Abstract
Background
Despite family carepartners of individuals post-stroke experiencing high levels of strain and reduced quality of life, stroke rehabilitation interventions rarely address carepartner well-being or offer training to support their engagement in therapeutic activities. Our group has developed creative intervention approaches to support families during stroke recovery, thereby improving physical and psychosocial outcomes for both carepartners and stroke survivors. The purpose of this study is to test the feasibility of an adapted, home-based intervention (Carepartner Collaborative Integrative Therapy for Gait-CARE-CITE-Gait) designed to facilitate positive carepartner involvement during home-based training targeting gait and mobility.
Methods
This two-phased design will determine the feasibility of CARE-CITE-Gait, a novel intervention that leverages principles from our previous carepartner-focused upper extremity intervention. During the 4-week CARE-CITE-Gait intervention, carepartners review online video-based modules designed to illustrate strategies for an autonomy-supportive environment during functional mobility task practice, and the study team completes two 2-h home visits for dyad collaborative goal setting. In phase I, content validity, usability, and acceptability of the CARE-CITE-Gait modules will be evaluated by stroke rehabilitation content experts and carepartners. In phase II, feasibility (based on measures of recruitment, retention, intervention adherence, and safety) will be measured. Preliminary effects of the CARE-CITE-Gait will be gathered using a single-group, quasi-experimental design with repeated measures (two baseline visits 1 week apart, posttest, and 1-month follow-up) with 15 carepartner and stroke survivor dyads. Outcome data collectors will be blinded. Outcomes include psychosocial variables (family conflict surrounding stroke recovery, strain, autonomy support, and quality of life) collected from carepartners and measures of functional mobility, gait speed, stepping activity, and health-related quality of life collected from stroke survivors.
Discussion
The findings of the feasibility testing and preliminary data on the effects of CARE-CITE-Gait will provide justification and information to guide a future definitive randomized clinical trial. The knowledge gained from this study will enhance our understanding of and aid the development of rehabilitation approaches that address both carepartner and stroke survivor needs during the stroke recovery process.
Trial registration
ClinicalTrials.gov, NCT 05257928. Registered 25 February 2022.
Trial status
This trial was registered on ClinicalTrials.gov (NCT 05257928) on March 25, 2022. Recruitment of participants was initiated on May 18, 2022.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Recognized as an independent risk factor for other comorbidities and a recurrent stroke [1], reduced physical activity in stroke survivors is a critical area of rehabilitation focus [2]. Few stroke survivors meet the current American Stroke Association guidelines [3] of 20–60 min of aerobic exercise 3 to 5 days per week. Typically, stroke survivors achieve only 63% of the recommended steps per day for people with disability (4078 versus 6500–8500) [4]. According to Fini et al. [2], motivation and carepartner support are potential factors to consider, underscoring self-management and behavior change to enhance engagement and sustainability of poststroke physical activity programs. However, lacking training and preparation to support the complexities of stroke rehabilitation [5,6,7], carepartners can experience high levels of care burden, reduced quality of life, and family conflict surrounding the poststroke recovery process [8, 9]. Designing interventions that support physical activity by engaging family carepartners may be a key factor for the sustainability of health-related behaviors.
Carepartner’s well-being can impact the health outcomes of both the carepartner and stroke survivor, as the physical, emotional, and psychological aspects of their daily living are intricately interconnected. With earlier discharge and a greater proportion of rehabilitation occurring at home, it is critical to find strategies to enable carepartners to support rehabilitation at home and in the community without adding to their own caregiving burden. Previous research studies incorporating carepartner training during stroke survivor rehabilitation have had promising results [10, 11]. The London Stroke Carers Training Course, a comprehensive competency training for carepartners, reduced health and social care costs, improved QOL, and reduced carepartner burden [11]. However, these findings were not replicated in a multi-site study [12], which may reflect the limitations of approaches that prescribe activities for carepartners instead of actively involving them as collaborators in the rehabilitation process. Work by Creasy et al. [13] suggests that carepartners are keenly aware of the critical nature of their role in the care of their loved one, and that they have expectations of being included in poststroke treatment planning. Carepartners underscored their need to have greater information about stroke and customization of rehabilitation training to more accurately address specific family needs [14]. Several studies have shown that specifically improving carepartner coping and life skills to care for a chronically ill family member leads to decreased caregiver burden and improved QoL [15,16,17]. Supporting previous recommendations [7, 17], a recent systematic review of stroke family carepartner and dyad interventions [18] emphasized that these interventions should combine skill building (e.g., problem-solving, goal setting, and stress management) with psychoeducational strategies and should be tailored to individualized needs of the carepartner. Collectively, these studies suggest that the engagement of carepartners offers valuable opportunities to enhance rehabilitation therapies.
