13853. doi:10.1371/journal.pone.0013853.
Langwig KE, Frick WF, Reynolds R, Parise KL, Drees KP, Hoyt JR, et al. Host and pathogen ecology drive the seasonal dynamics of a fungal disease, White-nose syndrome. Proc R Soc London B. 2015;282:20142335.
Bernard RF, McCracken GF. Winter behavior of bats and the progression of White-nose syndrome in the southeastern United States. Ecol Evol. 2017:1–10. doi:10.1002/ece3.2772.
Weather Underground. 2016. https://www.wunderground.com. Accessed 6 July 2016.
Washington Dept. of Fish and Wildlife, U.S. Fish and Wildlife Service, U.S. Geological Survey. Bat with White-nose syndrome confirmed in Washington State; 2016. p. 2.
Turmelle AS, Allen LC, Jackson FR, Kunz TH, Rupprecht CE, McCracken GF. Ecology of rabies virus exposure in colonies of Brazilian free-tailed bats (Tadarida brasiliensis) at natural and man-made roosts in Texas. Vector-Borne Zoonotic Dis. 2010;10:165–75. doi:10.1089/vbz.2008.0163.
Dimitrov DT, Hallam TG, Rupprecht CE, McCracken GF. Adaptive modeling of viral diseases in bats with a focus on rabies. J Theor Biol. 2008;255:69–80. doi:10.1016/j.jtbi.2008.08.007.
Dobony CA, Hicks AC, Langwig KE, von Linden RI, Okoniewski JC, Rainbolt RE. Little brown Myotis persist despite exposure to White-nose syndrome. J Fish Wildl Manag. 2011;2:190–5. doi:10.3996/022011-JFWM-014.
Flock B. Tennessee bat population monitoring and white nose syndrome surveillance. 2013. Retrieved from: http://www.tnbwg.org/Files/13-22%202013%20Bat%20Population%20Monitoring%20and%20White%20Nose%20Syndrome%20Surveillance.pdf
Holliday C. White-nose syndrome disease surveillance and bat population monitoring report. 2012. Retrieved from: http://www.tnbwg.org/2012%20White%20Nose%20Syndrome%20Report.pdf.
Samoray S. White-nose syndrome monitoring and bat population survey of hibernacula in Tennessee. 2011. Retrieved from: http://www.tnbwg.org/2011%20Tennessee%20Hibernaculum%20Survey%20Report_FINAL.pdf
Carr JA, Bernard RF, Stiver WH. Unusual bat behavior during winter in Great Smoky Mountains National Park. Southeast Nat. 2014;13:N18–21.
Zahn A, Rupp D. Ectoparasite load in European vespertilionid bats. J Zool. 2004;262:383–91. doi:10.1017/S0952836903004722.
Reichard JD, Kunz TH. White-nose syndrome inflicts lasting injuries to the wings of little brown myotis (Myotis lucifugus). Acta Chiropterologica. 2009;11:457–64. doi:10.3161/150811009X485684.
Turner GG, Meteyer CU, Barton H, Gumbs JF, Reeder DM, Overton B, et al. Nonlethal screening of bat-wing skin with the use of ultraviolet fluorescence to detect lesions indicative of White-nose syndrome. J Wildl Dis. 2014;50:566–73. doi:10.7589/2014-03-058.
McGuire LP, Turner JM, Warnecke L, McGregor G, Bollinger TK, Misra V, et al. White-nose syndrome disease severity and a comparison of diagnostic methods. EcoHealth. 2016;13:60–71. doi:10.1007/s10393-016-1107-y.
USGS National Wildlife Health Center. Bat White-nose Syndrome (WNS)/Pd surveillance submission guidelines. Madison: USGS National Wildlife Health Center; 2013. p. 36.
Shuey MM, Drees KP, Lindner DL, Keim P, Foster JT. Highly sensitive quantitative PCR for the detection and differentiation of Pseudogymnoascus destructans and other Pseudogymnoascus species. Appl Environ Microbiol. 2014;80:1726–31. doi:10.1128/AEM.02897-13.
Muller LK, Lorch JM, Lindner DL, O’Connor M, Gargas A, Blehert DS. Bat White-nose syndrome: a real-time TaqMan polymerase chain reaction test targeting the intergenic spacer region of Geomyces destructans. Mycologia. 2013;105:253–9. doi:10.3852/12-242.
Shelley V, Kaiser S, Shelley E, Williams T, Kramer M, Haman K, et al. Evaluation of strategies for the decontamination of equipment for Geomyces destructans, the causative agent of the White-nose syndrome (WNS). J Cave Karst Stud. 2013;75:1–10. doi:10.4311/2011LSC0249.
