Abstract
Background
Thysanolaena maxima (Roxb.) Kuntze, a perennial grass plant, is usually distributed in hilly regions of the Indian Subcontinent. Different parts of T. maxima have been used as herbal medicine by traditional healers of this region. In this present study, T. maxima plant extract has been screened for examination of its secondary metabolite content with their probable cytotoxic activity.
Methods
Secondary metabolites of the crude T. maxima plant extract were isolated by different chromatographic methods. The structures were elucidated by spectroscopic data (1H NMR, 13C NMR) as well as comparison with available literature sources. Antiradical activity by DPPH radical scavenging assay and antimicrobial activity by disc diffusion method of the fractions and cytotoxic activity by trypan blue exclusion method of the isolated compounds were also evaluated.
Results
Three phenolic compounds 4-hydroxybenzaldehyde (1), 4-hydroxycinnamic acid (2), 4-hydroxybenzoic acid (3) and two steroids stigmast-4-en-3-one (4) and β-stigmasterol (5) were isolated from the aerial part of T. maxima. Among the compounds 4-hydroxycinnamic acid, 4-hydroxybenzoic acid and stigmast-4-en-3-one exhibited notable cytotoxic activity against African Green Monkey Kidney Cell line (Vero cell).
Conclusion
Bioassay investigation of the isolated compounds and fractions suggested that T. maxima could be a potential source of bioactive secondary metabolites.
Graphical abstract

Similar content being viewed by others
Background
Thysanolaena maxima (Roxb.), Kuntze (Family: Poaceae) (Synonym: Thysanolaena latifolia, English name: Tiger grass), a perennial forest grass plant, is found in the hilly regions of Bangladesh, India, Thailand, Nepal and Bhutan. Many tribal populations of these countries have been using different parts of this plant traditionally for many years. The inflorescence paste of T. maxima was used for the treatment of eye infection by traditional healers of Meghalaya, India [1]. Soft part of young leaves and flower buds are used as raw form to cure flatulence and improve digestion by Dimasa tribes of Assam in India [2]. Young shoots and fresh roots are used in the treatment of tonsillitis, boils and skin diseases by the people of Darjeeling district, West Bengal, India [3]. Crushed flowers are taken with water as antiemetic and in the treatment of stomach trouble by Kanda tribal population of Sylhet in Bangladesh [4]. It has also been reported to be useful as anthelmintic and febrifuge by the local people of Sabah, Malaysia [5].
Pharmacological studies have demonstrated that ethanol extract of T. maxima roots possessed moderate DPPH radical scavenging activity (IC50 value: 250 μg/ml) and antibacterial activity against four bacterial strains [6]. Aqueous extract of T. maxima showed significant chemopreventive and hepatoprotective activity against CCl4 induced hepatotoxicity in rats [5]. Furthermore, ethanolic extract of leaves of T. maxima showed cytotoxic activity against HepG2 cancer cell line where the percentage inhibition was 29.50 ± 3.9 at the concentration of 50 μg/ml [3]. The bioactivities of the different solvent extract of T. maxima have evidenced it as a potent source of natural radical scavenger [7]. Few compounds have been successfully isolated from the florets of T. maxima in Nepal such as luteolin, isoswertisin, isoquercetin, ergosterol peroxide, β-sitosterol, 7α-hydroxysitosterol, benzoicacid, syringic acid, leonuriside A, 7R,8R-4-O-methylsyringylglycerol, butyl protocatechuate, jaboticabin acid, caffeic anhydride etc. [8]. For the established traditional use of this plant as herbal medicine by different tribal populations and for its distinguished pharmacological activities, it is, therefore, indispensable to separate and characterize its chemical constituents in terms of functionality. Therefore, the objective of this investigation was to isolate the metabolites produced by T. maxima available in Bangladesh and to assess the antiradical, antimicrobial and cytotoxic activity of the fractions and their isolated compounds.
Materials and methods
Apparatus and reagents
2,2-Diphenyl-1-picrylhydrazyl (DPPH) was purchased from Sigma-Aldrich Co., USA. Silica gel and silica gel F254 plates were purchased from Merck, Germany. Nutrient agar media, standard disc of kanamycin and ketoconazole were purchased from Hi media, India. The NMR spectra were documented by 400 MHz NMR spectrometer (Bruker, Switzerland) using deuterated chloroform and deuterated methanol purchased from Sigma-Aldrich Co., USA. All the chemicals and solvents used were of analytical grades.
Plant collection and identification
The aerial part of T. maxima was collected from Rangamati, Chittagong Hill Tracts, Bangladesh on August 20, 2015. The taxonomical identification of the tested plant was authenticated from Bangladesh National Herbarium (BNH), Mirpur, Dhaka and voucher specimen was deposited in the BNH with the accession number DACB 42267.
Preparation of the crude extract
A total of 1 kg powdered coarse plant materials were subjected to maceration in dichloromethane:methanol (1:1) mixture using 2.5 l of each solvent in an air-tight container for 5 days with intermittent shaking. The solvent mixture with most of the extractable compounds was collected by filtration with cotton plug followed by filter paper. The solvent was removed using rotary evaporator (Heidolph, Germany) at temperature around 40 °C - 50 °C under reduced pressure. The above process was repeated two times with fresh solvents to gather more concentrated extract. Finally, all the filtrates were mixed together to get the crude extract (19 g).
