Abstract
Background
Bisphenol exposure is widespread and correlated with diabetes and cardiovascular disease. Previous intervention studies have successfully lowered bisphenol exposure among women of normal weight. The primary objective of this study was to develop and test the feasibility of a 3-week behavioral change intervention, rooted in social cognitive theory, to lower a broad range of bisphenols (BPA, BPS, and BPF) in women with obesity.
Methods
Thirty women with obesity (31.1 ± 5.6 kg/m2, 21.1 ± 3.1 years) were randomly assigned to an intervention or control. The intervention included weekly face-to-face meetings to reduce bisphenol exposures from food, cosmetics, and packaged products. Fasting urinary bisphenols, creatinine, and weight were assessed at study entry and after 3 weeks.
Results
The intervention was evaluated as feasible (100% of enrollment and recruitment, 96% of retention and attendance at lesson plan visits, and 96% of a collection of urine samples). Adherence to the intervention was estimated based on completion of self-monitoring records; the number of daily records completed was 7.7 ± 1.3 (mean ± SD) after week 1, 7.1 ± 1.5 after week 2, and 4.4 ± 0.9 after week 3. In secondary analysis, there was a significant treatment × time effect on creatinine-corrected urinary BPS (− 1.42 μg/g creatinine in the intervention vs. − 0.09 μg/g creatinine in the control group).
Conclusion
In women with obesity, the 3-week intervention was considered feasible with promising preliminary results of decreasing BPS concentrations. These data warrant future large-scale clinical trial interventions to reduce bisphenol exposure and determine whether reductions in bisphenols positively impact diabetes and cardiovascular disease risk markers. This study was retroactively registered at ClinicalTrial.gov Identifier NCT03440307.
Similar content being viewed by others
Background
Non-persistent endocrine-disrupting chemicals, including bisphenol A (BPA) and analogs bisphenol S (BPS) and bisphenol F (BPF), are compounds routinely used in the production of plastics, appearing in the lining of food and beverage containers and several other products commonly used by consumers [1,2,3]. The human exposure to bisphenols is extensive; an analysis of the National Health and Nutrition Examination Survey (NHANES) data showed that 93% of the US population had detectable levels of BPA [4]. Observational data has shown positive associations between urinary BPA concentrations and type-2 diabetes [5], peripheral artery disease [6], metabolic syndrome [7], and obesity [8]. A recent study indicated that BPA exposure may be slightly declining whereas BPS exposure is increasing [9]. However, BPS and BPF are considered unsafe alternatives to BPA [10,11,12,13], highlighted by a recent review reporting BPS and BPF have similar potential endocrine-disrupting actions (high estrogenic and androgenic activity) as BPA [14].
Given the potential relationship between bisphenol exposure and adverse health outcomes, intervention studies targeting diet and education have tried to reduce exposures, with mixed results [15,16,17]. Sathyanarayana et al. observed that a 5-day randomized dietary replacement study actually increased urinary BPA [17], but the food provided to participants was likely contaminated with endocrine disruptors. In contrast, we showed that in women of normal weight, a 3-week intervention targeting BPA exposures from food, cosmetics, and other packaged products significantly (P < 0.05) reduced urinary BPA by 50% (− 1.06 ng/mL) whereas controls increased urinary BPA by 62% (+ 0.85 ng/mL), but BPS and BPF were not assessed [15]. Additionally, we noted a small, albeit significant difference in a 3-week weight (P = 0.03; − 0.28 kg weight loss vs. + 1.65 kg weight gain) in intervention vs. control participants, respectively, in our sample of normal-weight women; this might have been due to self-monitoring of caloric intake that occurred in the intervention group. These and other [16] data suggest that short-term interventions may successfully reduce BPA exposure in women with normal weight. Surprisingly, no published study to date has directly assessed whether a similar intervention can reduce bisphenol exposure in women with obesity. This is of concern because individuals with obesity may have higher urinary concentrations of bisphenols, and women in particular may be at the greatest risk [6, 18, 19]. Additionally, higher concentrations of BPA in women with obesity of reproductive age are associated with obesity, insulin resistance, and polycystic ovary syndrome and may lead to disruption of reproductive function [20, 21]. Thus, efficacious interventions to reduce bisphenol exposure in women with obesity are needed.
