Abstract
Background
Arterial thrombo-embolic complications (TEC) are still common during and after non-cardiac arterial procedures (NCAP). While unfractionated heparin has been used during NCAP for more than 70 years to prevent TEC, there is no consensus regarding the optimal dosing strategy. The aim of this pilot study was to test the effectiveness and feasibility of an activated clotting time (ACT)-guided heparinization protocol during open abdominal aortic aneurysm (AAA) surgery, in anticipation of a randomized controlled trial (RCT) investigating if ACT-guided heparinization leads to better clinical outcomes compared to a single bolus of 5000 IU of heparin.
Methods
A prospective multicentre pilot study was performed. All patients undergoing elective open repair for an AAA (distal of the superior mesenteric artery) between March 2017 and January 2020 were included. Two heparin dosage protocols were compared: ACT-guided heparinization with an initial dose of 100 IU/kg versus a bolus of 5000 IU. The primary outcome was the effectiveness and feasibility of an ACT-guided heparinization protocol with an initial heparin dose of 100 IU/kg during open AAA surgery. Bleeding complications, TEC, and mortality were investigated for safety purposes.
Results
A total of 50 patients were included in the current study. Eighteen patients received a single dose of 5000 IU of heparin and 32 patients received 100 IU/kg of heparin with additional doses based on the ACT. All patients who received the 100 IU/kg dosing protocol reached the target ACT of > 200 s. In the 5000 IU group, TEC occurred in three patients (17%), versus three patients (9.4%) in the 100 IU/kg group. Bleeding complications were found in six patients (33%) in the 5000 IU group and in 9 patients (28%) in the 100 IU/kg group. No mortality occurred in either group.
Conclusions
This pilot study demonstrated that ACT-guided heparinization with an initial dose of 100 IU/kg appears to be feasible and leads to adequate anticoagulation levels. Further randomized studies seem feasible and warranted to determine whether ACT-guided heparinization results in better outcomes after open AAA repair.
Similar content being viewed by others
Key messages regarding feasibility
-
What uncertainties existed regarding the feasibility?
The aim of this pilot study was to investigate whether an activated clotting time (ACT)-guided heparinization strategy with an initial heparin dose of 100 IU/kg is effective and safe during open AAA surgery. The feasibility of adherence to the heparinization protocol, adherence to the monitoring protocol, and the level of perprocedural anticoagulation using the ACT were tested.
-
What are the key feasibility findings?
Results showed that the ACT-guided heparinization protocol with initial heparin doos of 100 IU/kg seems safe and can be implemented successfully, leading to adequate ACT values.
-
What are the implications of the feasibility findings for the design of the main study?
The heparinization- and monitoring protocol were found to be feasible and effective. Therefore the same protocol is used for the ACTION-1 trial (clinicaltrials.gov NCT04061798), a randomized controlled trial on ACT-guided heparinization during open AAA repair, which started in March 2020.
Background
Throughout the years, outcomes of non-cardiac arterial procedures (NCAP) have improved [1, 2]. Yet, arterial thrombo-embolic complications (TEC) are still common pre- and post-procedural, especially during open abdominal aortic aneurysm (AAA) repair [3,4,5,6,7,8,9,10,11]. Unfractionated heparin has been used during NCAP for more than 70 years trying to prevent TEC [12]. However, there is no consensus regarding the optimal dosing strategy [13]. Heparin is heterogeneous in composition and thereby in pharmacokinetics. It has a non-linear dose–response curve and a non-linear elimination curve [14]. Moreover, there are differences in efficacy between different brands, and even in efficacy between batches of the same brand [15]. Therefore it is hard to predict the effect of heparin in the individual patient. In cardiac interventions, both open and endovascular, heparin is administered based on the weight of the patient, and the dose is adjusted during the procedure, using the activated clotting time (ACT) [16,17,18,19]. This is in contrast to NCAP, where a single bolus of 5000 IU of heparin without monitoring the effect of heparin is most often used [20]. Results from previous cohort studies suggest that ACT-guided heparinization may potentially lead to a lower incidence of TEC [21]. Large comparative studies are lacking, however, and published studies often have heterogeneous procedure types. Open AAA repair is a well-established procedure with a relatively standardized technique with a persistently high incidence of TEC and where anticoagulation and hemostasis are of vital importance [22,23,24,25,26,27,28]. It is therefore important to determine the best heparinization protocol during open AAA repair.
