Abstract
Background
Growing evidence supports that exercise therapy is effective for patellofemoral pain (PFP) rehabilitation. Nevertheless, the improvements have been reported not to be sustained in the long term, suggesting that the current protocols may not comprehend all required functional factors to provide a consistent recovery. A potential neglected factor in treatment protocols for PFP is postural control. However, it is unclear whether this population presents balance impairments or the influence of postural control on pain and function during rehabilitation programmes.
Objective
To investigate whether (Q1) balance is impaired in people with PFP compared to controls, (Q2) conservative interventions are effective to improve balance in people with PFP, and (Q3) balance exercises are effective to improve pain and function in people with PFP.
Data sources
Medline, Embase, CINAHL, SPORTDiscus, Web of Science and Cochrane Library, supplemented by hand searching of reference lists, citations and relevant systematic reviews in the field.
Methods
A systematic review with meta-analysis was conducted according to the Cochrane recommendations and reported according to the PRISMA statement recommendations. We included cross-sectional studies comparing balance between people with and without PFP; and randomised controlled trials verifying the effect of conservative intervention on balance and the effect of balance intervention on pain and function in people with PFP. The risk of bias was assessed using the Epidemiological Appraisal Instrument for cross-sectional studies and the Physiotherapy Evidence Database scale for randomised controlled trials.
Results
From 15,436 records, 57 studies (Q1 = 28, Q2 = 23, Q3 = 14) met the eligibility criteria. Meta-analyses indicated that people with PFP have worse anteroposterior (very low grade evidence, standardised mean difference [SMD] = 1.03, 95% CI 0.40–1.66) and mediolateral (moderate grade evidence, SMD = 0.87, 95% CI 0.31–1.42) balance compared to controls. Moderate grade evidence indicated that overall balance is not affected in people with PFP (SMD = 0.38, 95% CI − 0.05–0.82). Low to very low grade evidence indicates that interventions are ineffective for mediolateral (SMD = 0.01, 95% CI − 0.51–0.53) and overall (SMD = 0.49, 95% CI − 0.14–1.11) balance improvements, and low grade evidence indicates that interventions are effective to improve anteroposterior balance (SMD = 0.64, 95% CI 0.04–1.23). Moderate to low grade evidence indicated that balance interventions are effective to reduce pain (SMD = 0.82, 95% CI 0.26–1.38) and improve function (SMD = 0.44, 95% CI 0.09–0.80) when measured using questionnaires; and very low grade evidence indicated no efficacy for function measured via functional tests (SMD = 0.73, 95% CI − 0.16–1.61).
Conclusion
People with PFP likely present balance deficits compared to asymptomatic people. There was insufficient evidence to support the efficacy of interventions to improve or modify balance in people with PFP. Also, there was insufficient evidence to support the efficacy of balance exercises to improve pain and function in people with PFP.
Trial Registration The present systematic review was registered in PROSPERO (CRD42018091717).
Similar content being viewed by others
Key Points
-
Balance is likely impaired in people with patellofemoral pain compared to asymptomatic people.
-
It is uncertain whether conservative interventions are effective in improving balance in people with patellofemoral pain.
-
The efficacy of exercise programmes that included balance exercise to address pain or function in people with patellofemoral pain is arguable.
Introduction
Patellofemoral pain (PFP) is a frequent disorder in the general population, with an annual prevalence of up to 23% [1]. In the USA, more than two million people were diagnosed with PFP between 2007 and 2011 [2]. This condition has no spontaneous recovery [3, 4] and, therefore, requires treatment [5]. Growing evidence supports that exercise therapy protocols are effective rehabilitation for people with PFP [5,6,7,8]. However, pain and function improvements have been reported not to be sustained in the long term [6, 9, 10]. This indicates that the current protocols may not comprehend all required functional factors to provide a full and consistent recovery for that population.
