Abstract
Background
Obesity is a chronic disease and is an established risk factor for other chronic diseases and mortality. Young adulthood is a period when people may be highly amenable to healthy behavior change, develop lifelong healthy behaviors, and when primary prevention of obesity may be feasible. Interventions in early adulthood have the potential for primary or primordial prevention (i.e., preventing risk factors before disease onset). The primary objective of this study is to determine the feasibility of a 6-month behavioral and educational intervention to promote healthy behaviors for obesity prevention among young adults.
Methods
This is the study protocol for a pilot randomized controlled trial. Young adults (age 18–29) attending McMaster University, Hamilton, Canada, will be recruited and randomized to either the intervention or control. The intervention will include individual motivational interviewing sessions (online or in-person) with a trained interviewer plus educational materials (based on Canada’s food guide and physical activity recommendations). The control group will receive educational materials only. The primary feasibility outcomes that will be evaluated as part of this pilot study include enrollment, retention (≥ 80%), data completion (≥ 80% of weights measured, and surveys completed), and participant satisfaction. Secondary clinical outcomes will include body mass index (BMI) change from baseline to 6 months, physical activity, nutrition risk, health-related quality of life mental health, and economic outcomes. Outcomes will be measured remotely using activity trackers, and online questionnaires at baseline and every 2 months. Risk stratification will be applied at baseline to identify participants at high risk of obesity (e.g., due to family or personal history). Exit questionnaires will collect data on how participants felt about the study and cost analysis will be conducted.
Discussion
Our pilot randomized controlled trial will evaluate the feasibility of an obesity prevention intervention in early adulthood and will inform future larger studies for obesity prevention. The results of this study have the potential to directly contribute to the primary prevention of several types of cancer by testing an intervention that could be scalable to public health, post-secondary education, or primary care settings.
Trial registration
https://clinicaltrials.gov/ct2/show/NCT05264740. Registered on March 3, 2022.
Similar content being viewed by others
Background
Obesity is a chronic disease, and it is also a risk factor for other chronic diseases. In Canada, the prevalence of obesity among adults was 28% in 2018 [1, 2]. It has been estimated that the direct costs of overweight and obesity in Canada were $6 billion annually in 2006 [3]. By 2025 approximately 20% of adults worldwide will have obesity.[4] Obesity is defined as a body mass index (BMI) > 30 with classes of obesity severity defined as Class 1 (30–34), Class 2 (35–39), and Class 3 (≥ 40) [5]. Although there are limitations in the use of BMI cut points to define obesity, BMI is strongly correlated with body fat and remains the most practical measure for population-based studies [6, 7]. Obesity severity is important, with more severe obesity conferring a substantially greater risk of cancer, other chronic conditions, and poor mental health [8,9,10]. Obesity is a complex disease with a broad range of socio-ecological risk factors. Obesity treatment is challenging, and primary and secondary prevention interventions are urgently needed to reduce the incidence of obesity and decrease or maintain obesity severity [11].
The COVID-19 pandemic had a profound impact on activities of daily life, and many causes of weight gain, including chronic stress and physical inactivity, increased during the pandemic. Early indications suggest that 20–48% of people reported weight gain during the pandemic [12,13,14,15,16,17,18,19]. Some reasons include changes in food intake (increased food insecurity and consumption of “comfort” foods), decreased physical activity, increased sedentary time, and increased alcohol. There is some evidence of sex differences (greater weight gain in women) and increased weight gain among people with higher BMI pre-pandemic [12, 13]. One small US study found that pandemic shelter-in-place orders were associated with an average weight gain of 1.5 lbs per month [20]. It is established that obesity can develop slowly over time or as a result of a rapid weight gain [11]. Thus, it is important to investigate interventions that may contribute to modifying health behaviors for obesity prevention.
Young adulthood is a period when people may be highly amenable to healthy behavior change, develop lifelong healthy behaviors, and when the primary or secondary prevention of obesity may be feasible. Interventions in young adults have the potential for primary cancer prevention (i.e., stopping cancer risk factors before they develop). Young adulthood is a time when weight gain may occur rapidly [21] and developing healthy behaviors is critical to maintaining a healthy weight throughout adulthood. On average, Canadian adults gain 0.5 to 1 kg every 2 years and this is greater among young adults and those with higher BMI.[22] Systematic reviews suggest that students attending post-secondary education may experience greater weight gain [23, 24].