The aim of this study was to investigate whether an ACT-guided heparinization strategy with an initial heparin dose of 100 IU/kg is effective and safe during open AAA surgery, in view of determining if further studies are feasible and warranted to investigate whether ACT-guided heparinization may lead to better clinical outcomes compared to a single bolus of 5000 IU of heparin.
Methods
Study design
The MANCO study (measuring the ACT during non-cardiac arterial procedures, clinicaltrials.gov identifier: NCT03426293) is a prospective, multicentre cohort study for patients undergoing NCAP and receiving intraoperative heparin with ACT monitoring. The protocol was evaluated and approved by the Medical Ethical Committee Noord Holland. Local approval was obtained in both participating centers: Dijklander Hospital in Hoorn and Amsterdam UMC location VUmc, The Netherlands.
Patients were included in the pilot study if they underwent an open repair procedure for an AAA, originating distal of the superior mesenteric artery. Patients were included between March 2017 and January 2020. Exclusion criteria were acute interventions, previous open or endovascular intervention on the abdominal aorta, administration of heparin prior to surgery, allergy to heparin, eGFR < 30 ml/min, and medical history of coagulation disorders or heparin-induced thrombocytopenia.
Heparin protocol and anticoagulation monitoring
Patients included between March 2017 and July 2018 received a single bolus of 5000 IU of heparin. After July 2018, the heparin administration protocol was modified to ACT-guided heparinization with an initial heparin dose of 100 IU/kg in both hospitals. Therefore, between July 2018 and January 2020, patients received an ACT-guided heparinization protocol with an initial heparin dose of 100 IU/kg. The heparin bolus was administered intravenously after anesthetic induction and before arterial cross-clamping.
For the 5000 IU group, the ACT was measured 5 min after heparin administration and every 30 min thereafter until the end of the procedure.
In the 100 IU/kg group, the ACT was measured 5 min after the initial heparin gift. Depending on the ACT, additional heparin doses were administered according to the protocol (Fig. 1).
The ACT 5 min after administration of heparin in the 5000 IU group is expressed as ‘ACThep’. For the 100 IU/kg group ‘ACThep’ is defined as the first ACT > 200 s after the first heparin dosages.
Just before the end of surgery, the final ACT was measured and protamine was administered at the surgeon’s discretion. All ACT measurements were performed using the Haemostasis Management System (HMS) Plus (Medtronic®, Minneapolis, MN, USA). High-range cartridges were used during all procedures, the activator reagent was kaolin. For each measurement, a blood sample of 3 cc was drawn, obtained from an arterial line. Beforehand, 5 cc of blood was drawn and disposed of to prevent contamination of heparin residues.
Data collection
An encrypted database, using the cloud-based Electronic Data Capture platform ‘Castor EDC’, was created [29]. The following patient characteristics were included in the database: age (years), sex, weight (kg), BMI (kg/m2), smoking (current, cessation, never), cardiac disease (ischemic, arrhythmia, heart failure), cardiac intervention, hypertension (systolic > 140 mmHg, diastolic > 90 mmHg), hypercholesterolemia, chronic obstructive pulmonary disease (COPD)/lung fibrosis, transient ischemic attack (TIA)/cardiovascular accident (CVA), malignancy (actual/cured), diabetes mellitus, renal function (estimated glomerular filtration rate, mL/min), peripheral arterial obstructive disease, prior abdominal surgery, prior arterial intervention. Pre-procedural anticoagulation therapy, lab results, ASA (American Society of Anesthesiologists) classification, and largest aneurysm diameter were also registered. Information about the procedure (anatomic location, total blood loss, amount of heparin given), length of stay in hospital, and all complications within the same admission or 30 days postoperative were also collected.