Objectives
The primary objective of this study was to develop and test the feasibility of a 3-week behavioral change intervention, rooted in social cognitive theory [22, 23], to lower a broad range of bisphenols (BPA, BPS, and BPF) in women with obesity. The intervention was designed to targeted bisphenol exposures from food, personal hygiene products, cosmetics, and feminine hygiene products.
Secondary objectives were to examine weight changes and explored association with urinary bisphenols.
Methods
Participants
Thirty, healthy, premenopausal women with obesity from the California Polytechnic State University in San Luis Obispo, CA, were recruited on campus (Table 1) and assessed from November 2015 through November 2017. Eligibility included (1) female with obesity (> 30.0 kg/m2 BMI), (2) disease-free and non-smoking as assessed by a health history questionnaire, and (3) self-reported exposure to at least 5 potential dietary sources of bisphenols; for this, women completed a modified version of a 2-day diet practices survey to identify exposure to BPA as previously described [15, 16] and (4) self-reported daily use of at least 13 of 24 (> 50%) non-dietary product sources of BPA, including makeup products (foundation, mascara, eye shadow, etc.), hygiene products (hand sanitizer, face and body lotion, soap, shampoo, etc.), and feminine products (tampons, pads). This method was used successfully in our previous intervention trial where < 10% of participants at study entry had urinary BPA below the lowest detectable level [15]. The Institutional Review Board at California Polytechnic State University approved the study, and all women gave verbal and written consent. This study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Experimental protocol and design of intervention
After eligibility was determined, women reported to the Department of Kinesiology and Public Health at California Polytechnic State University in San Luis Obispo in the morning after an overnight fast (8–10 h). Research assistants blinded to treatment allocation completed all assessments. Weight was measured in duplicate at study entry and after 3 weeks in kilogram by a standard balance beam scale, and height by a stadiometer to the nearest centimeter. Women gave a fasting urine sample at study entry and after 3 weeks. The primary outcome measure was bisphenol (BPA, BPS, BPF) and creatinine concentrations, and the secondary outcome measure was weight.
This study adhered to the CONSORT guidelines for reporting randomized parallel pilot and feasibility studies [24]. At study entry, women were randomly assigned, by a computer-generated program, to the control group or the 3-week intervention group to decrease bisphenol exposure. The study statistician developed the randomization and provided concealed envelopes to study counselors with group assignments at study entry. The counselor did not know of the group assignment until the envelope was opened by the participant. Women in the control group received a weekly email newsletter that provided general information about bisphenol exposure, healthy eating, and beverages and did not receive any information about food or other packaging sources of bisphenol exposure. Women in the intervention group received all aspects of the control group, plus a behavioral intervention designed to decrease bisphenol exposure. The intervention was rooted in social cognitive theory [22, 23] and designed to reduce bisphenol exposure. The intervention promoted self-regulation skills (i.e., planning, self-monitoring, problem solving, goals, self-incentives) as well as positive reinforcement for adherence to behavior change goals and counselor feedback. The intervention included weekly face-to-face meetings with the counselor. At the first meeting, the counselor discussed the negative health consequences of bisphenols and how to avoid exposures through daily changes in diet and personal hygiene; strong emphasis was placed on the consumption of organic foods and tracking and changing intake of packaged foods. Women were encouraged to avoid canned/plastic containing food and beverages. Women were instructed to bring in their plastic containing products including Tupperware, dishware, cosmetics, and hygiene products (e.g., shampoo, condition, lotion), and these were returned after study completion. Participants were provided, free of charge, with replacement bisphenol-free glass Tupperware, water bottles, make-up, hygiene, and feminine products. The make-up, hygiene, and feminine products were packaged in BPA-free plastic containers, glass, and/or cardboard; this information was provided by the manufactures, and we did not directly test bisphenol levels in the packaging. Women were asked to self-monitor type (i.e., organic vs. non-organic) and packaging (plastic, glass, cardboard, other materials) of all food and beverages, but did not record quantity or caloric values of food. At the subsequent face-to-face visits, women were provided feedback on self-monitoring records and encouraged to continue self-monitoring and avoiding bisphenol containing products with the goal of all foods and drinks consumed packaged in bisphenol-free, glass, and cardboard containers or materials.