Chromatographic procedures
The crude extract was fractionated by vacuum liquid chromatography (VLC) on silica gel (60G) eluted with gradient solvent system as petroleum ether-CH2Cl2-MeOH to obtain 27 fractions. These fractions were screened by thin layer chromatography (TLC) with aluminum plates coated with silica gel (F254) and the plates were envisaged under UV light at 254 nm, 365 nm and by spraying with vanillin/1% H2SO4 solution followed by heating. The fractions having similar TLC patterns were combined to give 8 fractions (F-1 to F-8). Column chromatography (silica gel 70-230 and 230-400, mesh) and preparative TLC (PTLC) were performed for separation and purification of the compounds from the VLC fractions.
Bioassay screening of the fractions
DPPH free radical scavenging activity
The free radical scavenging ability of T. maxima VLC fractions were screened by measuring the reduced absorbance of methanolic DPPH solution [9]. Methanolic DPPH stock solution (20 μg/mL) was added (200 μL) to methanolic sample solution to obtain final 4 ml solution of different concentration (200 μg/mL to 12.5 μg/mL). The absorbance was measured at 517 nm by using UV-VIS spectrophotometer (Analytic Jena AG, Germany) after the solutions were mixed properly and kept in dark for 20 min. The result was expressed using the following formula as the percentage inhibition:
where A0 is the absorbance of the control and A1 is the absorbance of the fractions/standard.
Antimicrobial assay
Disc diffusion method was used for preliminary antimicrobial assay [10] against four bacterial strains (Bacillus megaterium, Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli) and two fungal strains (Aspergillus flavus and Aspergillus niger). A total of 100 μL of suspension of each microorganism containing approximately 100-150 CFU/mL was spread over the nutrient agar for bacteria and potato dextrose agar media for fungi. Filter paper discs around 6 mm diameter were sterilized, steeped with 200 μg of different fraction solutions and placed gently in the spreaded agar plates. Kanamycin (30 μg/disc) and ketoconazole (30 μg/disc) were used as positive control and solvent discs were used as negative control in this study. The zone of inhibition was measured as diameter in mm after 24-h incubation at 37 °C for bacteria and 48-h incubation at 28 °C for fungi.
Isolation of the compounds
Phytochemical investigation for compound isolation on fractions F-2, F-3 and F-4 were performed based on the preliminary chemical profiling using TLC, antimicrobial activity of F-2 and F-3 and antiradical activity of F-4. Polar fractions F-5 to F-8 eluted with CH2Cl2/0.1-8% MeOH showed very poor resolution of the compounds on TLC due to matrix effect. Therefore, phytochemical investigation for isolation of compounds from fraction F-5 to F-8 could not carry out in spite of their strong antiradical property.
An aliquot (80 mg) of VLC fraction F-3 (583.4 mg) eluted with petroleum ether/35-45% CH2Cl2was subjected to preparative thin layer chromatography (PTLC) (stationary phase: silica gel F254, mobile phase: toluene/10% EtOAc, two developments; thickness of plates: 0.25 mm) to afford compound 1 (3.0 mg, 3.75%). Compound 2 (2.5 mg, 3.12%) was obtained by subjecting 80 mg of the VLC fraction F-4 (1.38 g) eluted with petroleum ether/50-85% CH2Cl2 to PTLC (mobile phase: toluene/30% EtOAc, 2 developments). Fraction F-3 was subjected to silica gel column chromatography and eluted with n-hexane-CH2Cl2-MeOH solvent system with increasing polarity. Column fraction eluted with n-hexane/35-40% CH2Cl2 gave brown amorphous mass which was purified by solvent treatment to afford compound 3 (2.2 mg, 2.75%). Fraction F-4 was rechromatographed by silica gel column chromatography eluted with petroleum ether-CH2Cl2-MeOH in gradients of increasing polarities. Fraction obtained by petroleum ether/85-95% CH2Cl2 was further purified by PTLC (mobile phase: toluene/5% EtOAc, 2 developments) to yield compound 4 (3.0 mg). One slightly impure white crystal obtained from the VLC fraction of petroleum ether/20-30% CH2Cl2 (F-2, 287.5 mg) was purified by treatment with different solvents to get compound 5 (3.2 mg, 4%).
-
4-Hydroxybenzaldehyde (1): (3.0 mg, brown amorphous powder); 1H NMR (400 MHz, CDCl3): δ 9.78 (1H, s, −CHO), 7.74 (2H, d, J = 8.6 Hz, H-2, H-6), 6.91 (2H, d, J = 8.6 Hz, H-3, H-5). 13C NMR (100 MHz, CDCl3): δ 191.4 (−CHO), 163.2 (C-4), 132.4 (C-2, C-6), 128.8 (C-1), 115.8 (C-3, C-5).
-
4-Hydroxycinnamic acid (2): (2.5 mg, white amorphous powder); 1H NMR (400 MHz, CDCl3/1% CD3OD): δ 7.37 (2H, d, J = 8.2 Hz, H-2, H-6), 6.79 (2H, d, J = 8.2 Hz, H-3, H-5), 7.60 (1H, d, J = 16.0 Hz, H-7), 6.23 (1H, d, J = 16.0 Hz, H-8). 13C NMR (100 MHz, CDCl3): δ 169.3 (C-9), 158.8 (C-4), 145.3 (C-7),132.0 (C-2, C-6), 125.9 (C-1), 115.5 (C-3, C-5), 114.6 (C-8).