Indeed, the delivery of interventions without consideration of family context may limit the success of stroke recovery. To address this critical gap, we developed a theory-based, task-specific, and carepartner-focused intervention — Carepartner and Collaborative Integrated Therapy (CARE-CITE) [19]. Initially CARE-CITE was designed to enhance upper extremity functional task practice (repetitive performance of daily activities in the home setting to improve function) by instructing carepartners in methods for collaborative goal setting, problem-solving, and creating an autonomy-supportive environment. Arising from self-determination theory [20], CARE-CITE uses web-based interactive modules with exemplary videos created in collaboration with actual stroke dyads (carepartners and stroke survivors), which model autonomy supportive communication by offering choice, providing rationale, demonstrating empathy, and avoiding controlling language. Preliminary studies show the feasibility [21] and promising therapeutic benefits of CARE-CITE coupled with upper extremity constraint-induced movement therapy (CIMT). In our baseline data with chronic stroke survivors [22], we found that the majority of carepartners continue to experience family conflict surrounding stroke recovery which was related to higher levels of carepartner strain and less autonomy support provided to the stroke survivor during rehabilitation activities. These findings provide insights into the potential influence of family context on stroke survivor motivation and adherence. Outcomes from our study showed promising trends suggesting that carepartners receiving CARE-CITE had improved psychosocial outcomes, including improved quality of life, coupled with less strain, fatigue, and family conflict around stroke recovery [23, 24]. Although both stroke survivor control (CIMT-only) and intervention (CARE-CITE + CIMT) groups demonstrated improvements in upper extremity function and health-related quality of life [24], only the CARE-CITE + CIMT group maintained or continued to improve upper extremity function at 1-month follow-up testing. These results suggest that carepartner engagement may be instrumental for continued progress during stroke recovery.
Responding to the insights gained from the engagement of the carepartner in upper extremity rehabilitation, we now seek to broaden the scope of our intervention and further optimize poststroke recovery by pairing CARE-CITE with home-based gait and functional mobility training (CARE-CITE-Gait). The aim of this paper is to describe the CARE-CITE-Gait, two-phased, feasibility study design. The objective of Phase I of this trial is to evaluate the content validity and user satisfaction (usability and acceptability) of the CARE-CITE-Gait intervention. The primary objective of phase II is to determine the feasibility of CARE-CITE-Gait for stroke survivor and carepartner dyads, as indicated by participant recruitment and retention, adherence to the intervention, and safety (occurrence of stroke survivor or carepartner adverse events). The secondary objective of Phase II is to determine the preliminary effects of the CARE-CITE-Gait intervention on stroke survivors and carepartners. Carepartner psychosocial outcomes will include family conflict about stroke recovery, strain, autonomy support, and quality of life. Stroke survivor outcome measures include functional mobility, gait speed, stepping activity, and health-related quality of life. Insights from this study will guide the development of a randomized clinical trial to evaluate the efficacy of the CARE-CITE-Gait intervention.
Methods
Identification and reporting of relevant elements of this protocol are based on the Standard Protocol Items: Recommendations for Intervention Trials (SPIRIT) checklist [25] and Template for Intervention Description and Replication (TIDieR) guidelines for intervention descriptions [26]. This is the first published version of this clinical trial study protocol. Ethical approval was obtained by Emory University Institutional Review Board, and this protocol is registered on ClinicalTrials.gov (NCT05257928). Any protocol amendments will be immediately reported to the University Institutional Review Board for approval and to the funding agency as appropriate.
Study design and setting
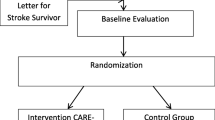
In Phase I, stroke rehabilitation content experts (physical and occupational therapists) and carepartners will evaluate the content validity and user satisfaction (usability and acceptability) of the CARE-CITE-Gait modules. In Phase II, feasibility (based on measures of recruitment, retention, intervention adherence, and safety) and preliminary effects of CARE-CITE-Gait will be measured using a one-group, quasi-experimental design with repeated measures (two baseline visits 1 week apart, posttest, and 1-month follow-up) with 15 stroke survivor and carepartner dyads. While the in-person portion of the intervention will take place in participant homes, the study recruitment, screening, and evaluations (evaluator blinded) for dyads will occur in a stroke research laboratory within an urban rehabilitation hospital located in Atlanta, GA, USA (Fig. 1 Study flow chart: evaluation of Carepartner-Integrated Telehealth Gait Rehabilitation Program for Persons with Stroke (CARE-CITE-Gait) N = 15)). Licensed physical therapists will conduct the evaluations and administer the 4-week home-based CARE-CITE-Gait intervention. During the intervention period, carepartners will access the web-based CARE-CITE-Gait modules independent of study staff involvement. The study schedule schematic design is presented in Table 1.