Urinary analysis
Fifteen milliliters of fasting urine were collected mid-stream in a bisphenol-free sterile specimen container and then aliquoted into 3 separate bisphenol-free polypropylene tubes and stored at − 80 °C until analyzed. All urine samples were assessed in duplicate by gas chromatographic-tandem mass spectrometric (GC-MS/MS) method using isotope dilution quantification using the established CDC protocol [18] and using good laboratory practices as previously described [25, 26]. Bisphenols were not detected in urine collection tubes and storage apparatus. The limit of detection (LOD) was 0.05 μg/L for BPA, BPS, and BPF. Urinary creatinine concentrations were assessed by a colorimetric assay. Urine collection occurred from November 2015 to November 2017, and samples were analyzed in batches no more than 2 months after collection. Samples were sent under strict protocols (overnight shipping on dry ice, 2 unique identifiers, etc.) to the Washington State Public Health Laboratories (CLIA certified) for analysis of bisphenols.
Statistical analyses
The sample size calculation for this study was based on our previous randomized controlled study [15] of 24 normal-weight women that showed that an intervention significantly reduced urinary BPA (− 1.06 ± 2.1 ng/mL) whereas controls increased urinary BPA (+ 0.85 ± 0.74 ng/mL). With 30 women at study entry and assuming 10% non-detectable BPA values at baseline, this study was projected to have > 99% power to detect a ≥ − 1.91 ng/mL difference in urine BPA concentrations between women in the intervention and control groups using a α = 0.05 and a 2-sided t test. Thus, recruitment was stopped at 30 participants. Based on NHANES [27] BPA median data of 2–3 ng/mL, a reduction of − 1.91 ng/mL in urine BPA is equivalent to > 35% decrease from the median.
A commercial software package from SPSS version 24 was used for statistical analysis of data. Summary statistics are reported as mean (SD) for participant characteristics; creatinine-corrected geometric mean, change in creatinine-corrected geometric mean, and 95% confidence interval for urinary BPA, BPS, and BPF concentrations; and mean ± SD for creatinine concentrations and body weight. Non-detectable BPA, BPS, and BPF concentrations were assigned the LOD (0.05 μg/L) [28]. Bisphenol concentrations were not normally distributed and were log-transformed prior to analysis. For secondary objectives, a RMANOVA was used to assess treatment × time effects on weight adjusting for study entry BMI and explored associations between weight and urinary bisphenol concentrations, adjusting for the group. An intent-to-treat approach (baseline data carried forward) was used for any missing data.
Results
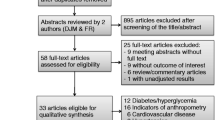
Figure 1 shows the CONSORT flow diagram. Over the course of the study, all women expressing interest in the study were randomized; thus, enrollment and recruitment were 100%. Overall retention was 96%, as one intervention woman was lost to follow-up due to unable to contact, and no women in the control group were lost to follow-up. Adherence to the bisphenol avoidance intervention was estimated based on the completion of self-monitoring records over time. In the intervention group, the number of daily records completed was 7.7 ± 1.3 (mean ± SD) after week 1, 7.1 ± 1.5 after week 2, and 4.4 ± 0.9 after week 3. Based on CONSORT guidelines, there were no harms or unintended effects for each treatment.
Table 2 presents geometric mean and 95% confidence intervals for urinary creatinine-corrected BPA, BPS, and BPF concentrations. Non-detectable levels of bisphenols were observed but did not significantly vary by groups. At study entry, 15 of 30 BPA concentrations (50%) were non-detectable (7 intervention, 8 control), 1 of 30 BPS concentrations (0.04%) was non-detectable, and 13 of 30 BPF concentrations (47%) were non-detectable (6 intervention, 7 control). Among the remaining participants with detectable values, creatinine-corrected BPA and BPF did not significantly differ between groups but differences in BPS were observed, with higher creatinine-corrected BPS concentrations in intervention vs. controls.