Narrowly defined lifestyle interventions for obesity prevention, such as those addressing only physical activity, have had limited success [25]. The 2020 Obesity Clinical Practice Guidelines recommend moving “beyond simplistic approaches of “eat less, move more,” and address the root drivers of obesity” [11]. Yet, there have been relatively few attempts to implement tailored, population-based obesity prevention interventions in young adults. Obesity interventions must be flexible to address the complex causes of obesity, and motivational interviewing may be a successful strategy [25]. Motivational interviewing (MI) is defined as “a collaborative conversation style for strengthening a person’s own motivation and commitment to change” [26]. Health care professionals have successfully used motivational interviewing for patients to set personalized goals for successful healthy life changes [27]. Motivational interviewing has shown some success for weight loss interventions in young adults, [25] but it has not been widely implemented for obesity prevention. It is unknown whether obesity prevention interventions are more successful among people at highest risk. Several obesity risk stratification tools have been developed and validated that identify young adults who are at greatest risk of obesity later in adulthood [28,29,30]. The use of these risk prediction tools to identify young adults who may benefit the most from an obesity prevention intervention may improve success and support broader population-level, precision public health or clinical interventions.
The primary study objective is:
-
1
To determine the feasibility (enrollment, retention, data completion, satisfaction) of a 6-month behavioral and educational intervention to promote healthy behaviors for obesity prevention among young adults.
The secondary objectives are:
-
2) To determine the effects of the 6-month behavioral and educational intervention, compared to an educational intervention only, on change in BMI, health behaviors (nutrition, physical activity, and sedentary time), health-related quality of life, and mental health (depression and anxiety).
-
3) To explore whether obesity risk stratification tools identify young adults who may be more successful in an obesity intervention.
Methods
Study design and population
We will conduct a pilot randomized controlled trial (RCT). The protocol is reported following the SPIRIT guidelines for RCT pilot studies, [31] the Template for Intervention Description and Replication (TIDieR) checklist and guideline, [32] and has been pre-registered (ClinicalTrials.gov Identifier: NCT05264740). Study participants will be young adults (age 18–29) who are students at McMaster University, Hamilton, Ontario, Canada. Recruitment posters will be posted in common areas on campus, shared on social media, and through email lists. The study website is available at https://motivate.healthsci.mcmaster.ca/.
The study inclusion criteria are:
-
English speaking and capable of providing informed consent.
-
McMaster University students 18–29 years of age.
-
BMI of at least 18.5 (BMI < 18.5 is considered underweight and will be excluded).
-
Access to wireless Internet (WiFi) at home.
Exclusion criteria are:
-
Physical and mental health conditions that would be contraindications for a weight management intervention, including eating disorders, pregnancy, cancer, or medications that affect body weight.
Randomization
Participants will be randomized to intervention and control arms in a 1:1 allocation ratio using a computer-generated randomization list that will be generated centrally by the Biostatistics Unit at St Joseph’s Healthcare Hamilton. Randomization will be stratified by sex and BMI (BMI < 25 vs BMI ≥ 25) to achieve some prognostic balance. We will use permuted blocks of random sizes to ensure equal numbers in each group. The block sizes and allocation will be concealed from the participants and trial staff, and the randomization codes will only be revealed after the participants have been recruited and baseline data has been collected. This is an open-label trial. Trial staff and participants will not be blinded to the intervention, but data analysts will be blinded.
Consent and ethics
Ethics approval was obtained from the Hamilton Integrated Research Ethics Board on July 25, 2022 (HiREB Project Number: 14675). Informed consent will be obtained from all participants at the first in-person visit. Participants may choose to withdraw from the study at any time and when possible, we will capture the reasons for withdrawal. Consideration has been given to issues of equity (advertisement and recruitment materials, research staff and investigators), and among this population of students, we do not anticipate that the English-language requirement will be a barrier.