Primary and secondary outcomes
The primary outcome was protocol feasibility, defined by the following outcomes: adherence to the heparinization protocol, adherence to the monitoring protocol, and level of perprocedural anticoagulation using the ACT.
The secondary outcome, for safety purposes, was the incidence of all TEC, bleeding complications, and all-cause mortality within 30 days of surgery or during the same admission in the hospital.
TEC included myocardial infarction, transient ischemic attack, cerebrovascular accident, deep venous thrombosis, pulmonary embolism, bowel ischemia, thrombo-embolic renal insufficiency (defined by RIFLE criteria: rise of serum creatinine > 100% or decrease of eGFR with 50%), athero-embolism, spinal cord ischemia, and graft thrombosis [30].
Bleeding complications were defined as European Multicentre Study on Coronary Artery Bypass Grafting (E-CABG) classification grade one or more (platelets, fresh frozen plasma, or ≥ 2 units of red blood cell transfusions) within 30 days of follow-up or during the same admission [31].
This pilot study was written according to the CONSORT (Consolidated Standards of Reporting Trials) statement, extension to pilot and feasibility trials.
Statistical analysis
Statistical analyses were performed using the SPSS statistical software package, version 28.0 [32]. The normality of continuous variables was tested using Kolmogorov–Smirnov test. The independent t-test was used for continuous variables with respect to two subgroups. In the case of skewed data, the Mann–Whitney test was performed. Outcomes were expressed as mean ± standard deviation. The chi-square test was used in the case of categorical variables. Outcomes were expressed as counts and percentages. A p value < 0.05 was considered significant.
Results
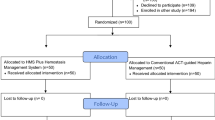
A total of 50 consecutive patients were included. In the same period, 40 other patients were treated but were not included in the study (Fig. 2).
Of the included patients, 18 patients received a single dose of 5000 IU heparin, and 32 patients received ACT-guided heparinization with an initial heparin dose of 100 IU/kg.
The patient demographics are depicted in Table 1. In the 5000 IU group, the mean age in the 5000 IU group was 68, and in the 100 IU/kg group 69. The majority of patients were male (61% in the 5000 IU group and 91% in the 100 IU/kg group, p = 0.012). Chronic obstructive pulmonary disease in medical history was found more often in the 5000 IU group than in the 100 IU/kg group (50% vs 19%, p = 0.021). Furthermore, no significant differences in medical history were found.
The preprocedural antithrombotic therapy is shown in Table 2. No differences between the groups were seen for any of the antithrombotic therapies.
Adherence to protocol
All patients in the intervention group received heparin doses according to the study protocol. ACT measurements were performed at the indicated time points.
ACT
Mean ACT values at ‘ACThep’ are presented in Table 3. Mean ACT value in the 5000 IU group was 181 ± 40 s and in the 100 IU/kg group 230 ± 23 s (p = 0.003). Peak ACTs were 186 ± 37 s in 5000 IU group and 238 ± 29 s in the ACT group (p = < 0.001).
Complications
In the 5000 IU group, TEC occurred in 17% of patients (n = 3). One patient developed sigmoidal ischemia requiring surgical resection. The other two patients had a peripheral thrombo-embolism and underwent thrombo-embolectomy. In the 100 IU/kg group TEC occurred in 9.4% of the patients (n = 3). The first patient had a transient ischemic attack that was treated conservatively. The second patient had graft thrombosis and underwent a surgical revision for the right distal anastomosis. The third patient had a peripheral thrombo-embolism requiring thrombo-embolectomy. Bleeding complications were found in 6 patients (33%) in the 5000 IU group and in 9 patients (28%) in the 100 IU/kg group. No mortality occurred in both groups. Outcomes can be found in Table 4.