-
4-Hydroxybenzoic acid (3): (2.2 mg, brown amorphous powder); 1H NMR (400 MHz, CD3OD): δ 7.88 (2H, d, J = 8.4 Hz, H-2, H-6), 6.78 (2H, d, J = 8.4 Hz, H-3, H-5). 13C NMR (CD3OD, 100 MHz): δ 171.4 (−COOH), 163.2 (C-4), 132.4 (C-2, C-6), 128.9 (C-1), 115.8 (C-3, C-5).
-
Stigmast-4-en-3-one (4): (3 mg, white crystal); 1H NMR (400 MHz, CDCl3): δ 5.75 (1H, s, H-4), 1.20 (3H, s, H-19), 0.94 (3H, d, J = 5.2 Hz, H-21), 0.91 (3H, d, J = 6.4 Hz, H-29), 0.87 (3H, d, J = 5.6 Hz, H-26), 0.84 (3H, d, J = 5.6 Hz, H-27), 0.73 (3H, s, H-18).13C NMR (100 MHz, CDCl3): δ 198.9 (C-3), 171.4 (C-5), 123.6 (C-4), 55.9 (C-17), 55.8 (C-14), 53.7 (C-9), 45.7 (C-24), 42.5 (C-13), 39.7 (C-12), 38.6 (C-10), 36.0 (C-20), 35.7 (C-8), 35.6 (C-1), 34.0 (C-22), 33.9 (C-2), 32.9 (C-6), 32.1 (C-7), 29.1 (C-25), 28.1 (C-16), 26.1 (C-23), 24.1 (C-15), 23.1 (C-28), 21.1 (C-11), 19.7 (C-26), 19.1 (C-27), 18.7 (C-21), 17.5 (C-19), 11.9 (C-18), 11.9 (C-29).
-
β-stigmasterol (5): (3.2 mg, white needle); 1H NMR (400 MHz, CDCl3): δ 5.37 (1H, d, J = 4.8 Hz, H-6), 5.18 (1H, dd, J = 15.0, 8.6 Hz, H-22), 5.04 (1H, dd, J = 15.2, 8.4 Hz, H-23), 3.55 (1H, m, H-3), 1.03 (3H, s, H-19), 0.94 (3H, d, J = 6.4 Hz, H-21), 0.86 (3H, d, J = 2.8 Hz, H-29), 0.85 (3H, d, J = 1.2 Hz, H-26), 0.82 (3H, d, J = 7.2 Hz, H-27), 0.71 (3H, s, H-18). 13C NMR (100 MHz, CDCl3): δ 140.7 (C-5), 138.4 (C-22), 129.2 (C-23), 121.6 (C-6), 71.8 (C-3), 56.9 (C-14), 56.8 (C-17), 51.2 (C-24), 50.1 (C-9), 45.8 (C-25), 42.3 (C-13), 42.2 (C-4), 40.4 (C-20), 39.8 (C-12), 37.2 (C-1), 36.4 (C-10), 33.9 (C-8), 31.7 (C-7), 29.2 (C-16), 28.1 (C-2), 24.4 (C-28), 24.3 (C-15), 21.1 (C-11), 21.0 (C-21), 19.7 (C-27), 19.3 (C-26), 19.0 (C-19), 12.1 (C-29), 12.0 (C-18).
Evaluation of cytotoxicity of pure compounds
Cytotoxic activity was studied against Vero cell line (Vero cell line, CLS 605372, Germany) using slight modification of the Trypan Blue Exclusion Method [11, 12]. Cells were cultivated in 75cm2 flasks in 5% (v/v) CO2 at 37 °C with media described at Khan et al, 2018 [10]. According to study design, cells were grouped into 3 with three replica each. Treatment groups were evaluated with vehicle group. Vero cells were split the day before experiments. The freshly prepared doses (0.1, 0.5, 1.0, 5.0, 10 and 20 μg/mL) were administered into 1 day before cultured T- flasks with approximately 2.5 × 106 cells. Negative control corresponds to the cells cultured with medium with 0.6% DMSO. After 24 h of incubation period cells were harvested using 0.5% trypsin. The number of dead cells was calculated by automated cell counter (LUNA-II™, South Korea) [13] using trypan blue (0.4% w/v). Percentage of dead cells was calculated following the mathematical formula:
Statistical analysis
All values for antioxidant and antimicrobial evaluation were determined as mean ± standard deviation (SD) where n = 3. Continuous variables between groups were compared with one-way analysis of variance (ANOVA) with post hoc tukey’s test for the analysis of the cytotoxicity results of pure compounds. Mean values between groups were compared using independent student t-test for equality of variances. Statistical significance was accepted when P < 0.001.
Results
Bioassay screening of fractions
DPPH free radical scavenging activity
The DPPH free radical scavenging method was exercised to investigate the antiradical activity of the fractions. Fraction F-7 exhibited the highest antiradical scavenging activity (IC50 = 21.39 ± 2.02 μg/mL) whereas F-1 showed the lowest activity (IC50 = 149.68 ± 1.91 μg/mL) (Table 1). The values of scavenging activity which denotes as IC50 of the fractions with standard (ascorbic acid) are shown in Table 1. The results indicated that fractions F-3 to F-8 possess antioxidant compounds that may play a significant role to prevent diseases caused by reactive oxygen species.
Antimicrobial assay
The antimicrobial activity of the fractions and the standards against different microorganisms is shown in Table 2. The fractions (200 μg/disc) showed weak to strong activities against some test microorganisms compared to the standard discs. Fraction F-3 exhibited notable antifungal activity against Aspergillus niger (19.3 ± 1.1 mm) and antibacterial activity against Bacillus megaterium (12.0 ± 0.5 mm) (Table 2). Fraction F-2 showed antibacterial activity against three bacterial strains (Bacillus megaterium, Pseudomonas aeruginosa and Escherichia coli) but found to be inactive against tested fungal strains. Other fractions (F-1, F-4 to F-8) were failed to present antimicrobial activity against the tested microorganisms.