Timing of visits, intervention, and control groups
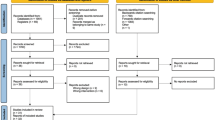
An overview of the study design is provided in Fig. 1. The first visit will occur in-person with a research assistant to obtain consent, collect baseline measures, provide participants with the study materials/equipment, and build a relationship with the study participants. For all subsequent visits, participants will have the option of in-person or virtual sessions.
The intervention will include motivational interviewing sessions with a trained research assistant/interviewer plus educational material. The motivational interviewing sessions will occur at baseline and once a month (7 visits in total). For this feasibility study, a priori criteria for the discontinuation or modification of the allocated intervention are not available.
The research assistant will receive training in motivational interviewing. Intervention fidelity (adherence to the principles of MI) will be assessed using recorded training interviews and established rating scales with continuing training as needed [33]. For training purposes, mock interviews conducted between members of the research team will be recorded and evaluated. Interviews with study participants will not be recorded.
The control group will receive educational material only. The same educational material will be provided to both the intervention and control group and will consist of evidence-based recommendations based on Canada’s food guide [34] and Canadian 24-h movement guidelines [35]. The SPIRIT schedule of enrolment, interventions, and assessments for this study is provided in Fig. 2.
Measurement of outcomes
Table 1 describes all outcomes. The primary feasibility outcomes that will be measured are recruitment, retention, data completion, and participant satisfaction. We will consider a larger trial feasible if recruitment rates are ≥ 50%, retention rates are ≥ 80%, and the data completion rate for all secondary clinical outcomes is ≥ 80%. Participant satisfaction with different aspects of the study will be measured at the exit questionnaire using a Likert scale and semi-structured qualitative free-text responses.
Secondary outcomes will include BMI change from baseline to 6-months. Interventions are more successful if longer than 4 months in duration [36]. All study participants will have one in-person visit with the research assistant at the start of the study where baseline weight and height will be measured using standardized methods with calibrated study instruments. Participants (both intervention and control) will then receive a Fitbit activity tracker to wear for the duration of the study. Study participants will be asked to visit the study office once a month to conduct a self weigh-in using a Fitbit electronic “smart” scale (e-scale). They will be asked to weigh themselves monthly, and reminders will be sent by email or text message. E-scales are feasible and valid for research conducted remotely and participant adherence to home weigh-ins is high [37,38,39], and as part of this feasibility study, we will assess the adherence to self weigh-ins when the scale is located in a private study office. Other secondary outcomes will include nutrition risk, physical activity (measured as 24-h movement, including sedentary time and sleep), and mental health collected using online questionnaires at baseline and every 2 months. Nutrition risk will be measured using the National Cancer Institute’s Dietary Screener Questionnaire (DSQ). The DSQ measures the frequency of intake of selected foods that are known to affect risk of cancer and obesity, including fruits, vegetables, whole grains, and sugary drinks. The DSQ has good validity compared to 24-h recalls [40]. Self-reported physical activity, sedentary time, recreational screen time, and sleep will be measured to describe adherence to recommended 24-h movement guidelines using questionnaires previously used in young adults [41], including the International Physical Activity Questionnaire (IPAQ) [42], and International Sedentary Assessment Tool (ISAT) [43]. The Insomnia Severity Index will also be included as a measure of perceived sleep quality [44]. Mental health is an important upstream determinant of health behaviors and is highly associated with obesity risk. We will measure depressive symptoms, anxiety, and health-related quality of life using the following validated tools: Centre for Epidemiologic Studies Depression Measure-10 (CESD-10) [45], Generalized Anxiety Disorder-7 (GAD-7) [46], and Euroqol-5D-5L (EQ-5D-5L) [47] We anticipate that some youth may also raise issues related to alcohol or substance use in the interviews; thus, we have included questions that ask about alcohol, smoking, cannabis, and medication use in the questionnaires. A single-item measure of body satisfaction is included in the study to evaluate any changes over the course of the study [48]. A validated two-item food insecurity screen is also included [49].