Discussion
This pilot study showed that an ACT-guided heparinization protocol with an initial dose of 100 IU/kg is feasible and leads to adequate levels of anticoagulation.
To date, only two studies have been performed on the effect of heparinization during elective open AAA procedures [33, 34]. The results of both studies showed no significant differences in blood loss or thrombosis, but the results of one study demonstrated a significantly higher percentage of patients suffering a fatal perioperative myocardial infarction in the non-heparinized group.
Although no hard scientific evidence is available for the benefit of using heparin during elective AAA surgery, heparin is widely advised and used. In the current clinical practice guidelines on open AAA repair, an initial heparin dose of 50–100 IU/kg is advised [35, 36]. The ESVS guideline mentioned that the ACT can be used to measure the effect of heparin; however, no target ACT was provided or which device to use [36].
Although the optimal target ACT has not been set in guidelines, research suggests an ACT between 200 and 250 s may be preferable during NCAP, to minimize the TEC risk without compromising procedural success or increasing bleeding complications [37, 38]. Previous studies on heparinization during NCAP found a lower incidence of TEC in patients receiving an ACT-guided heparinization protocol (9%) compared to a single heparin bolus of 5 000 IU (4.3%) [4, 21]. In addition, the ACT-guided heparinization protocol, similar to the 100 IU/kg protocol used in the current study, proved to be feasible and safe during NCAP, with more patients reaching an ACT > of 200 s [39]. The results of the current study seem consistent with this literature since a lower mean ACT of 181 s was found after a bolus of 5 000 IU of heparin with TEC in 17% of patients, whereas in the 100 IU/kg a higher mean ACT of 230 s was found with TEC in 9.4% of patients.
In the Dutch Surgical Aneurysm Audit (DSAA), a Dutch registry which is mandatory for all Dutch vascular surgeons who treat patients with an AAA, the rate of serious complications was 29% for all patients who underwent elective open AAA repair from 2014 to 2016 [40]. In addition, according to the Society for Vascular Surgery AAA 2018 guidelines, the incidence of perioperative TEC was between 15 and 36% [35]. The complication rate of the patients who received the standard heparin dose of 5000 IU in this pilot study is comparable to that.
Although ACT-guided heparinization is already being used in several hospitals during NCAP, this pilot study was of importance to explore whether all patients in the intervention group actually reached the target ACT. In addition, the incidence of bleeding complications in the intervention group was investigated to assess the safety of this protocol. Results showed that the ACT-guided heparinization protocol with an initial heparin dose of 100 IU/kg seems safe and can be implemented successfully, leading to adequate ACT values.
This is a pilot study that included 50 patients from two hospitals in the Netherlands, to test for feasibility. Due to the study being underpowered and the low incidence of complications (< 5 complications per group), it was not justified to present the p values. In addition, the groups were not matched for patient characteristics and other risk factors. Furthermore, the present pilot study did not include any blinding strategy. Bias may very well be present.
Therefore a randomized controlled trial should be performed to determine whether ACT-guided heparinization results in safe and optimal anticoagulation during open AAA repair, with a decrease of TEC and without an increase in bleeding complications compared to a standardized bolus of 5000 IU. Derived from the amount of TEC in this pilot study (100 IU/kg:9.4%, 5000 IU: 17%), using a continuity corrected chi-square test with a two-sided alpha of 5%, 310 patients are needed in each group of the trial to achieve a power of 80%. Taking dropout into account, a total of 750 patients is needed.
The decrease in TEC might lead to less mortality and morbidity, a lower number of re-operations, or better patency, all substantially improving the patient’s quality of health, the efficiency of medical care, and the quality of vascular medical care. Based on the feasibility of this pilot study, the ACTION-1 trial (clinicaltrials.gov NCT04061798), a randomized controlled trial on ACT-guided heparinization during open AAA repair, was started in March 2020 [41].