Phase I: Content validity and user satisfaction of CARE-CITE-Gait
The original CARE-CITE modules were designed to help the carepartner create a therapeutic home environment while encouraging the stroke survivor to use the weaker upper extremity during daily activities. We modified the existing upper extremity CARE-CITE intervention to address gait rehabilitation. Collaborating with Emory University’s Center for Digital Scholarship, the video modules were redesigned with a progressive therapeutic exercise plan to improve overall mobility and stepping-related physical activity. Additional content was added to address safety risks and falls (appropriate use of gait belts, supervision, etc.) associated with gait and balance exercises. The gait revisions maintained the core CARE-CITE theoretical framework (the concept of autonomy support), with text and video that demonstrate ways to encourage empathy (video examples of discussions of carepartners with stroke survivors acknowledging the difficulty of the exercise), collaborating on problem-solving (examples of methods to increase or decrease the difficulty of activities together), emphasizing the importance of offering the stroke survivor choice in activities to practice (examples of joint goal setting), and ways to provide noncontrolling language (scenarios showing controlling vs. noncontrolling language).
In Phase I, we will expand upon the established validity and satisfaction demonstrated for the original upper extremity CARE-CITE intervention [21] using similar procedures to evaluate the adaptation of the modules to gait and mobility examples. To determine content validity, six to seven stroke rehabilitation content experts will review modules for content accuracy, problem relevance, and ease of use with forms adapted based on work by Bakas and colleagues [27]. Qualitative feedback with general comments and suggestions for improvement will be gathered from open-ended questions.
To assess user satisfaction (usability, acceptability, and overall satisfaction), three carepartners will complete questions at the end of each module rating the following areas: (1) usefulness of overall content, (2) usefulness of written text, (3) usefulness of videos, (4) ease of use, and (5) acceptability using a 5-point Likert-type response scale. Time required for module review and general comments and improvement suggestions will be collected. In our previous work [21], carepartners required approximately 5–20 min to complete each of the six modules. Data gathered about content validity and user satisfaction will guide any additional refinements of intervention before the initiation of Phase II study recruitment.
Phase II: Feasibility and preliminary effects of CARE-CITE-Gait
For Phase II, the study coordinator will make a clinic appointment at Emory Rehabilitation Hospital for interested participants. If screening criteria are met, written informed consent will be obtained from the stroke survivor and carepartner by the study PI or study coordinator. Medical clearance from the stroke survivor physician will be obtained before study participation.
Recruitment
Participants
Inclusion criteria
All participants must be greater than 21 years of age, able to read and write English, and able to provide informed consent. Stroke survivors will be > 3-month postischemic or hemorrhagic event and discharged home, able to walk 10 m with or without an assistive device, have no severe cognitive deficits (mini-mental test > 24 [28]) or physician-determined major medical problems that would limit participation, and have the presence of a carepartner. Carepartners will be self-identified as a spouse/partner or family member dwelling in the same household who has the role as the primary caregiver of the stroke survivor. The primary carepartner inclusion criteria include interest and willingness to support the stroke survivor during the study activities, ability to provide any necessary supervision with safety-related mobility activities, and no significant cognitive deficits (as demonstrated by their ability to explain the study purpose to the PI during the informed consent discussion). Participants will be requested not to participate in other research studies during the study intervention and evaluation period.
Recruitment and retention strategies
Through weekly monitoring of the inpatient and outpatient rehabilitation stroke census, potential participants will be identified by study staff, and eligibility criteria will be confirmed based on physician and physical therapy electronic medical records. To broaden recruitment outreach, stroke information in services will be provided to regional hospitals and community support groups as well as partnering with other regional stroke research collaborators to facilitate shared recruitment efforts. Targeted enrollment rate is two to three dyads per month. To support participant adherence and retention, the study coordinator and PI will be available to respond promptly to any participant concern or issue (via email or phone) and will provide regular check-in telephone calls or texts for appointment confirmation reminders. Each participant receives US $50 (US $100/dyad) for study participation.
Sample size estimation
For Phase II, given the time required to adapt the intervention, the proposal timeline, and preliminary data from our upper extremity intervention, a sample size of 15 dyads is proposed to evaluate feasibility. An attrition rate of ~ 8% is projected based on the literature and our previous work [29]; thus, we will enroll 16 dyads to achieve a final sample of 15. Assuming a two-tailed alpha = 0.05, we will have 80% power to detect an effect size = 0.78 (Cohen, large) and can calculate 95% confidence intervals within 0.64 standard deviations. These data will provide precision to the estimates of mean changes, variability, and effect sizes for key outcomes. Importantly, these data will inform sample size estimation and identify feasibility lessons to guide our planned next step—a randomized clinical trial evaluating the efficacy of CARE-CITE-Gait.