For objective 3, we will evaluate whether feasibility and other secondary outcomes differed for participants who were identified as high versus low risk using existing obesity risk stratification tools. Three validated risk stratification tools will be applied to the data to explore these differences [28,29,30]. The Edmonton Obesity Staging System will also be used [50]. The required variables for each of the risk tools will be collected from the baseline questionnaire (e.g., parent obesity, history of child obesity, alcohol, smoking, medical history). Feasibility data to inform costing and economic analyses for a larger scale trial will be collected. In addition to the EQ-5D-5L, self-reported healthcare use, and time missed from school/work will be collected on the surveys. For this pilot study a descriptive cost analysis will be conducted.
Data collection and management
All data will be stored in a secure REDCap database. Surveys will be collected online using secure electronic data capture in REDcap. The final trial dataset will only be available to select members of the research team (LA, LM, TI). The final dataset will not be made publicly available to protect the confidentiality of participants, but de-identified data could be made available upon request. Baseline surveys will collect detailed data on socioeconomic variables, including age, sex, gender identity, income, and food security, medical history, and the secondary outcomes described above (nutrition, physical activity, sedentary time, mental health). Follow-up surveys administered every 2 months (at 2, 4, and 6 months) will collect updated data on secondary outcomes. Participants in both the control and intervention groups will receive a $30 gift card at the time of each survey completion. We will continue to collect data from participants if they deviate from protocol unless there is withdrawal from the study. To ensure confidentiality, a unique study id number will be used for the weight and activity data and linked at McMaster by the research team to the main study database.
Safety protocols and unanticipated outcomes
Possible adverse outcomes may include concerns related to mental health, including eating disorders or frustration due to weight gain/no change. Expectations of the research will be laid out before enrollment and feelings such as these can be addressed as part of the motivational interviewing process. Our research team including the research assistants/interviewers and physician co-investigator (EA) are equipped to assess the situation and provide necessary referrals. Medical and counseling services are available on campus for all students. If participants raise any concerns of self-harm or harm to others, then safety protocols will be followed.
Data analysis
The analysis and reporting of our results will be done according to the CONSORT guidelines [51]. The data analyst will be blinded. Patient screening, randomization, allocation, and follow-up numbers will be illustrated in a flow diagram. Baseline data will be reported in a table for both groups (intervention and control) and summarized as means (standard deviation) or median (first quartile, third quartile) for continuous variables and counts (percent) for categorical variables. Data on feasibility outcomes will be measured and compared to pre-determined thresholds for interpretation. The primary analysis will be by intention-to-treat (data from participants will be analyzed according to their allocation irrespective of whether they received that intervention). Comparison of groups for secondary outcomes will be reported descriptively for this pilot study. No formal significance testing for comparisons of the secondary clinical outcomes will be made because this is a pilot study with a priori sample size determined based on feasibility outcomes only. It is well established that there are sex and gender differences in obesity [52]. Disaggregated data on sex and gender will be reported.
Sample size
Our sample size estimation was based on feasibility considerations and calculated using WINPEPI [53]. Using a 95% confidence level, assuming that 80% of the participants will complete the study with a 7.5% margin of error, 110 participants are needed (55 intervention and 55 control). Since the randomization will be stratified by sex and BMI (BMI < 25 vs BMI ≥ 25), we will have approximately equal numbers in each group. This sample size is not sufficiently powered to make between-group comparisons but will provide accurate data on feasibility and estimates of effect size variability that will inform the sample size calculation of a larger definitive trial.
Reporting and interpretation
Study results will be reported following the CONSORT extension for randomized pilot and feasibility trials guideline [54]. For each feasibility outcome, we will report whether the feasibility threshold was met and use a traffic light system to inform progression to a larger trial. Green, proceed to larger trial; yellow, proceed with some modifications; and red, larger trial not feasible. At least one feasibility outcome must be green. Any deviations to the protocol will be recorded and reported in the final manuscript. Study results will be shared with participants through a final report and published in a peer-reviewed manuscript.
Conclusions
Although it is well recognized that the causes of obesity are complex, many obesity trials have evaluated a narrowly defined “one-size-fits-all” intervention. We will evaluate the feasibility of motivational interviewing sessions that will be flexible and allow participants to discuss the health behaviors that are most important to them, thus tailoring the intervention to their needs. This approach will allow participants to set their own behavioral change goals. Further, we will explore the feasibility of a risk stratification approach to an obesity intervention to determine if higher-risk young adults benefit more from the intervention.