Secondary metabolites from Thysanolaena maxima
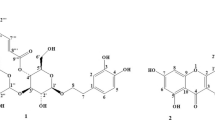
Compounds 1 - 5 were isolated from dichloromethane:methanol (1:1) extract of T. maxima using different chromatographic methods. The structures of these compounds were elucidated by comparing their NMR data with those in the related literature (Fig. 1). To the best of our knowledge, compounds 1, 2, 4 and 5 were isolated from T. maxima for the first time.
Compound 1 was obtained as brown amorphous solid. The 1H NMR spectrum of compound 1 showed two doublets at δ 7.74 and δ 6.91 ppm characteristic of a para disubstituted aromatic compound and a signal for an aldehydic proton at δ 9.78 ppm. The 13C NMR showed one aldehydic carbon at δ 191.4 ppm, one oxygenated carbon signal at δ 163.2 ppm and four olefins at δ 132.4 and 115.8 ppm. These spectroscopic data are found to be identical with the spectroscopic data of 4-hydroxybenzaldehyde [14].
Compound 2 was obtained as white amorphous solid. The 1H NMR spectrum of compound 2 displayed proton signals at δ 7.37 and δ 6.79 ppm and two trans-olefinic proton signals at δ 7.60 and δ 6.23 ppm. The 13C NMR spectrum of compound 2 showed nine signals corresponding to one carboxylic acid signal at δ 169.3 ppm, one oxygenated quaternary carbon signal at δ 158.8 ppm, and six methine carbon signals at δ 132.0, 115.5, 145.3 and 114.6 ppm. Thus, compound 2 was identified as 4-hydroxycinnamic acid by comparing these data with the reported values [15].
Compound 3 was obtained as brown amorphous solid. The 1H NMR spectrum of compound 3 showed ortho-coupled proton signals at δ 7.88 and δ 6.78 ppm. The 13C NMR of compound 3 contained one carboxyl carbon signal at δ 171.4 ppm, one oxygenated carbon signal at δ 163.2 ppm, and four methine carbon signals at δ 132.4 and δ 115.8 ppm. According to this analysis and compared with published spectroscopic data [14], compound 3 was suggested as 4-hydroxybenzoic acid.
Compound 4 was obtained as white crystals. The 1H NMR spectrum of compound 4 showed one-proton singlet in the olefinic region at δ 5.75 ppm, two singlets at δ 0.73 and 1.20 ppm and four three proton doublets at δ 0.94, 0.87, 0.84 and 0.91 ppm. The presence of 29 carbons in the molecule clearly indicated by the 29 signals in the 13C NMR spectrum including a carbonyl peak at δ 198.9 ppm and two olefinic carbon peaks at δ 123.6 and δ 171.4 ppm. These spectral data indicated that the compound 4 is a steroidal molecule containing a ketone group, one double bond and six methyl groups which was in complete agreement with the literature data [16] suggesting the compound as stigmast-4-en-3-one.
Compound 5 was obtained as white needle. The 1H NMR spectrum of compound 5 showed three one-proton peaks in the olefinic region at δ 5.37, 5.18 and 5.04 ppm and one one-proton multiplet at δ 3.55 ppm. The spectrum also displayed one three-proton doublet at δ 0.71 ppm and one three proton singlet at δ 1.03 ppm, four-three proton doublets at δ 0.94, 0.85, 0.82 and 0.86 ppm. The 13C NMR spectrum displayed an oxymethine carbon at δ 71.8 ppm and four signals at δ 140.7, 121.6, 138.4 and 129.2 ppm clearly indicated the presence of one hydroxyl group and two double bonds in compound 5. This analysis and previous literature [17] were concluded recognizing this structure as β-stigmasterol.
Evaluation of cytotoxicity of pure compounds
The half-maximal inhibitory value of each compound was measured from the analysis of dose response curve. The IC50 value of each compound is summarized in Table 3. Stigmast-4-en-3-one (4) exhibited highest cytotoxic activity with IC50 value of 5.82 μg/mL among the tested compounds. Another compound 4-hydroxybenzaldehyde (1) also presented a significant inhibition with IC50 value of 7.6 μg/mL. 4-hydroxybenzoic acid (3) and β-stigmasterol (5) were overall ineffective in Vero cell line failed to show significant change in cell viability at the tested concentrations (Fig. 2).
In Vero cell line, compound 4-hydroxycinnamic acid (2) at 5.0 μg/mL reduced cell viability (22.7%) whereas compound 4-hydroxy benzoic acid (3) showed maximum cell proliferation (88.8%) at the same concentration. It is noteworthy that these two compounds are phenolic compounds, but their bioactivity characteristics are different presumably due to their different functional groups. More investigations like applying in silico method and functional group study, animal study are required to confirm this statement.