Intervention
CARE-CITE-Gait intervention
The CARE-CITE-Gait intervention will occur over 4 weeks in the dyad’s home. In-person visits by the interventionist will occur at weeks 1 and 4, consisting of facilitation of collaborative goal setting, development of a personalized home exercise program based on daily activities related to gait and mobility, and assessment of gait speed and balance. Telephone check-ins occurring in weeks 2 and 3 will consist of a 10-min phone call with the carepartner to address any questions and identify challenges and opportunities with the implementation of autonomy-supportive strategies and problem-solving with the stroke survivor. Carepartners will complete 6 online CARE-CITE Gait modules throughout the course of the intervention using a web platform. Modules will consist of demonstration videos and instructive content covering the following topics: collaborative goal setting that addresses both stroke survivor and carepartner goals, principles of functional task practice (practice of daily activities), and strategies for task adaptation and progression to drive neuroplasticity (see Table 2). A central theme of the modules is helping carepartners to provide autonomy support for the stroke survivor by fostering empathy, incorporating choice in activities, and providing instruction in the use of noncontrolling language. An additional module encourages carepartners to create their own self-care goals.
Standardization
To increase rigor and minimize bias, study staff collecting data will be blinded to study intervention and trained in outcome measure administration with regular assessments of competence every 1.5 months by the PI. Variability and risk for interrater reliability concerns will be minimized by using the same study staff for all assessments of a dyad when possible. The PI will provide supervision of in-home visits and phone calls for the first five dyads and will communicate through email or phone with the interventionist after each visit for each subsequent dyad to ensure consistency of intervention delivery. Evaluation and intervention data collection forms will be standardized to facilitate protocol adherence. In addition to adherence with good laboratory practice [30] principles for clinical research, all study personnel will receive ongoing university diversity, equity, and inclusion training to foster education, self-awareness, communication skills, and community engagement.
Outcomes
Feasibility
Feasibility of the study protocol will be assessed by participant recruitment, retention, intervention adherence, and safety as defined in Table 3. Justification of ineligibility, declining to participate, or study withdrawal following enrollment and obtaining signed consent will be recorded in the study flowchart.
In addition to the above feasibility measures, at the end of the 1-month follow-up evaluation, carepartners will complete the standardized Post-Study System Usability Questionnaire [31] and a study exit interview questionnaire. This exit interview questionnaire was developed by the PI based on post-study participation interviews in similar stroke research [32] and was reviewed by stroke caregiving experts for content validity. The interview evaluates the carepartner’s perceptions of their confidence in providing care, value of participation in the study, and helpfulness of the intervention with a mix of rating scales and open-ended questions.
Outcomes for carepartner and stroke survivor
Carepartner psychosocial outcome measures and stroke survivor physical function and health-related quality of life outcome measures will be administered in separate rooms at the stroke research laboratory before and immediately after the 4-week intervention and at 1-month follow-up. After each assessment visit, stroke survivors will wear ankle accelerometers to measure home and community stepping activity over 7 days. Each outcome measure has been tested in the stroke population previously (Table 4 lists outcome measure descriptions with established reliability and validity).
Additional assessments
Stroke survivor medical records and dyad information questionnaires will be used to document data about participant demographics (age, gender), marital status, education level, income, work status, self-identified race and ethnicity, comorbidities, COVID (past history of testing positive and vaccinations), and current medications. Using the dyad zip code, we will determine the Area Deprivation Index (ADI) [33], which characterizes the relative disadvantage of an individual or social network using several US Ccensus indicators of employment, housing, poverty, and education [34]. Taken together, this collection of data will guide recruitment to ensure a diverse and inclusive participant sample.
Step activity data
To gain information about real-world stepping activity at home and in the community, the stroke survivor will be provided with a step activity monitor (Actigraph GT3X + (Pensacola, FL, USA)), to be worn on the paretic and non-paretic ankles for 7 days in order to obtain at least 3 days of step activity data. Participants will be provided with verbal and written instructions to don the device upon waking and doff prior to sleeping, removing the device during the day only for bathing, or taking part in water-based activities. Daily stepping activity for the paretic and non-paretic leg before and after the intervention period will be calculated.
Data management and analysis
REDCap (Research Electronic Data Capture) [35] electronic database will be used to store the quantitative data. REDCAP is a secure (compliant with United States healthcare confidentiality legislation requirements), web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for importing data from external sources. The study coordinator will be trained in REDCap processes and will complete all data entry. Data will be double-checked for verification by a second REDCap-trained study staff. REDCap creates a comma-separated value file and SAS program to create an analytic dataset. These files and all resulting datasets, programs, and results are stored on a HIPAA-compliant Emory University Rollins School of Public Health server.