Sikes RS, ACUC. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J Mammal. 2016;97:663–88. doi:10.1093/jmammal/gyw078.
Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using “Eigen” and S4. In: R package version 1.1–13. 2017.
R Core Team. 2017. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. https://www.r-project.org/.
Bernard RF, Foster JTJT, Willcox EV, Parise KLKL, Mccracken GF. Molecular detections of the causative agent of White-nose syndrome on Rafinesque’s big-eared bats (Corynorhinus rafinesquii) and two species of migratory bats in the Southeastern USA. J Wildl Dis. 2015;51:519–22. doi:10.7589/2014-08-202.
Reeder DM, Frank CL, Turner GG, Meteyer CU, Kurta A, Britzke ER, et al. Frequent arousal from hibernation linked to severity of infection and mortality in bats with White-nose syndrome. PLoS One. 2012;7:e38920. doi:10.1371/journal.pone.0038920.
Jonasson KA, Willis CKR. Changes in body condition of hibernating bats support the thrifty female hypothesis and predict consequences for populations with White-nose syndrome. PLoS One. 2011;6:e21061. doi:10.1371/journal.pone.0021061.
Reichard JD. Wing-damage index used for characterizing wing condition of bats affected by White-nose Syndrome. Retrieved from https://www.fws.gov/northeast/PDF/Reichard_Scarring%20index%20bat%20wings.pdf.
Begon M, Bennett M, Bowers RG, French NP, Hazel SM, Turner J. A clarification of transmission terms in host-microparasite models: numbers, densities and areas. Epidemiol Infect. 2002;129:147–53. doi:10.1017/S0950268802007148.
Pikula J, Bandouchova H, Novotny L, Meteyer CU, Zukal J, Irwin NR, et al. Histopathology confirms White-nose syndrome in bats in Europe. J Wildl Dis. 2012;48:207–11.
U.S. Fish and Wildlife Service. Gray bat recovery plan. St. Louis: Fish and Wildlife Serivce; 1982. p. 143.
U.S. Fish and Wildlife Service. Gray Bat (Myotis grisescens) 5-year review: summary and evaluation. Columbia: Fish and Wildlife Service; 2009. p. 34.
Verant ML, Boyles JG, Waldrep W, Wibbelt G, Blehert DS. Temperature-dependent growth of Geomyces destructans, the fungus that causes bat White-nose syndrome. PLoS One. 2012;7:e46280. doi:10.1371/journal.pone.0046280.
Stevenson DE, Tuttle MD. Survivorship in the endangered gray bat (Myotis grisescens). J Mammal. 1981;62:244–57.
Tuttle MD. Population ecology of the gray bat (Myotis grisescens): factors influencing growth and survival of newly volant young. Ecology. 1976;57:587–95.
Perkins CE. Microclimate and bat occupation trends at Gorman cave, Colorado Bend State Park, San Saba county, Texas durin 2010–2011. 2011. Report to Texas Parks and Wildlife Department, Colorado Bend State Park, Texas, USA. 2011. p. 28.
Fujita MS, Kunz TH. Pipistrellus subflavus. Mammalian Species. 1984. pp. 1–6. doi:10.2307/3504021
Briggler JT, Prather JW. Seasonal use and selection of caves by the eastern pipistrelle bat (Pipistrellus subflavus). Am Midl Nat. 2003;149:406–12. doi:0003/0003-0031(2003)149[0406:SUASOC]2.0.CO;2.
Campbell J. Tennessee winter bat population and White-nose syndrome monitoring report for 2014-2015 and 2015-2016. 2016. Retrieved from: http://www.tnbwg.org/2016%20Annual%20Monitoring%20Report.pdf
Briggs CJ, Knapp RA, Vredenburg VT. Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc Natl Acad Sci. 2010;107:9695–700. doi:10.1073/pnas.0912886107.
Frick WF, Cheng TL, Langwig KE, Hoyt JR, Janicki AF, Parise KL, et al. Pathogen dynamics during invasion and establishment of White-nose syndrome explain mechanisms of host persistence. Ecology. 2017;98:624–31. doi:10.1002/ecy.1706.
Langwig KE, Hoyt JR, Parise KL, Frick WF, Foster JT, Kilpatrick AM, et al. Resistance in persisting bat populations after White-nose syndrome invasion. Philos Trans R Soc B. 2017;372:20160044.
Pounds JA, Bustamante MR, Coloma LA, Consuegra JA, Fogden MPL, Foster PN, et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439:161–7. doi:10.1038/nature04246.