Discussion
Polyphenols are well known for their radical quenching activity. The presence of multiple hydroxyl groups on their structure makes them able to donate single electron to the highly reactive oxygen species (ROS). These ROS are highly reactive free radicals who are developed in our body as a byproduct of natural ATP producing pathway. The damaging effects of ROS are mainly result from their ability to oxidize cell membrane and biomolecules like lipid, proteins and DNA. This ROS generated oxidative damage silently triggers the development of various degenerative diseases like diabetes, hypertension, atherosclerosis, Alzheimer’s disease and cancer [18, 19]. Free radical DPPH reduces to its stable hydrazine form by accepting hydrogen/electron from potent antioxidant substances. In this assay, the VLC fractions of T. maxima exhibited strong radical scavenging capability which can be attributed to its isolated phenolic compounds as a positive correlation is reported between the phenolic compounds and free radical scavenging capacity [20]. Furthermore, stigmasterols are also reported to have strong antioxidant and free radical scavenging property [21]. Therefore, the strong radical scavenging activity of the VLC fractions may be attributed to the phenols and sterols present in the plant making it a potent source of antioxidants. This result is also consistent with the previous studies where presence of high amount of phenolic constituents and potent DPPH free radicals scavenging activity of this plant was reported [5, 6].
In the present study, some of the VLC fractions (F-2, F-3) showed low to moderate antibacterial activity against four strains of tested bacteria. Studies have shown that T. maxima leaf extract and its essential oils have potential antimicrobial activity which also strengthens our present findings [6, 22]. The fraction F-3 showed the maximum antifungal activity against Aspergillus flavus which make it a potential antifungal candidate. The antimicrobial properties of some individual sterols of plant origin were reported previously [23, 24]. So, the antimicrobial activities of these fractions may be related to the isolated sterols; though further studies are required to confirm this.
This study reports isolation of three phenolic compounds 4-hydroxybenzaldehyde (1), 4-hydroxycinnamic acid (2), 4-hydroxybenzoic acid (3) and two steroids stigmast-4-en-3-one (4) and β-stigmasterol (5) in which compounds 1, 2, 4 and 5 were isolated from T. maxima for the first time. Shrestha and co-investigators isolated 34 known compounds and a new compound 6″-O-acetylorientin-2″-O-α-L-rhamnopyranoside from this plant found in Nepal [8]. On the other hand, 21 components were identified by GC-MS from the essential oil extracted from this plant available in Southern China [22].
The highest cytotoxic activity against Vero cell line was obtained with stigmast-4-en-3-one (IC50 = 5.82 ± 0.66 μg/mL); this activity could be explained by the presence of a carbonyl group at C3 position in the steroidal nucleus compared to the stigmasterol (IC50 = 28.96 ± 0.24 μg/mL) having a hydroxyl group at C3 position. Another significant cytotoxic activity was observed with 4-hydroxy benzaldehyde (IC50 = 7.60 ± 0.66 μg/mL), presumably due to the presence of an aldehyde group at C1 position in the benzene ring whereas a carboxylic acidic group was present in other two phenolic compounds, 4-hydroxycinnamic acid (IC50 = 10.0 ± 0.09 μg/mL) and 4-hydroxy benzoic acid (IC50 = 38.85 ± 0.33 μg/mL). 4-Hydroxybenzaldehyde, stigmast-4-en-3-one, β-stigmasterol showed monophasic dose response curve whereas 4-hydroxycinnamic acid and 4-hydroxybenzoic acid showed bi-phasic dose response curve (Fig. 2), indicated that different mechanisms may involve for individual compound’s cytotoxic activity due to their structural and functional group differences. We observed that compound 2 (4-hydroxycinnamic acid) and compound 3 (4-hydroxybenzoic acid) reduced cell viability with the increased concentration but after certain concentration cell viability increased with the higher concentration. This event suggests that at higher concentrations of compound 2 and 3, an additional pathway is activated but what this might be unknown. These two compounds were acidic among five compounds which might have influenced on the pH level of media that might affect cell viability. On the other hand, some macromolecules like caspase inhibitors may release in the media when used high concentration of compound 2 and 3which may reduce cell death [25]. In the last few years it has been reported that hydroxycinnamic acid and its derivatives have potential inhibitory effects on metastasis and cancer invasion [23, 26]. Scientists have been paid great attention to elucidate the possible mechanisms involved in anticancer effects on phenolic compounds including hydroxycinnamic acids [26,27,28]. β-stigmasterol (5), which is commonly distributed in many medicinal plants are reported to have anti-osteoarthritic [29], anti-hypercholesterolemia [30], antitumor [31], hypoglycemic [32], antimutagenic [33] and anti-inflammatory activity [34]. Stigmast-4-en-3-one (4) which is a derivative of stigmasterol, showed potent hypoglycemic effect on alloxan-induced diabetic rats [32]. The incidence of stigmast-4-en-3-one is described for the first time from T. maxima and provided evidence as another source of natural hypoglycemic agent. Preliminary TLC revealed the presence of several metabolites in each of fractions F-2, F-3 and F-4. This means that these fractions might contain metabolite(s) responsible to exert antiradical or antimicrobial activity. It is also possible to assume that there may present synergistic effects of some of these compounds or individual effect of the single compound of these fractions.