Conclusion
This pilot study demonstrates that ACT-guided heparinization with an initial heparin dose of 100 IU/kg appears to be feasible and leads to adequate anticoagulation levels. However, the potential beneficial effects of perioperative ACT-guided heparinization over a standardized bolus of 5000 IU on clinical outcomes (TEC and mortality) and adverse events during and after open AAA repair should be demonstrated in future large randomized studies. The ACTION-1 trial will address this [41].
Availability of data and materials
The data supporting the findings of this study are available from the corresponding author on request.
References
Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol. 2017;2(2):181–7. https://doi.org/10.1001/JAMACARDIO.2016.4792.
Egorova NN, Guillerme S, Gelijns A, Morrissey N, Dayal R, McKinsey JF, et al. An analysis of the outcomes of a decade of experience with lower extremity revascularization including limb salvage, lengths of stay, and safety J Vasc Surg 2010;51(4). https://doi.org/10.1016/J.JVS.2009.10.102.
AlOthman O, Bobat S. Comparison of the short and long-term outcomes of endovascular repair and open surgical repair in the treatment of unruptured abdominal aortic aneurysms: meta-analysis and systematic review. Cureus 2020;12(8). https://doi.org/10.7759/CUREUS.9683.
Doganer O, Jongkind V, Blankensteijn JD, Yeung KK, Wiersema AM. A standardized bolus of 5 000 IU of heparin does not lead to adequate heparinization during non-cardiac arterial procedures. Ann Vasc Surg. 2020;0(0):1–7. https://doi.org/10.1016/j.avsg.2020.07.035.
Kontopodis N, Antoniou SA, Georgakarakos E, Ioannou CV. Endovascular vs open aneurysm repair in the young: systematic review and meta-analysis. J Endovasc Ther. 2015;22(6):897–904. https://doi.org/10.1177/1526602815606937.
Saedon M, Mt-Isa S, Saratzis A, Leung E, Mahmood A. Outcome of open versus endovascular abdominal aortic aneurysm repair in obese patients: a systemic review and meta-analysis. Int Angiol. 2014;34(1):9–15.
Rayt HS, Sutton AJ, London NJM, Sayers RD, Bown MJ. A systematic review and meta-analysis of endovascular repair (EVAR) for ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2008;36(5):536–44. https://doi.org/10.1016/J.EJVS.2008.08.008.
Sweeting MJ, Balm R, Desgranges P, Ulug P, Powell JT. Individual-patient meta-analysis of three randomized trials comparing endovascular versus open repair for ruptured abdominal aortic aneurysm. Br J Surg. 2015;102(10):1229–39. https://doi.org/10.1002/BJS.9852.
Antoniou GA, Georgiadis GS, Antoniou SA, Pavlidis P, Maras D, Sfyroeras GS, et al. Endovascular repair for ruptured abdominal aortic aneurysm confers an early survival benefit over open repair. J Vasc Surg. 2013;58(4):1091–105. https://doi.org/10.1016/J.JVS.2013.07.109.
Antoniou GA, Ahmed N, Georgiadis GS, Torella F. Is endovascular repair of ruptured abdominal aortic aneurysms associated with improved in-hospital mortality compared with surgical repair? Interact Cardiovasc Thorac Surg. 2015;20(1):135–9. https://doi.org/10.1093/ICVTS/IVU329.
Badger SA, Harkin DW, Blair PH, Ellis PK, Kee F, Forster R. Endovascular repair or open repair for ruptured abdominal aortic aneurysm: a Cochrane systematic review. BMJ Open. 2016;6(2):e008391. https://doi.org/10.1136/bmjopen-2015-008391.
Murray G. Heparin in surgical treatment of blood vessels. Arch Surg. 1940;40(2):307. https://doi.org/10.1001/archsurg.1940.04240010147010.
Doganer O, Wiersema AM, Scholtes V, Blankensteijn JD, Yeung KK, Jongkind V. No concluding evidence on optimal activated clotting time for non-cardiac arterial procedures. Eur J Vasc Endovasc Surg 2020:137–47. https://doi.org/10.1016/j.ejvs.2019.08.007.