A potential neglected factor in treatment protocols for PFP is postural control [5, 11,12,13,14]. Postural control involves a complex integration of visual, vestibular and somatosensory systems based on reflex actions occurring to maintain balance [15,16,17]. Considering people with PFP have impaired H-reflex [18, 19], it is reasonable to expect that people with PFP will have alterations in other neuromuscular reflexes which may impact balance. Additionally, the presence of pain in people with PFP may also lead to impairments in postural control [20, 21]. The nociceptive information potentially impairs information from mechanoreceptors [20, 21], and consequently, may delay reflexes and actions required to maintain balance [20,21,22]. Some studies have evaluated balance in people with PFP; however, the respective results are conflicting [23,24,25,26,27]. For example, Saad et al. [27] reported that females with PFP have a greater centre of pressure (CoP) displacement during a stair ascent task compared to asymptomatic females. Contrastingly, Silva et al. [26] reported that females with PFP have decreased CoP displacement during the same task compared to asymptomatic females. Therefore, there is uncertainty regarding balance impairments in the population with PFP. Interestingly, some research investigating the efficacy of interventions for PFP has used balance measures as outcomes, e.g. CoP behaviour during different tasks [28, 29] and Star Excursion Balance Test (SEBT) [30], although it is unclear if people with PFP actually present balance deficits and if balance is a modifiable outcome in this population. Also, some research studies included balance exercises in their protocols [28, 31, 32] and activities challenging the control of centre of gravity inside the base of support [33, 34], and their importance for PFP rehabilitation remains unknown.
Excessive dynamic knee valgus, including excessive movements at the hip [35, 36] and at the ankle [37], during activities is thought to be an important biomechanical factor for PFP due to a potential increase in the lateral force acting on the patella [35, 36, 38]. Although hip and ankle kinematic alterations are not risk factors for PFP development [39], people with PFP have presented excessive hip and ankle movements during activities [11, 40, 41]. As a consequence, interventions targeting hip and ankle joints have been suggested and reported to be effective for PFP rehabilitation, such as hip muscle strengthening [6] and foot orthoses [42]. Interestingly, adjustment movements at the ankle and hip, also called “ankle strategy” and “hip strategy”, are adopted to keep the centre of gravity close to the support base in order to maintain balance in asymptomatic people [43, 44]. Perhaps, excessive hip and ankle movements observed in people with PFP [11] may be compensations for potential impairments in postural control, and therefore, interventions targeting balance could also be beneficial for people with PFP. However, little is known about the addition of balance exercises in protocols for PFP treatment.
To understand the importance of postural control for the management of PFP, this systematic review aimed to answer three questions: (Q1) Is balance impaired in people with PFP compared to asymptomatic people? (Q2) Are conservative interventions effective to improve potential balance impairments in people with PFP? (Q3) Are balance exercises effective to improve pain and function in people with PFP?
Methods
Design
The review was conducted according to the Cochrane recommendations [45], reported according to PRISMA statement recommendations [46] and a priori registered at PROSPERO (CRD42018091717).
Deviation from Protocol
We assessed the evidence quality using GRADE (Grading of Recommendations Assessment, Development and Evaluation), in order to perform a broader assessment of evidence quality [47, 48], instead of a modified version of the van Tulder criteria. [49]. Searches in the Cochrane library were also included.
Eligibility Criteria
-
Q1: studies were included if (i) investigation was conducted with people with PFP and asymptomatic people; (ii) evaluated balance impairments in people with PFP compared to asymptomatic people using any instrument or tool; and (iii) used a cross-sectional design or other design that permitted cross-sectional data for PFP and control groups to be extracted. We considered as balance evaluations those assessing postural stability during standing (static balance) or performing activities (dynamic balance) by using spatial–temporal measures of sway, i.e. assessments related to the centre of gravity behaviour, including displacement, velocity, area, etc., via force platform or computerised posturography; or using clinical tests, e.g. SEBT [50].