The biosynthesis of phenol compounds usually occurs at the membrane surface of the endoplasmic reticulum (ER) in plant cells [35]. Phenylalanine (L-Phe) is the main precursor for the biosynthesis of different groups of phenolic compounds such as flavonoids, coumarins, phenolic acids, tannins, lignins etc. L-Tyrosine (L-Tyr) and L-Tryptophan (L-Trp) also play an important role to produce phenolic compounds when needed by the plants [36, 37]. Several enzymes namely erythrose-4-phosphatase, phenyl alanine ammonia lyase (PAL), cinnamate-4-hydroxylase (C4H), p-coumarate-3-hydroxylase (C3H) and o-methyltransferase etc. are directly involved in different steps for the biosynthesis of phenolic compounds [38]. It is presumed that the biosynthesis of phenolic compounds begins by releasing an ammonia group of phenylalanine with the direct involvement of phenylalanine ammonia lyase (PAL) and this step produces trans-cinnamic acid by creating a double bond (Fig. 3). This trans-cinnamic acid is then shifted in 4-hydroxycinnamic acid with the activity of cinnamate-4-hydroxylase by introducing a hydroxyl group into the aromatic ring. The 4-hydroxycinnamic acid is then converted into 4-hydroxybenzaldehyde by 4-hydroxybenzaldehyde synthase and 4-hydroxybenzaldehyde is shifted into 4-hydroxybenzoic acid with the activity of 4-hydroxybenzaldehyde hydroxylase by introducing a hydroxyl group into side chain.
Schematic representation of the possible biosynthetic pathways for 4-hydroxybenzaldehyde (1), 4-hydroxybenzoic acid (2), 4-hydroxycinnamic acid (3) in Thysanolaena maxima Roxb. E1-3-deoxy-7-phosphoheptulonate synthase, E2-3-dehydroquinate synthase, E3-shikimate dehydrogenase, E4-shikimate kinase, E5-3-phosphoshikimate 1-carboxyvinyltransferase, E6-chorismate synthase, E7-chorismate mutase, E8-arogenate dehydratase, E9-bifunctional aspartate aminotransferase, E10-histidinol-phosphate aminotransferase, E11-tyrosine aminotransferase, E12-phenylalanine ammonia-lyase, E13- cinnamate-4-hydroxylase, E14-4-hydroxybenzaldehyde synthase, E15-4-hydroxybenzaldehyde hydroxylase
It is thought that the biosynthesis of phytosterols produces in the isoprenoid pathway where C5-unit originates from the acetate-mevalonate or mevalonate-independent pathways (Fig. 4). In this biosynthetic pathway 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) plays key role to convert 3-hydroxy-3-methylglutaryl-CoA to mevalonate which is the main precursor for the biosynthesis of phytosterols [39]. A series of enzymes are involved to convert mevalonate to cycloartenol which is still unexplored. In this step cycloartenol is converted to 24-methylene cycloartenol by the activity of C24-methyl transferase. Isoflucosterol is produced from the 24-methylene cycloartenol by the involvement of different types of demethylase, reductase, transferase enzymes. Isoflucosterol is shifted to β-sitosterol with the activity of C-24 reductase. β-sitosterol is converted to stigmasterol when C-24 reductase is involved and stigmasta-4-en-3-one is originated when C-3 reductase is involved [40].
Conclusion
This study is suggestive that T. maxima is a rich source of different phenolic and steroid compounds which can be used as a source of antioxidant, antimicrobial and cytotoxic agents in the development of new therapeutic agents. Further work on isolation of compounds with bioactivities is on progress and will be reported in due course.
Availability of data and materials
The datasets used and/or analysed during the current study are mostly included in the submitted manuscript. Any other information will be available from the corresponding author upon request.
References
Hynniewta SR, Kumar Y. Herbal remedies among the Khasi traditional healers and village folks in Meghalaya. Indian J Tradit Knowl. 2008;7:581–6 http://nopr.niscair.res.in/handle/123456789/2383.
Rout J, Sajem AL, Nath M. Medicinal plants of north Cachar hills district of Assam used by the Dimasa tribe. Indian J Tradit Knowl. 2012;11:520–7 http://nopr.niscair.res.in/handle/123456789/14395.
Tiwary BK, Bihani S, Kumar A, Chakraborty R, Ghosh R. In vitro cytotoxic activity of ethno-pharmacological important plants of Darjeeling district of West Bengal against different human cancer cell lines. BMC Complement Altern Med. 2015;15:1–10.
Rahmatullah M, Ayman U, Akter F, Sarker M, Sifa R, Sarker B, Chyti HN, Jahan FI, Chowdhury MH, Chowdhury SA. Medicinal formulations of a Kanda tribal healer - a tribe on the verge of disappearance in Bangladesh. Afr J Tradit Complement Altern Med. 2012;10:213–22. https://doi.org/10.4314/ajtcam.v10i2.5https://www.ncbi.nlm.nih.gov/pubmed/24146444.
Gnanaraj C, Haque ATME, Iqbal M. The chemopreventive effects of Thysanolaena latifolia against carbon tetrachloride (CCl4)-induced oxidative stress in rats. J Exp Integr Med. 2012;2:345–55. https://doi.org/10.5455/jeim.030912.or.045https://www.researchgate.net/publication/265059034.
Subba B, Basnet P. Antimicrobial and antioxidant activity of some indigenous plants of Nepal. J Pharmacogn Phytochem. 2014;3:62–7 http://www.phytojournal.com/archives/?year=2014&vol=3&issue=1&ArticleId=312.
Hoque N, Sohrab MH, Debnath T, Rana MS. Antioxidant, antibacterial and cytotoxic activities of various extracts of Thysanolaena maxima (Roxb.) kuntze available in Chittagong hill tracts of Bangladesh. Int J Pharm Pharm Sci. 2016;8:168–72 https://innovareacademics.in/journals/index.php/ijpps/article/view/11490.