This intervention is designed to be flexible to focus on a broad range of health behaviors so study participants can set behavioral change goals related to the behaviors that they think are most important to them. While for some participants, this may focus on traditional, obesity-related risk factors such as diet or physical activity, for other participants this may include behaviors related to sleep, mental health, substance use, or time management. All these behaviors have the potential to either directly or indirectly impact obesity prevention.
Our feasibility trial will inform future larger RCTs for obesity prevention. The results of this study have the potential to directly contribute to the primary prevention of several types of cancer by testing an intervention that could be scalable to public health, post-secondary education, or primary care settings. This study is pragmatic with the option of remote visits for MI. Unlike traditional MI, this format with a trained interviewer is feasible and the results of the risk stratification objective may inform who would benefit the most from a larger-scale program. This pilot study will provide proof of concept for an intervention that could be expanded to other settings such as public health, post-secondary education, or primary care. If successful, this program could be implemented in university wellness services and may have a profound impact on the primary prevention of an established and highly prevalent chronic disease risk factor.
Availability of data and materials
Not applicable.
Abbreviations
- BMI:
-
Body mass index
- CESD-10:
-
Centre for Epidemiologic Studies Depression Measure-10
- DSQ:
-
Dietary Screener Questionnaire
- EQ-5D-5L:
-
Euroqol-5D
- GAD-7:
-
Generalized Anxiety Disorder -7
- HiREB:
-
Hamilton Integrated Research Ethics Board
- IPAQ:
-
International Physical Activity Questionnaire
- ISAT:
-
International Sedentary Assessment Tool
- MI:
-
Motivational Interviewing
- RCT:
-
Randomized controlled trial
References
Rigobon AV, Birtwhistle R, Khan S, Barber D, Biro S, Morkem R, et al. Adult obesity prevalence in primary care users: an exploration using Canadian Primary Care Sentinel Surveillance Network (CPCSSN) data. Can J Public Health. 2015;106(5):e283–9.
Statistics Canada. Adult body mass index - Health Canada classification, inactive. Government of Canada; [cited 2021 Mar 11]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310032301
Anis AH, Zhang W, Bansback N, Guh DP, Amarsi Z, Birmingham CL. Obesity and overweight in Canada: an updated cost-of-illness study. Obes Rev Off J Int Assoc Study Obes. 2010;11(1):31–40.
NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387(10026):1377–96.
CDC. Defining Adult Overweight and obesity. Centers for Disease Control and Prevention. 2021 [cited 2021 Jul 14]. Available from: https://www.cdc.gov/obesity/adult/defining.html
Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes. 2008;32 Suppl 3:S56-59.
Andreacchi AT, Griffith LE, Guindon GE, Mayhew A, Bassim C, Pigeyre M, et al. Body mass index, waist circumference, waist-to-hip ratio, and body fat in relation to health care use in the Canadian Longitudinal Study on Aging. Int J Obes. 2021;45(3):666–76.
Katz DA, McHorney CA, Atkinson RL. Impact of obesity on health-related quality of life in patients with chronic illness. J Gen Intern Med. 2000;15(11):789–96.
Thygesen LC, Grønbaek M, Johansen C, Fuchs CS, Willett WC, Giovannucci E. Prospective weight change and colon cancer risk in male US health professionals. Int J Cancer. 2008;123(5):1160–5.
de Mutsert R, Sun Q, Willett WC, Hu FB, van Dam RM. Overweight in early adulthood, adult weight change, and risk of type 2 diabetes, cardiovascular diseases, and certain cancers in men: a cohort study. Am J Epidemiol. 2014;179(11):1353–65.
Wharton S, Lau DCW, Vallis M, Sharma AM, Biertho L, Campbell-Scherer D, et al. Obesity in adults: a clinical practice guideline. Can Med Assoc J. 2020;192(31):E875–91.
Reyes-Olavarría D, Latorre-Román PÁ, Guzmán-Guzmán IP, Jerez-Mayorga D, Caamaño-Navarrete F, Delgado-Floody P. Positive and negative changes in food habits, physical activity patterns, and weight status during COVID-19 confinement: associated factors in the Chilean population. Int J Environ Res Public Health. 2020;17(15):5431.