Bosch J, Carrascal LM, Duran L, Walker S, Fisher MC. Climate change and outbreaks of amphibian chytridiomycosis in a montane area of Central Spain; is there a link? Proc R Soc B. 2007;274:253–60. doi:10.1098/rspb.2006.3713.
Reichard JD, Fuller NW, Bennett AB, Darling SR, Moore MS, Langwig KE, et al. Interannual survival of Myotis lucifugus (Chiroptera: Vespertilionidae) near the epicenter of White-nose syndrome. Northeast Nat. 2014;21:N56–9.
Lilley TM, Johnson JS, Ruokolainen L, Rogers EJ, Wilson CA, Schell SM, et al. White-nose syndrome survivors do not exhibit frequent arousals associated with Pseudogymnoascus destructans infection. Front Zool. 2016;13:12. doi:10.1186/s12983-016-0143-3.
Lorch JM, Meteyer CU, Behr MJ, Boyles JG, Cryan PM, Hicks AC, et al. Experimental infection of bats with Geomyces destructans causes White-nose syndrome. Nature. 2011;480:376–8. doi:10.1038/nature10590.
Cornelison CT, Keel MK, Gabriel KT, Barlament CK, Tucker TA, Pierce GE, et al. A preliminary report on the contact-independent antagonism of Pseudogymnoascus destructans by Rhodococcus rhodochrous strain DAP96253. BMC Microbiol. 2014;14:246.
Langwig KE, Frick WF, Reynolds R, Parise KL, Drees KP, Hoyt JR, et al. Data from: host and pathogen ecology drive the seasonal dynamics of a fungal disease, White-nose syndrome. Dryad Digit Repos. 2014;282:20142335.
Acknowledgements
We would like to thank Anna Chow, Max Cox, Neil Giffen, Reilly Jackson, Devin Jones, Kitty McCracken, Mariah Patton, and Ana Reboredo-Segovia for help in the field; permitting and field help from Great Smoky Mountains National Park, Tennessee Wildlife Resources Agency, Tennessee chapter of The Nature Conservancy, Tennessee Department of Environmental Conservation, and the Tennessee regional office of the US Fish and Wildlife Service. We would also like to thank Colin Sobek for assisting with lab work at Northern Arizona University, and the White-nose syndrome and North American Society for Bat Research (NASBR) communities for countless discussions, advice, and continued support throughout this study. We would like to thank Melquisedec Gamba-Rios for graphic support and assistance in R, as well as keeping RFB sane while finishing her degree. Finally, we would like to thank all reviewers for their comments on earlier versions of the manuscript.
Funding
The research presented in this manuscript was funded by the White-nose syndrome research grant through Basically Bats Wildlife Conservation, Inc., University of Tennessee Institute of Agriculture Center for Wildlife Health, University of Tennessee Department of Ecology and Evolutionary Biology, and the US Geological Survey.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the figshare repository, https://figshare.com/s/e84c3d6178fbe7294d6b.
Author information
Authors and Affiliations
Contributions
RFB designed the project, collected all field samples, analyzed all data, collaborated on obtaining research funding, and wrote the manuscript. EVW assisted with collection of field samples and collaborated on obtaining research funding. JTF and KLP performed the genetic analyses. GFM assisted with the project design, collaborated on obtaining research funding, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We followed field decontamination protocols in accordance with the United States Fish and Wildlife Service WNS Decontamination Guidelines and recommendations by the state of Tennessee [54]. All capture and handling techniques were approved by the University of Tennessee Institute of Animal Care and Use Committee (IACUC 2026–0514) and were consistent with the guidelines issued by the American Society of Mammalogists [55]. We obtained both federal (USFWS TE-71613A; GRSM-2013-SCI-1053; GRSM-2014-SCI-1053) and state (TWRA 3716; TDEC 2011–031) permits to capture and handle bats at winter hibernacula for this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1: Figure S1.
Peak load of P. destructans on bats captured leaving hibernacula. Maximum monthly load of P. destructans for seven species captured at five cave sites in Tennessee during the 2012–2013 and 2013–2014 hibernation period (October–April). Circles indicate months where Pd positive individuals were captured. Species acronym codes: EPFU – Eptesicus fuscus, MYGR – Myotis grisescens, MYLE – Myotis leibii, MYLU – Myotis lucifugus, MYSE – Myotis septentrionalis, MYSO – Myotis sodalis, PESU – Perimyotis subflavus. (PNG 264 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bernard, R.F., Willcox, E.V., Parise, K.L. et al. White-nose syndrome fungus, Pseudogymnoascus destructans, on bats captured emerging from caves during winter in the southeastern United States. BMC Zool 2, 12 (2017). https://doi.org/10.1186/s40850-017-0021-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40850-017-0021-2