-
Q2: studies were included if (i) investigation was conducted with people with PFP; (ii) investigated the effect of any conservative intervention for PFP; (iii) compared the experimental intervention to any alternative, control or no intervention; (iv) outcomes included balance assessed using any instrument; and (v) used a randomised controlled trial design, including crossover design. Conservative interventions were defined as any non-pharmacological and/or non-surgical interventions, including (but not limited to) exercise therapy, taping or braces [51].
-
Q3: studies were included if (i) investigation was conducted with people with PFP; (ii) investigated the effect of balance exercises or programmes which include balance exercises targeting people with PFP; (iii) compared the experimental intervention to any intervention without balance exercises; (iv) evaluated intervention effects on pain and/or physical function using patient-reported outcome measures, e.g. visual analogue scale or questionnaires, or applying clinical tests, e.g. hop tests; and (v) used a randomised controlled trial design. Balance exercises were defined as activities which induce difficulties in controlling an adequate alignment between the centre of gravity and the base of support with the aim of improving postural control [22, 33, 34, 52]. These activities include exercises that reduce the base of support, e.g. single-legged stance; or challenge the control of the centre of gravity, e.g. exercises performed on unstable surfaces or with participants closing their eyes [22, 33, 34, 52]. We included studies that clearly described the presence of balance exercises or when it was possible to identify exercises that were specifically prescribed to improve postural control. Activities in which balance is a potential component, such as single-legged squat or landing tasks, were considered balance exercise if the study clearly stated that the exercise targeted postural control. These tasks may target different aspects of functionality, such as movement control, muscle capacity or impact absorption, and participants could be allowed to make use of varied external support elements to remain balanced, and therefore, the exercise would not target balance improvements.
We considered as PFP those participants who were clinically diagnosed with at least the presence of retropatellar or peripatellar pain, not related to traumatic events, which was aggravated during activities that overload the patellofemoral joint [13]. Protocols, reviews, letters, academic theses, congress abstracts, and case series studies were excluded. Only English-language publications were considered. No restriction on publication period was adopted.
Search Strategy
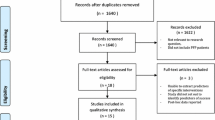
Electronic searches were conducted in six databases: Medline via OVID, Embase via Elsevier, CINAHL and SPORTDiscus via EBSCO, Web of Science, and Cochrane Library from inception to August 2022. Terms related to “patellofemoral pain” and “balance” (indexed and free-text terms) were used to prepare the search strategy (Additional file 1). Reference lists of included articles, citation lists of included articles using Google Scholar, and the included studies of relevant systematic reviews [5, 7, 8, 53] were screened for eligibility (Fig. 1).
Study Selection
First, duplicates were identified and excluded using the reference software Endnote X9 (Clarivate Analytics, Philadelphia, USA). Then, the records were exported to the software Rayyan QCRI (Qatar Computing Research Institute, Doha, Qatar) [54] which was used for the screening process. The selection was performed by two independent reviewers by titles, then by abstracts and lastly by full text (Fig. 1). The reviewers presented substantial agreement regarding the study eligibility (kappa = 0.79, based on a pilot screening with 543 records) [45, 55]. At all steps, disagreements between reviewers were resolved by consulting a third reviewer.
Data Extraction
Data extraction was performed by two independent reviewers, and disagreements were resolved by consensus. Data extracted were: study characteristics (design, study duration, aims, sample size, funding source and settings), participant characteristics based on the REPORT-PFP Checklist (sex, age, symptoms duration, pain severity, weight, height, body mass index and recruitment settings) [56], outcomes (balance measures for Q1 and Q2; pain and function measures for Q3), analysis (data on central tendency and dispersion), and intervention characteristics (description for Q2 and Q3). Where data were missing, incompletely or unclearly reported, we contacted authors for clarification or to request the missing data. The data from two studies [57, 58] were extracted from graphs using the web-based programme WebPlotDigitizer [59, 60], as the respective authors did not respond to our request. Two studies presented the data on central tendency as median [61, 62] and one study presented the dispersion as quartiles [62]. For these cases, mean and standard deviation were estimated using the Box-Cox method [63].