Standard data cleaning, identification of missing data, and internal consistency reliability for standardized scales will be completed. We will investigate whether missing data is related to treatment and baseline factors but do not anticipate missing data being a limitation if lost-to-follow-up rates in this new clinical trial are similar to other stroke studies performed in the PI (SB) and Co-I (TK) labs. Primary quantitative statistical analyses will be performed by the study statistician (GC) using SAS statistics for Windows (Version 9.4), and accelerometry data analysis (stroke survivor stepping activity) will be performed using ActiLife software (Version 6.13.4). Descriptive statistics (e.g., frequencies, means, ranges, standard deviations) will be calculated for all relevant variables, as well as to identify unusual or suspect values requiring review and confirmation. Additional descriptive statistics will be calculated for feasibility measures of recruitment rate, retention, and intervention adherence (described under the “Feasibility” section). To determine estimates of variability (for postulating effect sizes to design next phase clinical trials), the mean and median changes from baseline to 1 month and standard errors of the means will be calculated. To provide preliminary information about possible feasibility study intervention effects, confidence intervals for the difference in mean changes for major study variables will be reported as descriptive statistics. Graphical methods will be used to investigate outliers and investigate the consistency of the changes over time. Estimates of intercorrelations among the study variables will be calculated to gain insight into potential variables that could influence response to the intervention and guide the design of future studies. To understand potential relationships and possible confounding factors, we will examine stroke survivor comorbidities and the biological variables of carepartner age, sex, gender, and relationship of carepartner to stroke survivor. The second phase of the analysis will include one-way repeated measures analysis of variance (ANOVA) to evaluate differences between baseline, post, and follow-up timepoints. Tukey’s pairwise comparisons will be used to determine which time points are different if necessary. Level of significance of 0.05 will be used. No adjustments will be used to control for multiple study variables.
Safety
While no significant risks have been associated with home-based gait and mobility training, inherent risks associated with the intervention involve fatigue, muscle soreness, and falls. To minimize these risks, additional content related to mobility safety has been incorporated into the CARE-CITE-Gait modules. Safety issues will be discussed during home visits with support for collaborative problem-solving around maximizing safety during mobility training, and gait belts will be used to reduce fall risks during evaluations and as appropriate during home visits. Additionally, stroke survivor vital signs will be monitored during all study assessments and visits. The interventionist will monitor adverse events throughout the intervention period with frequent assessments of pain, fatigue, and falls as well as any medical appointments that may reveal changes in medications or medical conditions. Adverse events will be documented and categorized based on the Emory University Internal Review Board Protocol. The PI will be notified to evaluate and determine if an event is serious or related to the intervention and, in collaboration with the study team, will determine whether safety concerns warrant termination of participation in the study. Safety considerations arising throughout the study will be discussed regularly during study meetings. Though risks associated with carepartner participation in the review of the CARE-CITE-Gait modules are minimal, psychosocial assessments evaluating quality of life and mood will be monitored. In the case of any areas of concern (e.g., potential depressive symptoms), the PI will recommend a referral to their primary healthcare provider for further assessment. Due to the minimal study participation risks, a Data Safety and Monitoring Board was not established.
Auditing
All study records will be available for review by authorized representatives of the Foundation for Physical Therapy Research, regulatory agencies, and the Emory University Institutional Review Board to monitor study safety, progress, and procedures for quality assurance.
Dissemination
Dissemination of study results to academic communities will occur through peer-reviewed manuscripts and regional and national conference presentations. The study team will work collaboratively with the funding agency to share results based on agency requirements. The PI will meet regularly with the study team to revise the proposed dissemination plan and discuss authorship guidelines. For nonacademic communities, the PI will schedule presentations with community stroke groups and therapy clinics involved in study recruitment. A lay summary of study findings will be created for distribution based on participant requests.
Discussion
This protocol describes the methodology to evaluate the feasibility of a Carepartner-Integrated Telehealth Gait Rehabilitation Program during stroke recovery. The study findings will provide valuable insights regarding innovative home-based rehabilitation that engages carepartners and support a future study testing the efficacy of CARE-CITE-Gait intervention. Web-based approaches like CARE-CITE-Gait increase accessibility to content outside of traditional clinic hours and reduce transportation-related barriers. While many carepartners may still lack resources and skills to use web-based approaches, the pandemic has rapidly increased the adoption of these technologies, and a recent study [36] found that 86.1% of stroke survivors and carepartners had Internet access. Implementing telerehabilitation delivery of carepartner interventions offers promising and scalable alternatives to improve access to care. Results will provide important preliminary estimates of efficacy and components of variability as well as inform future randomized clinical trial sample size power calculations. Data gathered regarding recruitment, retention, adherence rates, adverse events, outcome measure appropriateness, and participant exit interviews will inform the feasibility and justification of a definitive trial of CARE-CITE-Gait. Our proposal will lay the foundations for several research trajectories with the long-term goal of developing more personalized, precise, and efficacious family-focused rehabilitation interventions.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to some data containing information that could compromise research participant privacy/consent but are available from the corresponding author [sb] on reasonable request.