In the secondary analysis, using an intent-to-treat approach and adjusted repeated measures analyses, there was a significant treatment × time (P = 0.01) effect on creatinine-corrected urinary BPS concentrations. From study entry to 3 weeks, geometric mean creatinine-corrected urinary BPS concentrations significantly declined by 1.42 μg/g creatinine in the intervention group and were reduced slightly by 0.09 μg/g creatinine in the control group. There were no significant main or treatment × time effects on creatinine-corrected urinary BPA and BPF concentrations between groups. Our analysis assumed a LOD of 0.05 μg/L for each bisphenol, and in post hoc power analysis, the study was not sufficiently powered (32% power for both BPA and BPF) excluding the large amount of non-detectable. Completers in the intervention had − 0.31 μg/g creatinine and − 2.26 μg/g creatinine reduction in BPA and BPF vs. completers in the control had + 0.21 μg/g creatinine and − 0.80 μg/g creatinine in BPA and BPS, but there was no significant difference between groups. Examining urinary creatinine, there was no significant treatment × time effect observed from study entry to 3 weeks in the intervention (980 ± 778 and 1270 ± 891 mg/l, respectively) and control (1637 ± 954 and 1468 ± 1156 mg/l, respectively) groups.
There were no significant main or treatment × time effects on weight; the mean weight change in the intervention was 0.1 ± 1.0 kg and in the control was − 0.2 ± 1.2 kg. Changes in creatinine-corrected BPA, BPS, and BPF were not significantly associated with each other or associated with weight changes.
Discussion
The primary objective of this study was to develop and test the feasibility of a 3-week behavioral intervention to reduce bisphenols. The intervention, rooted in social cognitive theory, included weekly face-to-face meetings to reduce bisphenol exposures from food, cosmetics, and packaged products. The intervention was evaluated as feasible. Recruitment and enrollment were 100%, and overall retention was 96% as one intervention woman was lost to follow-up and no women in the control group were lost to follow-up. Adherence to the bisphenol avoidance intervention, estimated based on completion of self-monitoring records, was high early on and decreased over-time. In the intervention group, the number of daily records completed on average was 7.7 after week 1, 7.1 after week 2, and 4.4 after week 3. Based on these feasibility results, a large scale, clinical trial intervention to reduce bisphenol exposure is warranted.
In the secondary analysis, the 3-week behavioral intervention reduced BPS exposure relative to controls in women with obesity. Geometric mean urinary creatinine-corrected BPS declined by 1.42 μg/g creatinine in the intervention and slightly decreased by 0.09 μg/g creatinine in controls, underscoring the short-term ability of an intervention to decrease urinary bisphenols in college-aged women with obesity. These data need to be interpreted with caution given the relatively small sample size in each group, and the large number of non-detectable BPA and BPF concentrations in both groups.
Prior studies have examined interventions to reduce bisphenol exposure in women of normal weight. One non-randomized study found significant reductions in BPA and phthalates after a 3-day “fresh food” diet [16]. Another 5-day randomized dietary replacement study showed an actual increase in BPA [17], but the food provided to the intervention group was DEHP contaminated and possibly also had BPA contamination. Our previous randomized controlled study showed that a similar 3-week intervention successfully reduced BPA concentrations in women of normal weight, but BPS and BPF were not assessed [15]. Surprisingly, no published study to date has assessed whether a similar intervention may lower bisphenols in women with obesity. The current study adds to the literature suggesting that an intervention may potentially impact BPS in women with obesity.
In the current study, we chose to enroll women with obesity as preliminary evidence suggests that this population is typically exposed to higher bisphenol concentrations compared to women of normal-weight and men [4]. Higher BPA exposure in women with obesity of reproductive age is associated with obesity, insulin resistance, and polycystic ovary syndrome and may lead to disruption of reproductive function [20, 21]. However, the exact mechanism(s) of higher exposure in women with obesity is unclear, although diet and acquisition of canned foods are primary exposure to bisphenols [29]. Future intervention studies are needed to determine whether a similar intervention would be beneficial to others including men, and at-risk groups including diabetics.
BPS and BPF, analogs of BPA with similar chemical structures, are increasingly used as substitutes for BPA in packaging and industry products. In particular, BPS is a stronger acid and more stable than BPA, allowing it to be more resistant to heat and sunlight [30]. Studies have shown that BPS is present in food packaging and foodstuff [31], paper products [32], and a variety of personal care products including body wash, hair care, skin lotion, and shampoo [33]. A recent observational study indicated that BPA exposure in the USA may be slightly declining whereas BPS exposure is increasing [9]. However, BPS and BPF are considered unsafe alternatives to BPA [10,11,12,13], highlighted by a current review reporting BPS and BPF have endocrine-disrupting actions [14]. In particular, animal data suggest that BPS has estrogenic activity, impairs blood functioning and increases cardiovascular risk, and has a stronger effect on reducing testosterone concentrations than BPA [14, 34, 35]. Thus, interventions to reduce a broad range of bisphenols, and not necessarily just BPA, are needed.