Risk of Bias Assessment
The risk of bias (RoB) was assessed by two independent reviewers, and disagreements were resolved by a third reviewer. For cross-sectional studies (Q1), 24 relevant questions from the Epidemiological Appraisal Instrument (EAI) were used [64]. The questions were selected according to our aims and relevancy for cross-sectional design [65, 66]. Each question was scored as “yes” (2 points), “partial” (1 point), “no” (0 point), or “unable to determine” (0 point). Each study received a final score calculated by dividing its total score (from 0 to 48) by 24 (total number of questions), and final scores higher than one point were considered as studies with low RoB [65, 66]. For randomised controlled trials (Q2 and Q3), specific criteria from the Physiotherapy Evidence Database scale (PEDro) were used [67]: adequacy of randomisation, allocation concealment, between-group baseline comparability, blinding of assessors, adequate follow-up, and intention-to-treat analysis. Each question was scored as “yes” (1 point) or “no” (0 point). The sum of all criteria was used in the analysis, and studies were classified as having a low RoB (≥ 5 points), moderate RoB (3–4 points) or high RoB (≤ 2 points) [67].
Statistical Analysis
Meta-analyses for all questions were carried out using sample size, mean and standard deviation for the outcomes analysed. Only continuous data were used. Balance measures indicating postural stability indices were pooled accordingly to the direction/axis of body movement, i.e. anteroposterior, mediolateral, posteromedial, posterolateral and overall postural stability, which were measures without a defined direction, e.g. CoP area, or a composition of all measured directions, e.g. SEBT index [68,69,70]. Additionally, balance measures were divided into postural stability indices related to displacement, e.g. CoP area, CoP displacement or SEBT; and related to velocity, i.e. CoP velocity [68,69,70]. For Q2 and Q3, end-of treatment results were applied in meta-analysis. For meta-analyses related to Q3, results from studies that included more than one group with balance exercises were combined [71].
Analyses were conducted with R statistical software 4.1.1 (package meta), using random-effects modes estimated via the DerSimonian and Laird method [45]. Results are presented as standardised mean differences (SMD) with a 95% confidence interval (CI) (Hedges’ g) due to differences in instruments and/or units of measure. Pooled SMDs were categorised as trivial (< 0.2), small (≥ 0.2 to < 0.5), moderate (≥ 0.5 to < 0.8), large (≥ 0.8 to < 1.20) and very large (≥ 1.2) [72].
The statistical heterogeneity of the meta-analyses was assessed using the Higgins’ I2 measure, and analyses presenting I2 > 50% were considered as high heterogeneity [71]. Some reports suggest that meta-analysis with high heterogeneity should be omitted because the high variability among the included studies could compromise the reliability and clinical applicability [73, 74]. However, there is no consensus regarding the limit of acceptable heterogeneity for meaningful meta-analysis, and omitting meta-analysis results or excluding studies to reach homogeneity could prevent understanding of the real state of the literature [74,75,76]. Therefore, we reported meta-analyses with high heterogeneity and explored possible sources of heterogeneity by performing subgroup and meta-regression analyses for the meta-analyses including more than 10 studies [45].
For Q1, subgroup analyses included sex (females and male/female combined), assessment method, and task (static or dynamic); and meta-regression analysis included age as a potential moderator. For Q2, subgroup analyses included intervention characteristics (passive or exercise), comparator type (sham/no-intervention or exercise), design (parallel or crossover) and intervention characteristics (intervention targeting balance or non-specific for balance); and meta-regression analyses included age and treatment duration (weeks) as potential moderators. For Q3, subgroup analyses included comparator type (sham/no-intervention or exercise), and study aim (effect of balance or not specific for balance effects); and meta-regression analyses included age and treatment duration (weeks) as potential moderators.