Flanagan EW, Beyl RA, Fearnbach SN, Altazan AD, Martin CK, Redman LM. The impact of COVID-19 stay-at-home orders on health behaviors in adults. Obesity. 2021;29(2):438–45.
Shimokihara S, Maruta M, Hidaka Y, Akasaki Y, Tokuda K, Han G, et al. Relationship of decrease in frequency of socialization to daily life, social life, and physical function in community-dwelling adults aged 60 and over after the COVID-19 pandemic. Int J Environ Res Public Health. 2021;18(5):2573.
Deschasaux-Tanguy M, Druesne-Pecollo N, Esseddik Y, de Edelenyi FS, Allès B, Andreeva VA, et al. Diet and physical activity during the coronavirus disease 2019 (COVID-19) lockdown (March–May 2020): results from the French NutriNet-Santé cohort study. Am J Clin Nutr. [cited 2021 Mar 12]; Available from: https://academic.oup.com/ajcn/advance-article/doi/https://doi.org/10.1093/ajcn/nqaa336/6155959
Scarmozzino F, Visioli F. COVID-19 and the subsequent lockdown modified dietary habits of almost half the population in an Italian sample. Foods. 2020;9(5):675.
Zachary Z, Brianna F, Brianna L, Garrett P, Jade W, Alyssa D, et al. Self-quarantine and weight gain related risk factors during the COVID-19 pandemic. Obes Res Clin Pract. 2020;14(3):210–6.
Di Renzo L, Gualtieri P, Pivari F, Soldati L, Attinà A, Cinelli G, et al. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J Transl Med. 2020;18(1):229.
Bhutani S, vanDellen MR, Cooper JA. Longitudinal weight gain and related risk behaviors during the COVID-19 pandemic in adults in the US. Nutrients. 2021;13(2):671.
Lin AL, Vittinghoff E, Olgin JE, Pletcher MJ, Marcus GM. Body weight changes during pandemic-related shelter-in-place in a longitudinal cohort study. JAMA Netw Open. 2021;4(3): e212536.
Gordon-Larsen P, The NS, Adair LS. Longitudinal trends in obesity in the United States from adolescence to the third decade of life. Obes Silver Spring Md. 2010;18(9):1801–4.
Orpana HM, Tremblay MS, Finès P. Trends in weight change among Canadian adults. Health Rep. 2007;18(2):9–16.
Fedewa MV, Das BM, Evans EM, Dishman RK. Change in weight and adiposity in college students: a systematic review and meta-analysis. Am J Prev Med. 2014;47(5):641–52.
Vella-Zarb RA, Elgar FJ. The “freshman 5”: a meta-analysis of weight gain in the freshman year of college. J Am Coll Health J ACH. 2009;58(2):161–6.
Poobalan AS, Aucott LS, Precious E, Crombie IK, Smith WCS. Weight loss interventions in young people (18 to 25 year olds): a systematic review: Weight loss interventions in young people. Obes Rev. 2009;11(8):580–92.
Miller WR, Rollnick S. Motivational interviewing: helping people change. New York; Guilford Press; 2012. p. 497.
Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol. 2009;64(6):527–37.
Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337(13):869–73.
Potter CM, Ulijaszek SJ. Predicting adult obesity from measures in earlier life. J Epidemiol Community Health. 2013;67(12):1032–7.
Lebenbaum M, Espin-Garcia O, Li Y, Rosella LC. Development and validation of a population based risk algorithm for obesity: The Obesity Population Risk Tool (OPoRT). PLoS ONE. 2018;13(1): e0191169.
Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346(jan08 15):e7586–e7586.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348(mar07 3):g1687–g1687.
Mbuagbaw L, Ye C, Thabane L. Motivational interviewing for improving outcomes in youth living with HIV. Cochrane Database Syst Rev. 2012;9:CD009748.