Oates JA, Wood AJJ, Hirsh J. Heparin. N Engl J Med. 1991:1565–74. https://doi.org/10.1056/NEJM199105303242206.
Arsenault KA, Paikin JS, Hirsh J, Dale B, Whitlock RP, Teoh K, et al. Subtle differences in commercial heparins can have serious consequences for cardiopulmonary bypass patients: a randomized controlled trial. J Thorac Cardiovasc Surg. 2012;144(4):944-950.e3. https://doi.org/10.1016/j.jtcvs.2012.05.065.
Hattersley PG. Activated coagulation time of whole blood. JAMA J Am Med Assoc. 1966;196(5):436–40. https://doi.org/10.1001/jama.1966.03100180108036.
Despotis GJ, Gravlee G, Filos K, Levy J. Anticoagulation monitoring during cardiac surgery: a review of current and emerging techniques. Anesthesiology 1999:1122–51. https://doi.org/10.1097/00000542-199910000-00031.
Shore-Lesserson L. Evidence-based coagulation monitors: heparin monitoring, thromboelastography, and platelet function. Semin Cardiothorac Vasc Anesth 2005:41–52. https://doi.org/10.1177/108925320500900105.
Goldhammer JE, Zimmerman D. Pro: activated clotting time should be monitored during heparinization for vascular surgery. J Cardiothorac Vasc Anesth. 2018:1494–6. https://doi.org/10.1053/j.jvca.2017.04.047.
Wiersema A, Bruijninckx C, Reijnen M, Vos J, Van Delden O, Vahl A, et al. Perioperative prophylactic antithrombotic strategies in vascular surgery: current practice in the Netherlands. J Cardiovasc Surg (Torino). 2015;56(1):119–25.
Doganer O, Roosendaal LC, Wiersema AM, Blankensteijn JD, Yeung KK, Jongkind V. Weight based heparin dosage with activated clotting time monitoring leads to adequate and safe anticoagulation in non-cardiac arterial procedures. Ann Vasc Surg. 2022;84:327–35. https://doi.org/10.1016/J.AVSG.2022.01.029.
Burgers LT, Vahl AC, Severens JL, Wiersema AM, Cuypers PWM, Verhagen HJM, et al. Cost-effectiveness of elective endovascular aneurysm repair versus open surgical repair of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2016;52(1):29–40. https://doi.org/10.1016/j.ejvs.2016.03.001.
Behrendt CA, Sedrakyan A, Rieß HC, Heidemann F, Kölbel T, Petersen J, et al. Short-term and long-term results of endovascular and open repair of abdominal aortic aneurysms in Germany. J Vasc Surg. 2017;66(6):1704-1711.e3. https://doi.org/10.1016/j.jvs.2017.04.040.
Behrendt CA, Rieß HC, Schwaneberg T, Larena-Avellaneda A, Kölbel T, Tsilimparis N, et al. Incidence, predictors, and outcomes of colonic ischaemia in abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2018;56(4):507–13. https://doi.org/10.1016/j.ejvs.2018.06.010.
Deery SE, O’Donnell TFX, Bodewes TCF, Dalebout BA, Pothof AB, Shean KE, et al. Early reintervention after open and endovascular abdominal aortic aneurysm repair is associated with high mortality. J Vasc Surg. 2018;67(2):433-440.e1. https://doi.org/10.1016/j.jvs.2017.06.104.
Trenner M, Haller B, Storck M, Reutersberg B, Kallmayer MA, Eckstein HH. Trends in patient safety of intact abdominal aortic aneurysm repair: German Registry Data on 36,594 Procedures. Eur J Vasc Endovasc Surg. 2017;53(5):641–7. https://doi.org/10.1016/j.ejvs.2016.12.024.
Hynes CF, Endicott KM, Iranmanesh S, Amdur RL, Macsata R. Reoperation rates after open and endovascular abdominal aortic aneurysm repairs. J Vasc Surg. 2017;65(5):1323–8. https://doi.org/10.1016/j.jvs.2016.09.053.