Abbreviations
- CARE-CITE-Gait:
-
Carepartner and Collaborative Integrated Therapy-Gait
- FCCS:
-
Family Caregiver Conflict Scale about Stroke Recovery
- FCCQ:
-
Family Care Climate Questionnaire
- CSI:
-
Caregiver Strain Index
- BCOS:
-
Bakas Caregiving Outcomes Scale
- SIS:
-
Stroke Impact Scale
- 5STS:
-
5-Times sit to stand
- TUG:
-
Timed up and go test
- SIS:
-
Stroke Impact Scale
- ABC:
-
Activities-specific Balance Confidence Scale
- PI:
-
Principal investigator
- Co-I:
-
Co-investigator
- CP:
-
Carepartner
- SS:
-
Stroke survivor
References
Turan TN, Nizam A, Lynn MJ, Egan BM, Le NA, Lopes-Virella MF, Hermayer KL, Harrell J, Derdeyn CP, Fiorella D, et al. Relationship between risk factor control and vascular events in the SAMMPRIS trial. Neurology. 2017;88(4):379–85.
Fini NA, Bernhardt J, Said CM, Billinger SA. How to address physical activity participation after stroke in research and clinical practice. Stroke. 2021;52(6):e274–7.
Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, MacKay-Lyons M, Macko RF, Mead GE, Roth EJ, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(8):2532–53.
Fini NA, Holland AE, Keating J, Simek J, Bernhardt J. How physically active are people following stroke? Systematic review and quantitative synthesis. Phys Ther. 2017;97(7):707–17.
Grant JS, Hunt CW, Steadman L. Common caregiver issues and nursing interventions after a stroke. Stroke. 2014;45(8):e151-153.
Miller EL, Murray L, Richards L, Zorowitz RD, Bakas T, Clark P, Billinger SA. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: a scientific statement from the American Heart Association. Stroke. 2010;41(10):2402–48.
Bakas T, McCarthy M, Miller ET. Update on the state of the evidence for stroke family caregiver and dyad interventions. Stroke. 2017;48(5):e122–5.
Clark PC, Dunbar SB, Shields CG, Viswanathan B, Aycock DM, Wolf SL. Influence of stroke survivor characteristics and family conflict surrounding recovery on caregivers’ mental and physical health. Nurs Res. 2004;53(6):406–13.
Haley WE, Allen JY, Grant JS, Clay OJ, Perkins M, Roth DL. Problems and benefits reported by stroke family caregivers: results from a prospective epidemiological study. Stroke. 2009;40(6):2129–33.
ATTEND Collaborative Group. Family-led rehabilitation after stroke in India (ATTEND): a randomised controlled trial. Lancet. 2017;390(10094):588–99. https://doi.org/10.1016/S0140-6736(17)31447-2.
Kalra L, Evans A, Perez I, Melbourn A, Patel A, Knapp M, Donaldson N. Training carers of stroke patients: randomised controlled trial. BMJ. 2004;328(7448):1099.
Forster A, Dickerson J, Young J, Patel A, Kalra L, Nixon J, Smithard D, Knapp M, Holloway I, Anwar S, et al. A cluster randomised controlled trial and economic evaluation of a structured training programme for caregivers of inpatients after stroke: the TRACS trial. Health Technol Assess. 2013;17(46):1–216.
Creasy KR, Lutz BJ, Young ME, Ford A, Martz C. The impact of interactions with providers on stroke caregivers’ needs. Rehabil Nurs. 2013;38(2):88–98.
Monaghan J, Channell K, McDowell D, Sharma AK. Improving patient and carer communication, multidisciplinary team working and goal-setting in stroke rehabilitation. Clin Rehabil. 2005;19(2):194–9.
Bakas T, Farran CJ, Austin JK, Given BA, Johnson EA, Williams LS. Stroke caregiver outcomes from the Telephone Assessment and Skill-Building Kit (TASK). Top Stroke Rehab. 2009;16(2):105–21.
Lutz BJ, Young ME, Creasy KR, Martz C, Eisenbrandt L, Brunny JN, Cook C. Improving stroke caregiver readiness for transition from inpatient rehabilitation to home. Gerontologist. 2017;57(5):880–9.
Bakas T, Clark PC, Kelly-Hayes M, King RB, Lutz BJ, Miller EL. American Heart Association Council on C, Stroke N, the Stroke C: Evidence for stroke family caregiver and dyad interventions: a statement for healthcare professionals from the American Heart Association and American Stroke Association. Stroke. 2014;45(9):2836–52.
Bakas T, McCarthy MJ, Miller EL. Systematic review of the evidence for stroke family caregiver and dyad interventions. Stroke. 2022;53(6):2093–102.