Shrestha S, Park JH, Cho JG, Lee DY, Jeong RH, Song MC, Cho SK, Lee DS, Baek NI. Phytochemical constituents from the florets of tiger grass Thysanolaena latifolia from Nepal. J Asian Nat Prod Res. 2016;18:206–13. https://doi.org/10.1080/10286020.2015.1062757.
Braca A, De Tommasi N, Di Bari L, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia terapotensis. J Nat Prod. 2001;64:892–5. https://doi.org/10.1021/np0100845https://pubs.acs.org/doi/abs/10.1021/np0100845.
Khan N, Afroz F, Begum MN, Rony SR, Sharmin S, Moni F, Hasan CM, Shaha K, Sohrab MH. Endophytic Fusarium solani: a rich source of cytotoxic and antimicrobial napthoquinone and aza-anthraquinone derivatives. Toxicol Rep. 2018;5:970–6. https://doi.org/10.1016/j.toxrep.2018.08.016https://www.ncbi.nlm.nih.gov/pubmed/30294556/.
Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001. https://doi.org/10.1002/0471142735.ima03bs111https://www.ncbi.nlm.nih.gov/pubmed/18432654.
Wilson CH, Ali ES, Scrimgeour N, Martin AM, Hua J, Tallis GA, Rychkov GY, Barritt GJ. Steatosis inhibits liver cell store-operated Ca2+ entry and reduces ER Ca2+ through a protein kinase C-dependent mechanism. Biochem J. 2015;466:379–90. https://doi.org/10.1042/BJ20140881https://www.ncbi.nlm.nih.gov/pubmed/25422863.
Berry MN, Barritt GJ, Edwards AM. Isolated hepatocytes: preparation, properties and applications. New York: Elsevier Science; 1991. https://www.elsevier.com/books/978-0-444-81302-2.
Nguyen DH, Zhao BT, Le DD, Kim KY, Kim YH, Yoon YH, Ko JY, Woo KS, Woo MH. Phenolic constituents and their anti-inflammatory activity from Echinochloa utilis grains. Nat Prod Sci. 2016;22:140–5. https://doi.org/10.20307/nps.2016.22.2.140https://www.researchgate.net/publication/305461509.
Yi B, Hu L, Mei W, Zhou K, Wang H, Luo Y, Wei X, Dai H. Antioxidant phenolic compounds of cassava (Manihot esculenta) from Hainan. Molecules. 2011;16:10157–67. https://doi.org/10.3390/molecules161210157https://www.ncbi.nlm.nih.gov/pubmed/22157579.
DellaGreca M, Monaco P, Previtera L. Stigmasterols from Typha latifolia. J Nat Prod. 1990;53:1430–5. https://doi.org/10.1021/np50072a005.
Ahmed Y, Rahman S, Akhtar P, Islam F, Rahman M, Yaako Z. Isolation of steroids from n-hexane extract of the leaves of Saurauia roxburghii. Int Food Res J. 2013;20:2939–43 https://www.researchgate.net/publication/259969879.
Liao K, Yin M. Individual and combined antioxidant effects of seven phenolic agents in human erythrocyte membrane ghosts and phosphatidylcholine liposome system: the importance of the partition coefficient. J Agric Food Chem. 2000;48:2266–70. https://doi.org/10.1021/jf990946whttps://www.ncbi.nlm.nih.gov/pubmed/10888534.
Iqbal E, Salim KA, Lim LB. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J King Saud Univ Sci. 2015;27:224–32. https://doi.org/10.1016/j.jksus.2015.02.003https://www.researchgate.net/publication/272297904.
McDonald S, Prenzler PD, Antolovich M, Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001;73:73–84. https://doi.org/10.1016/S0308-8146(00)00288-0.
Kaur N, Chaudhary J, Jain A, Kishore L. Stigmasterol: a comprehensive review. Int J Pharm Sci Res. 2011;2:2259–65. https://doi.org/10.13040/IJPSR.0975-8232.2(9).2259-65.
Li R, Hu HB, Li XF, Zhang P, Xu YK, Yang JJ, Wang YF. Essential oils composition and bioactivities of two species leaves used as packaging materials in Xishuangbanna, China. Food Control. 2015;51:9–14.
Kuete V, Nguemeving JR, Beng VP, Azebaze AG, Etoa FX, Meyer M, Bodo B, Nkengfack AE. Antimicrobial activity of the methanolic extracts and compounds from Vismia laurentii De Wild (Guttiferae). J Ethnopharmacol. 2007;109:372–9. https://doi.org/10.1016/j.jep.2006.07.044https://www.ncbi.nlm.nih.gov/pubmed/16971076.
Djoukeng JD, Abou-Mansour E, Tabacchi R, Tapondjou AL, Bouda H, Lontsi D. Antibacterial triterpenes from Syzygium guineense (Myrtaceae). J Ethnopharmacol. 2005;101:283–6. https://doi.org/10.1016/j.jep.2005.05.008https://www.researchgate.net/publication/7775047.
Quesney S, Marvel J, Marc A, Gerdil C, Meignier B. Characterization of Vero cell growth and death in bioreactor with serum-containing and serum-free media. Cytotechnology. 2001;35:115–25.
Weng CJ, Yen GC. Chemo preventive effects of dietary phytochemicals against cancer invasion and metastasis: phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat Rev. 2012;38:76–87. https://doi.org/10.1016/j.ctrv.2011.03.001https://www.ncbi.nlm.nih.gov/pubmed/21481535.