Prinssen M, Verhoeven ELG, Buth J, Cuypers PWM, Van Sambeek MRHM, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351(16):1607–18. https://doi.org/10.1056/NEJMoa042002.
Castor Electronic Data Capture 2019. Available from: https://www.castoredc.com/.
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, workgroup the A. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204. https://doi.org/10.1186/CC2872.
Biancari F, Ruggieri VG, Perrotti A, Svenarud P, Dalén M, Onorati F, et al. European Multicenter Study on Coronary Artery Bypass Grafting (E-CABG registry): study protocol for a prospective clinical registry and proposal of classification of postoperative complications. J Cardiothorac Surg. 2015;10(1). https://doi.org/10.1186/s13019-015-0292-z.
IBM Corp. IBM SPSS Statistics for Windows 2020.
Thompson JF, Mullee MA, Bell PRF, Campbell WB, Chant ADB, Darke SG, et al. Intraoperative heparinisation, blood loss and myocardial infarction during aortic aneurysm surgery: a Joint Vascular Research Group study. Eur J Vasc Endovasc Surg. 1996;12(1):86–90. https://doi.org/10.1016/S1078-5884(96)80281-4.
Johnston KW. Multicenter prospective study of nonruptured abdominal aortic aneurysm. Part II. Variables predicting morbidity and mortality. J Vasc Surg. 1989;9(3):437–47. https://doi.org/10.1016/S0741-5214(89)70007-0.
Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(1):2-77.e2. https://doi.org/10.1016/j.jvs.2017.10.044.
Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, et al. European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur J Vasc Endovasc Surg. 2018;(2018). https://doi.org/10.1016/j.ejvs.2018.09.020.
Nissborg E, Wahlgren C-M. Anticoagulant effect of standard dose heparin during peripheral endovascular intervention. Ann Vasc Surg. 2019;60:286–92. https://doi.org/10.1016/j.avsg.2019.02.033.
Roosendaal LC, Wiersema AM, Smit JW, Doganer O, Blankensteijn JD, Jongkind V. Sex differences in response to administration of heparin during non-cardiac arterial procedures. Eur J Vasc Endovasc Surg. 2022. https://doi.org/10.1016/J.EJVS.2022.08.005.
Doganer O, Wiersema AM, Pierie M, Blankensteijn JD, Yeung KK, Jongkind V. More effective anticoagulation during non-cardiac arterial procedures using activated clotting time guided heparin administration. Ann Vasc Surg. 2021;76:378–88. https://doi.org/10.1016/J.AVSG.2021.04.023.
Dutch Insitute for Clinical Auditing. DICA Jaarrapportage. https://dica.nl/jaarrapportage-2016/home/dsaa.
Wiersema AM, Roosendaal LC, Koelemaij MJW, Tijssen JGP, van Dieren S, Blankensteijn JD, et al. ACTION-1: study protocol for a randomised controlled trial on ACT-guided heparinization during open abdominal aortic aneurysm repair. Trials. 2021;22(1):1–16. https://doi.org/10.1186/S13063-021-05552-7/FIGURES/3.
Funding
This research was supported by a grant from Medtronic (reference number: A1395092/SH-3950). Medtronic had no involvement in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
LR drafted the manuscript, analysis, and interpretation of data. MH substantially revised the manuscript and interpretation of data. AW contributed to the conception, supervision, and interpretation of data, and substantially revised the manuscript. JB contributed to the supervision and interpretation of data and substantially revised the manuscript. VJ contributed to the conception, design, and interpretation of data and substantially revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol was evaluated and approved by the Medical Ethical Committee Noord Holland. Local approval was obtained in both participating centers.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Roosendaal, L.C., Hoebink, M., Wiersema, A.M. et al. Activated clotting time-guided heparinization during open AAA surgery: a pilot study. Pilot Feasibility Stud 10, 73 (2024). https://doi.org/10.1186/s40814-024-01500-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40814-024-01500-9