Blanton S, Clark PC, Dunbar SB. Feasibility of a carepartner integrated telehealth rehabilitation program for stroke: a case series [Poster Abstract]. American Physical Therapy Association Combined Sections Conference. San Antonio; 2017. https://journals.lww.com/jnpt/Documents/Poster%20Abstracts%20CSM%202017.pdf.
Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. 2000;55(1):68–78.
Blanton S, Dunbar S, Clark PC. Content validity and satisfaction with a caregiver-integrated web-based rehabilitation intervention for persons with stroke. Top Stroke Rehabil. 2018;25(3):168–73.
Blanton S, Clark PC, Cotsonis G, Dunbar SB. Factors associated with depressive symptoms of carepartners of stroke survivors after discharge from rehabilitation therapy. Top Stroke Rehabil. 2020;27(8):590–600.
Blanton S, C P, Cotsonis G. A Carepartner-Integrated Telehealth Rehabilitation Program influences carepartner life changes, strain, and fatigue. Orlando: In: American Physical Therapy Association Combined Sections Conference; 2021.
Blanton S, C P, Dunbar S. Evaluation of a Carepartner-Integrated Telehealth Rehabilitation Program for persons with stroke. 2020: In: American Physical Therapy Association Combined Sections Conference; Denver.
Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza-Jeric K, Hrobjartsson A, Mann H, Dickersin K, Berlin JA, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687.
Bakas T, Farran CJ, Austin JK, Given BA, Johnson EA, Williams LS. Content validity and satisfaction with a stroke caregiver intervention program. J Nurs Scholarsh. 2009;41(4):368–75.
Agrell B, Dehlin O. Mini mental state examination in geriatric stroke patients. Validity, differences between subgroups of patients, and relationships to somatic and mental variables. Aging (Milan, Italy). 2000;12(6):439–44.
Blanton S, Morris DM, Prettyman MG, McCulloch K, Redmond S, Light KE, Wolf SL. Lessons learned in participant recruitment and retention: the EXCITE trial. Phys Ther. 2006;86(11):1520–33.
Ezzelle J, Rodriguez-Chavez IR, Darden JM, Stirewalt M, Kunwar N, Hitchcock R, Walter T, D’Souza MP. Guidelines on good clinical laboratory practice: bridging operations between research and clinical research laboratories. J Pharm Biomed Anal. 2008;46(1):18–29.
Lewis JR. Psychometric evaluation of the PSSUQ using data from five years of usability studies. Int J Hum Comput Interact. 2002;14(3–4):463–88.
Winstein CJ, Wolf SL, Dromerick AW, Lane CJ, Nelsen MA, Lewthwaite R, Blanton S, Scott C, Reiss A, Cen SY, et al. Interdisciplinary Comprehensive Arm Rehabilitation Evaluation (ICARE): a randomized controlled trial protocol. BMC Neurol. 2013;13:5.
Kind AJ, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, Greenberg C, Smith M. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765–74.
Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible - the Neighborhood Atlas. N Engl J Med. 2018;378(26):2456–8.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Naqvi IA, Montiel TC, Bittar Y, Hunter N, Okpala M, Johnson C, Weiner MG, Savitz S, Sharrief A, Beauchamp JES. Internet access and usage among stroke survivors and their informal caregivers: cross-sectional study. JMIR Form Res. 2021;5(3):e25123.
Collen FM, Wade DT, Bradshaw CM. Mobility after stroke: reliability of measures of impairment and disability. Int Disabil Stud. 1990;12(1):6–9.
Flansbjer UB, Holmbäck AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005;37(2):75–82.
Lin JH, Hsu MJ, Hsu HW, Wu HC, Hsieh CL. Psychometric comparisons of 3 functional ambulation measures for patients with stroke. Stroke. 2010;41(9):2021–5.
Eng JJ, Dawson AS, Chu KS. Submaximal exercise in persons with stroke: test-retest reliability and concurrent validity with maximal oxygen consumption. Arch Phys Med Rehabil. 2004;85(1):113–8.
Fulk GD, Echternach JL. Test-retest reliability and minimal detectable change of gait speed in individuals undergoing rehabilitation after stroke. J Neurol Phys Ther. 2008;32(1):8–13.
Mong Y, Teo TW, Ng SS. 5-repetition sit-to-stand test in subjects with chronic stroke: reliability and validity. Arch Phys Med Rehabil. 2010;91(3):407–13.
Beninato M, Portney LG, Sullivan PE. Using the International Classification of Functioning, Disability and Health as a framework to examine the association between falls and clinical assessment tools in people with stroke. Phys Ther. 2009;89(8):816–25.