Puangpraphant S, Berhow MA, Vermillion K, Potts G, Gonzalez de Mejia E. Dicaffeoylquinic acids in Yerba mate (Ilex paraguariensis St. Hilaire) inhibit NF-κB nucleus translocation in macrophages and induce apoptosis by activating caspases-8 and-3 in human colon cancer cells. Mol Nutr Food Res. 2011;55:1509–22. https://doi.org/10.1002/mnfr.201100128https://www.ncbi.nlm.nih.gov/pubmed/21656672.
Rosa LS, Silva NJ, Soares NC, Monteiro MC, Teodoro AJ. Anticancer properties of phenolic acids in colon cancer - a review. J Food Sci Nutr. 2016;6:10–4172. https://doi.org/10.4172/2155-9600.1000468https://www.longdom.org/abstract/anticancer-properties-of-phenolic-acids-in-colon-cancer-a-review-35403.html.
Gabay O, Sanchez C, Salvat C, Chevy F, Breton M, Nourissat G, Wolf C, Jacques C, Berenbaum F. Stigmasterol: a phytosterol with potential antiosteoarthritic properties. Osteoarthr Cartilage. 2010;18:106–16 https://www.sciencedirect.com/science/article/pii/S1063458409002179.
Chandler RF, Hooper SN, Ismail A. Antihypercholesterolemic studies with sterols: beta-sitosterol and stigmasterol. J Pharm Sci. 1979;68:245–7 https://www.sciencedirect.com/science/article/abs/pii/S0022354915424621.
Kasahara Y, Kumaki K, Katagiri S, Yasukawa K, Yamanouchi S, Takido M, Akihisa T, Tamura T. Carthami flos extract and its component, stigmasterol, inhibit tumour promotion in mouse skin two stage carcinogenesis. Phytother Res. 1994;8:327–31. https://doi.org/10.1002/ptr.2650080603.
Jamaluddin F, Mohamed S, Lajis MN. Hypoglycaemic effect of Parkia speciosa seeds due to the synergistic action of β-sitosterol and stigmasterol. Food Chem. 1994;49:339–45. https://doi.org/10.1016/0308-8146(94)90002-7https://www.sciencedirect.com/science/article/pii/0308814694900027.
Jae-Chul L, Jong Hee P, Milos B, Alexander K, Yeong-Hwan H, Byung-Soo K, et al. Antimutagenic constituents from the thorns of Gleditsia sinensis. Chem Pharm Bull. 2005;53:561–4.
Garcia MD, Saenz MT, Gomez MA, Fernandez MA. Topical anti-inflammatory activity of phytosterols isolated from Eryngium foetidum on chronic and acute inflammation models. Phytother Res. 1999;13:78–80. https://doi.org/10.1002/(SICI)1099-1573(199902)13:1<78::AID-PTR384>3.0.CO;2-Fhttps://onlinelibrary.wiley.com/doi/pdf/10.1002/(SICI)1099-1573(199902).
Shahidi F, Yeo J. Bioactivities of phenolics by focusing on suppression of chronic diseases: a review. Int J Mol Sci. 2018;19:1573. https://doi.org/10.3390/ijms19061573https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6032343/.
Weaver LM, Herrmann KM. Dynamics of the shikimate pathway in plants. Trends Plant Sci. 1997;2:346–51. https://doi.org/10.1016/S1360-1385(97)84622-5.
Jiang X, Liu Y, Li W, Zhao L, Meng F, Wang Y, Tan H, Yang H, Wei C, Wan X, Gao L, Xia T. Tissue-specific, development-dependent phenolic compounds accumulation profile and gene expression pattern in tea plant (Camellia sinensis). PLoS One. 2013;8:e62315. https://doi.org/10.1371/journal.pone.0062315.
Shahidi F, Naczk M. Phenolics in food and nutraceuticals. Boca Raton: CRC press; 2003. ISBN 978036739509.
Harker M, Holmberg N, Clayton JC, Gibbard CL, Wallace AD, Rawlins S, Hellyer SA, Lanot A, Safford R. Enhancement of seed phytosterol levels by expression of an N-terminal truncated Hevea brasiliensis (rubber tree) 3-hydroxy-3-methylglutaryl-CoA reductase. Plant Biotechnol J. 2003;1:113–21. https://doi.org/10.1046/j.1467-7652.2003.00011.xhttps://www.researchgate.net/publication/6650929.
Seki H, Ohyama K, Nishizawa T, Yoshida S, Muranaka T. The “all-in-one” rol-type binary vectors as a tool for functional genomic studies using hairy roots. Plant Biotechnol-Nar. 2008;25:347–55. https://doi.org/10.5511/plantbiotechnology.25.347https://www.researchgate.net/publication/271887385.
Acknowledgements
Authors are grateful to Pharmaceutical Science Research Division, BCSIR Laboratories Dhaka for providing with the necessary laboratory and instrumental facilities and also for all the chemical and reagent supports.
Funding
Authors receive no fund for the research work.
Author information
Authors and Affiliations
Contributions
All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content of the manuscript. Author MHS conceived the presented research project and supervised all the work along with FA, SR and CMH. Author NH, SRR managed the literature study. Author NH, SRR, SS and FM performed the experimental work as well as performed the statistical analysis. Author NH wrote the first draft of the manuscript which was corrected by FA and SS. Authors FA, SR, and MHS finalized the final drafting of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hoque, N., Sohrab, M.H., Afroz, F. et al. Cytotoxic metabolites from Thysanolaena maxima Roxb. available in Bangladesh. Clin Phytosci 6, 89 (2020). https://doi.org/10.1186/s40816-020-00226-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40816-020-00226-4