We observed no significant intervention changes in BPA and BPF concentrations, which likely reflect the high rate of baseline non-detectable levels of these bisphenols. At baseline, 50% and 47% of participants had non-detectable BPA and BPF, respectively, which is higher than previous observational studies [18]. In post hoc power analysis, this study was insufficiently powered to detect changes in BPA and BPF due to the large non-detects. Unlike the current study, in our previous intervention study in women with normal weight, < 10% of all baseline samples had non-detectable BPA and geometric mean baseline samples were similar to the reference NHANES data [27]. In the current study in women with obesity, the adipose tissue may be acting as a bisphenol storage site, thereby removing bisphenols from circulation (and ultimately urinary excretion), particularly in individuals with obesity [36]. Alternatively, the large non-detectable could reflect a decline in exposure to these bisphenols occurring nationally [9]. To assess bisphenol exposure prior to randomization, we used a survey from prior studies [15, 16] and future research should consider directly assessing urinary bisphenol concentrations during screening to ensure baseline exposure consistent with the general population prior to randomization.
In secondary analyses, the bisphenol intervention had no impact on weight changes, and there was no relationship between changes in weight and bisphenol concentrations. Previously, we showed that a BPA intervention, compared to controls, significantly reduced a 3-week weight gain (− 0.28 kg weight loss vs. 1.65 kg weight gain) [15]. In an effort to increase awareness of BPA-containing food packaging, our previous intervention encouraged self-monitoring of food and calorie intake with daily diaries [37], and this may have unintentionally led to weight changes between groups. In the current study, we modified the intervention and instructed women to record food packing only and women did not record calories; we observed no significant weight changes. However, it is important to note that the current study did not control for many diet variables, which potentially confounds the relationship between bisphenols and weight. Future research is needed to untangle the relationships between bisphenol exposure, dietary changes, and weight status.
There are notable strengths and limitations of the current study. We experimentally tested the feasibility, using a randomized controlled trial consistent with CONSORT guidelines [38], of an intervention to reduce a broad range of bisphenol exposures that included promoting healthy organic foods, and daily self-monitoring of bisphenol exposures and provided product labeled BPA-free in women with obesity. Despite the success of the intervention to reduce BPS exposure, results should be interpreted with caution given the relatively small sample size of this study and the large number of non-detectable BPA and BPF concentrations. Only spot urine samples were collected, which may have been insufficient to reliably estimate a long-term urinary bisphenol exposure as a recent study suggested that multiple samples over several days are needed [39]. Previous studies have shown that urinary BPA concentrations vary daily [40] and may be influenced by recent food intake [41]. To minimize these potential confounds, we took great care in collecting urine samples and used good laboratory practices in our analyses (e.g., assessment of blanks, collection, and storage tubes, etc.). Also, randomization led to higher BPS exposure in the intervention group vs. control, although this was adjusted for statistically. We did not assess whether intervention reductions in bisphenols were related to improvements in cardiometabolic health risk markers (e.g., glucose, insulin, lipid profile). We did not assess the intervention on other non-persistent endocrine-disrupting chemicals with known negative health consequences (e.g., phthalates) [42, 43]. Finally, we recruited a convenient sample of college-aged women with obesity, and it is unclear whether the intervention would have the ability to reduce bisphenol exposure in men and other at-risk groups including diabetics.
Conclusion
In conclusion, the primary objective of this study is to develop and test the feasibility of a 3-week behavioral change intervention to lower bisphenols were met. Specifically, we developed a novel behavioral intervention, rooted in social cognitive theory, with high retention and adherence in college-aged women with obesity. A future large-scale clinical randomized trial in women is currently in development collecting repeat urine samples over multiple days, assessing a broad range of endocrine-disrupting chemicals, and determining whether reducing exposures to endocrine-disrupting chemicals has a positive impact on health outcomes related to the pathogenesis of cardiovascular disease and type 2 diabetes.
Availability of data and materials
Datasets used and/or analyzed during the current study are freely available from the California Polytechnic State University Digital Commons repository (https://digitalcommons.calpoly.edu/kine_fac/133/).