Madhavan S, Sivaramakrishnan A, Bowden MG, Chumbler NR, Field-Fote EC, Kesar TM. Commentary: remote assessments of gait and balance - Implications for research during and beyond Covid-19. Top Stroke Rehabil. 2022;29(1):74–81.
Knorr S, Brouwer B, Garland SJ. Validity of the Community Balance and Mobility Scale in community-dwelling persons after stroke. Arch Phys Med Rehabil. 2010;91(6):890–6.
Ng SS, Hui-Chan CW. The timed up & go test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch Phys Med Rehabil. 2005;86(8):1641–7.
Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke A J Cerebral Circulation. 1999;30(10):2131–40.
Salbach NM, Mayo NE, Hanley JA, Richards CL, Wood-Dauphinee S. Psychometric evaluation of the original and Canadian French version of the Activities-specific Balance Confidence Scale among people with stroke. Arch Phys Med Rehabil. 2006;87(12):1597–604.
Botner EM, Miller WC, Eng JJ. Measurement properties of the Activities-specific Balance Confidence Scale among individuals with stroke. Disabil Rehabil. 2005;27(4):156–63.
Mudge S, Stott NS. Test–retest reliability of the StepWatch Activity Monitor outputs in individuals with chronic stroke. Clin Rehabil. 2008;22(10–11):871–7.
Mudge S, Stott NS, Walt SE. Criterion validity of the StepWatch Activity Monitor as a measure of walking activity in patients after stroke. Arch Phys Med Rehabil. 2007;88(12):1710–5.
Haeuber E, Shaughnessy M, Forrester LW, Coleman KL, Macko RF. Accelerometer monitoring of home- and community-based ambulatory activity after stroke. Arch Phys Med Rehabil. 2004;85(12):1997–2001.
Lee JY, Kwon S, Kim WS, Hahn SJ, Park J, Paik NJ. Feasibility, reliability, and validity of using accelerometers to measure physical activities of patients with stroke during inpatient rehabilitation. PLoS ONE. 2018;13(12):e0209607.
Clark P, Dunbar SB. Preliminary reliability and validity of a Family Care Climate Questionnaire for heart failure. Fam Syst Health. 2003;21(3):281–91.
Kruithof WJ, Post MW, Visser-Meily JM. Measuring negative and positive caregiving experiences: a psychometric analysis of the Caregiver Strain Index Expanded. Clin Rehabil. 2015;29(12):1224–33.
Robinson BC. Validation of a Caregiver Strain Index. J Gerontol. 1983;38(3):344–8.
van Exel NJ. Scholte op Reimer WJ, Brouwer WB, van den Berg B, Koopmanschap MA, van den Bos GA: Instruments for assessing the burden of informal caregiving for stroke patients in clinical practice: a comparison of CSI, CRA SCQ and self-rated burden. Clin Rehabil. 2004;18(2):203–14.
Post MW, Festen H, van de Port IG, Visser-Meily JM. Reproducibility of the Caregiver Strain Index and the Caregiver Reaction Assessment in partners of stroke patients living in the Dutch community. Clin Rehabil. 2007;21(11):1050–5.
Clark PC, Shields CG, Aycock D, Wolf SL. Preliminary reliability and validity of a family caregiver conflict scale for stroke. Prog Cardiovasc Nurs. 2003;18(2):77.
Bakas T, Champion V, Perkins SM, Farran CJ, Williams LS. Psychometric testing of the revised 15-item Bakas Caregiving Outcomes Scale. Nurs Res. 2006;55(5):346–55.
Acknowledgements
The authors would like to thank the stroke survivors and carepartners who assisted in the development of the CARE-CITE-Gait video intervention and the research study participants.
Funding
2021 Paris Patla Physical Therapy Research Grant, Foundation for Physical Therapy Research, Emory Library Information Technology Services grant support (UL1 TR000424) for REDCap data management system.
Author information
Authors and Affiliations
Contributions
Protocol design by SB, TK, PC, and GC; SB wrote the protocol which was edited by all authors; SB and TK oversee study implementation; PC and AJ served as consultants for SB and TK during study development; PC and SB developed the original upper extremity CARE-CITE intervention; SB, TK, SC, and KB adapted CARE-CITE to CARE-CITE-Gait; GC developed and will conduct statistical analysis; AJ and DR provided consultation on accelerometry methodology and data analysis; and RS assisted in accelerometry data collection procedures and will conduct data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was obtained by Emory University Institutional Review Board (IRB00070957), and this protocol is registered on ClinicalTrials.gov (NCT02703532).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Blanton, S., Cotsonis, G., Brennan, K. et al. Evaluation of a carepartner-integrated telehealth gait rehabilitation program for persons with stroke: study protocol for a feasibility study. Pilot Feasibility Stud 9, 192 (2023). https://doi.org/10.1186/s40814-023-01411-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40814-023-01411-1