Canada H. Welcome to Canada’s food guide. 2021 [cited 2021 Jul 9]. Available from: https://food-guide.canada.ca/en/
Ross R, Chaput JP, Giangregorio LM, Janssen I, Saunders TJ, Kho ME, et al. Canadian 24-hour movement guidelines for adults aged 18–64 years and adults aged 65 years or older: an integration of physical activity, sedentary behaviour, and sleep. Appl Physiol Nutr Metab Physiol Appl Nutr Metab. 2020;45(10 (Suppl. 2)):S57-102.
Hebden L, Chey T, Allman-Farinelli M. Lifestyle intervention for preventing weight gain in young adults: a systematic review and meta-analysis of RCTs: weight gain prevention in young adults. Obes Rev. 2012;13(8):692–710.
Krukowski RA, Ross KM. Measuring weight with electronic scales in clinical and research settings during the coronavirus disease 2019 pandemic. Obesity. 2020;28(7):1182–3.
Ross KM, Qiu P, You L, Wing RR. Characterizing the pattern of weight loss and regain in adults enrolled in a 12-week internet-based weight management program. Obes Silver Spring Md. 2018;26(2):318–23.
Bertz F, Pacanowski CR, Levitsky DA. Frequent self-weighing with electronic graphic feedback to prevent age-related weight gain in young adults: frequent self-weighing prevents weight gain. Obesity. 2015;23(10):2009–14.
Thompson FE, Midthune D, Kahle L, Dodd KW. Development and evaluation of the national cancer institute’s dietary screener questionnaire scoring algorithms. J Nutr. 2017;147(6):1226–33.
Weatherson KA, Joopally H, Wunderlich K, Kwan MY, Tomasone JR, Faulkner G. Post-secondary students’ adherence to the Canadian 24-hour movement guidelines for adults: results from the first deployment of the Canadian Campus Wellbeing Survey (CCWS). Health Promot Chronic Dis Prev Can Res Policy Pract. 2021;41(6):173–81.
Murphy JJ, Murphy MH, MacDonncha C, Murphy N, Nevill AM, Woods CB. Validity and reliability of three self-report instruments for assessing attainment of physical activity guidelines in university students. Meas Phys Educ Exerc Sci. 2017;21(3):134–41.
Prince SA, LeBlanc AG, Colley RC, Saunders TJ. Measurement of sedentary behaviour in population health surveys: a review and recommendations. PeerJ. 2017;11(5): e4130.
Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307.
Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10(2):77–84.
Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7.
Herdman M, Gudex C, Lloyd A, Janssen Mf, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–36.
Carter A, Forrest JI, Kaida A. Association between internet use and body dissatisfaction among young females: cross-sectional analysis of the Canadian Community Health Survey. J Med Internet Res. 2017;19(2): e39.
Hager ER, Quigg AM, Black MM, Coleman SM, Heeren T, Rose-Jacobs R, et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics. 2010;126(1):e26-32.
Canning KL, Brown RE, Wharton S, Sharma AM, Kuk JL. Edmonton obesity staging system prevalence and association with weight loss in a publicly funded referral-based obesity clinic. J Obes. 2015;2015:1–7.
Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340(mar23 1):c869–c869.
Shah B, Tombeau Cost K, Fuller A, Birken CS, Anderson LN. Sex and gender differences in childhood obesity: contributing to the research agenda. BMJ Nutr Prev Health. 2020;3(2):387–90.
Abramson JH. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov EPI. 2011;8(1):1.
Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;24: i5239.
Funding
This research is funded by a Proof-of-Concept Intervention Grant in Primary Prevention of Cancer (Action Grant) of the Canadian Cancer Society and the Canadian Institutes of Health Research-Institute for Cancer Research (CCS grant #707228/CIHR-ICR grant # POC-181032).
Author information
Authors and Affiliations
Contributions
All authors contributed to the development of the study design and contributed to writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Study participants will provide written consent and this study has been approved by the Hamilton Integrated Research Ethics Board (HiREB Project Number: 14675).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Anderson, L.N., Alvarez, E., Incze, T. et al. Motivational interviewing to promote healthy behaviors for obesity prevention in young adults (MOTIVATE): a pilot randomized controlled trial protocol. Pilot Feasibility Stud 9, 156 (2023). https://doi.org/10.1186/s40814-023-01385-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40814-023-01385-0