References
Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol. 2007;24(2):139–77.
Kang JH, Kondo F, Katayama Y. Human exposure to bisphenol A. Toxicology. 2006;226(2-3):79–89.
Simoneau C, Valzacchi S, Morkunas V, Van den Eede L. Comparison of migration from polyethersulphone and polycarbonate baby bottles. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28(12):1763–8.
Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116(1):39–44.
Silver MK, O'Neill MS, Sowers MR, Park SK. Urinary bisphenol A and type-2 diabetes in U.S. adults: data from NHANES 2003–2008. PLoS One. 2011;6(10):e26868.
Shankar A, Teppala S, Sabanayagam C. Bisphenol A and peripheral arterial disease: results from the NHANES. Environ Health Perspect. 2012;120(9):1297–300.
Teppala S, Madhavan S, Shankar A. Bisphenol A and metabolic syndrome: results from NHANES. Int J Endocrinol. 2012;2012:598180.
Shankar A, Teppala S, Sabanayagam C. Urinary bisphenol a levels and measures of obesity: results from the national health and nutrition examination survey 2003–2008. ISRN Endocrinol. 2012;2012:965243.
Ye X, Wong LY, Kramer J, Zhou X, Jia T, Calafat AM. Urinary concentrations of bisphenol a and three other bisphenols in convenience samples of U.S. adults during 2000–2014. Environ Sci Technol. 2015;49(19):11834–9.
Macczak A, Cyrkler M, Bukowska B, Michalowicz J. Eryptosis-inducing activity of bisphenol A and its analogs in human red blood cells (in vitro study). J Hazard Mater. 2016;307:328–35.
Kataria A, Levine D, Wertenteil S, Vento S, Xue J, Rajendiran K, Kannan K, Thurman JM, Morrison D, Brody R, et al. Exposure to bisphenols and phthalates and association with oxidant stress, insulin resistance, and endothelial dysfunction in children. Pediatr Res. 2017;81(6):857–64.
Desdoits-Lethimonier C, Lesne L, Gaudriault P, Zalko D, Antignac JP, Deceuninck Y, Platel C, Dejucq-Rainsford N, Mazaud-Guittot S, Jegou B. Parallel assessment of the effects of bisphenol A and several of its analogs on the adult human testis. Hum Reprod. 2017;32(7):1465–73.
Vinas R, Watson CS. Bisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: effects on cell functions. Environ Health Perspect. 2013;121(3):352–8.
Rochester JR, Bolden AL. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect. 2015;123(7):643–50.
Hagobian T, Smouse A, Streeter M, Wurst C, Schaffner A, Phelan S. Randomized intervention trial to decrease bisphenol A urine concentrations in women: pilot study. J Women's Health (Larchmt). 2017;26(2):128–32.
Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, Rizzo J, Nudelman JL, Brody JG. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2011;119(7):914–20.
Sathyanarayana S, Alcedo G, Saelens BE, Zhou C, Dills RL, Yu J, Lanphear B. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. J Expo Sci Environ Epidemiol. 2013;23(4):378–84.
Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113(4):391–5.
Wang T, Li M, Chen B, Xu M, Xu Y, Huang Y, Lu J, Chen Y, Wang W, Li X, et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97(2):E223–7.
Hong SH, Sung YA, Hong YS, Ha E, Jeong K, Chung H, Lee H. Urinary bisphenol A is associated with insulin resistance and obesity in reproductive-aged women. Clin Endocrinol. 2017;86(4):506–12.
Rutkowska AZ, Diamanti-Kandarakis E. Polycystic ovary syndrome and environmental toxins. Fertil Steril. 2016;106(4):948–58.
Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215.
Bandura A. Social Learning Theory. Englewood Cliffs: Prentice-Hall; 1977.
Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, Lancaster GA, group Pc. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016;2:64.
Teeguarden JG, Twaddle NC, Churchwell MI, Doerge DR. Urine and serum biomonitoring of exposure to environmental estrogens I: bisphenol A in pregnant women. Food Chem Toxicol. 2016;92:129–42.
Ye X, Zhou X, Hennings R, Kramer J, Calafat AM. Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: an elusive laboratory challenge. Environ Health Perspect. 2013;121(3):283–6.
Fourth report on human exposure to environmental chemicals, updated tables. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention http://wwwcdcgov/exposurereport/ February, 2015.
Wendelberger J, Campbell K. Nondetect data in environmental investigations. Am Stat Assoc. 1994:1–12.
Lorber M, Schecter A, Paepke O, Shropshire W, Christensen K, Birnbaum L. Exposure assessment of adult intake of bisphenol A (BPA) with emphasis on canned food dietary exposures. Environ Int. 2015;77:55–62.
Wu LH, Zhang XM, Wang F, Gao CJ, Chen D, Palumbo JR, Guo Y, Zeng EY. Occurrence of bisphenol S in the environment and implications for human exposure: a short review. Sci Total Environ. 2018;615:87–98.
Liao C, Kannan K. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J Agric Food Chem. 2013;61(19):4655–62.
Liao C, Kannan K. Widespread occurrence of bisphenol A in paper and paper products: implications for human exposure. Environ Sci Technol. 2011;45(21):9372–9.
Liao C, Kannan K. A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States. Arch Environ Contam Toxicol. 2014;67(1):50–9.
Eladak S, Grisin T, Moison D, Guerquin MJ, N'Tumba-Byn T, Pozzi-Gaudin S, Benachi A, Livera G, Rouiller-Fabre V, Habert R. A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril. 2015;103(1):11–21.
Pal S, Sarkar K, Nath PP, Mondal M, Khatun A, Paul G. Bisphenol S impairs blood functions and induces cardiovascular risks in rats. Toxicol Rep. 2017;4:560–5.
Wang L, Xue J, Kannan K. Widespread occurrence and accumulation of bisphenol A diglycidyl ether (BADGE), bisphenol F diglycidyl ether (BFDGE) and their derivatives in human blood and adipose fat. Environ Sci Technol. 2015;49(5):3150–7.
Teixeira PJ, Carraca EV, Marques MM, Rutter H, Oppert JM, De Bourdeaudhuij I, Lakerveld J, Brug J. Successful behavior change in obesity interventions in adults: a systematic review of self-regulation mediators. BMC Med. 2015;13:84.
Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Obstet Gynecol. 2010;115(5):1063–70.
Morgan MK, Nash M, Barr DB, Starr JM, Scott Clifton M, Sobus JR. Distribution, variability, and predictors of urinary bisphenol A levels in 50 North Carolina adults over a six-week monitoring period. Environ Int. 2017;112:85–99.
Ye X, Wong LY, Bishop AM, Calafat AM. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-hour collections. Environ Health Perspect. 2011;119(7):983–8.
Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113(2):192–200.
Yaghjyan L, Sites S, Ruan Y, Chang SH. Associations of urinary phthalates with body mass index, waist circumference and serum lipids among females: National Health and Nutrition Examination Survey 1999-2004. Int J Obes. 2015;39(6):994–1000.
Singh S, Li SS. Bisphenol A and phthalates exhibit similar toxicogenomics and health effects. Gene. 2012;494(1):85–91.
Acknowledgements
We thank Blaine Rhodes and Caroline West of Washington State Public Health Laboratories for aiding with bisphenol analysis. We thank all the participants in this study.
Endnotes
Not applicable.
Funding
This study was partially supported by the William and Linda Frost Research Fund at Cal Poly.
Author information
Authors and Affiliations
Contributions
TH oversaw all the aspects of the study, developed the study design and methodology, analyzed the data, and wrote the manuscript. ZDP, AM, and MG collected the data and was a contributor in writing the manuscript. AB aided in the data analysis and was a contributor in writing the manuscript. SP aided in the study design, intervention, and analyses and was a major contributor in writing the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board at California Polytechnic State University approved the study, and all women gave verbal and written consent. This study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). This study was retroactively registered at ClinicalTrial.gov Identifier NCT03440307.
Consent for publication
Not applicable.
Competing interests
SP has received a grant from Weight Watchers International. All other authors declare no other actual or potential conflict of interest and no competing financial interests in relation to the work described.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hagobian, T., Delli-Bovi, Z., Mercado, A. et al. Development and feasibility of randomized trial to reduce urinary bisphenols in women with obesity. Pilot Feasibility Stud 7, 24 (2021). https://doi.org/10.1186/s40814-020-00744-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40814-020-00744-5