The test proposed by Egger [45, 77] was used to evaluate the presence of publication bias for the meta-analyses including more than 10 studies [45]. When publication bias was detected (Egger’s test p ≤ 0.05), two sensitivity analyses were conducted to verify the impact of this bias: (i) trim-and-fill analyses in which effect sizes are imputed to balance the influence of small-study effects until funnel plot symmetry is reached [78]; and (ii) considering the limitations of the trim-and-fill approach [79,80,81], we also performed analyses by removing the outliers detected in the trim-and-fill analysis [82, 83].
Level of Evidence
The level of evidence was assessed by two independent reviewers using the GRADE tool, and disagreements were resolved by a third reviewer. The level of GRADE evidence was downgraded if meta-analysis: (i) included > 25% of studies with high RoB (1 level) or only studies with high RoB (2 levels); (ii) were heterogeneous as assessed by I2 (> 50%) (1 level); (iii) did not include direct evidence related to the main questions, i.e. generalisation (1 level); (iv) included less than 100 participants per group (1 level); and (v) presented publication bias according to Egger’s test (p ≤ 0.05) (1 level) [47, 48, 84]. For analysis with less than 10 studies, publication bias was not considered [47, 48]. The evidence quality was classified as high (no downgraded level), moderate (downgraded 1 level), low (downgraded 2), or very low (downgraded ≥ 3 levels) [47, 48].
Results
Study Selection
From 15,436 records, 57 studies were included, of which 28 papers met the eligibility criteria for Q1, 23 papers for Q2 and 14 papers for Q3 (Fig. 1). Excluded studies during full-text screening are presented in Additional file 2 along with the reasons for exclusion. Meta-analyses for Q1 included 26 papers, for Q2 included 15 papers and for Q3 included 14 papers. Funnel plots are presented in Additional file 3, and detailed GRADE scores are presented in Additional file 4.
Question 1: PFP Versus Control (Asymptomatic) for Balance Measures
-
Study characteristics: From 28 included studies, 679 people with PFP and 616 people without PFP were evaluated regarding balance performance (Additional file 5). Posturography (single- and double-legged stance), CoP behaviour, and SEBT-related tasks were used to assess balance performance; CoP behaviour was assessed during single-legged squat, step tasks, seated position, single-legged landing, single- and double-legged stance (Additional file 5).
-
Risk of bias: The mean EIA score was 1.2 (0.3), with 75% of included studies presenting low RoB (n = 21), and 25% presenting high RoB (n = 7) (Additional file 6).
-
Anteroposterior (AP) postural stability (19 studies [24, 25, 30, 85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100]): Very low level evidence indicated that people with PFP present worse AP balance with a large effect compared to controls (SMD 1.03, 95% CI 0.40–1.66; Fig. 2 and Table 1). Publication bias was detected (Egger’s test p = 0.018), and the sensitivity analyses indicated different results. Trim-and-fill analysis indicated a moderate and non-significant effect (low-level evidence, SMD 0.64, 95% CI − 0.23–1.52; Table 1) and by removing the outliers [96, 97], the analysis indicated a moderate and significant effect for worse balance in people with PFP (moderate level evidence, SMD 0.65, 95% CI 0.30–0.99; Table 1) (Additional file 3). Subgroup analyses indicated that the assessment method is a potential source of heterogeneity (Additional file 7). Meta-regression indicated that age is not a source of heterogeneity (Additional file 7).
-
Mediolateral (ML) postural stability (13 studies [24, 29, 58, 86,87,88,89,90,91,92, 96, 99, 100]): Moderate level evidence indicated that people with PFP present worse ML balance with a large effect compared to controls (SMD 0.87, 95% CI 0.31–1.42; Fig. 3 and Table 1). The Egger’s test was not significant for publication bias (p = 0.059); however, as we found a marginal p value, we performed the sensitivity analyses. Trim-and-fill analysis (moderate level evidence, SMD 0.70, 95% CI 0.23–1.37; Table 1) and by removing the outlier [96] (moderate level evidence, SMD 0.69, 95% CI 0.23–1.16; Table 1) also indicated a moderate effect for worse balance in people with PFP (Additional file 3). Subgroup and meta-regression analyses did not indicate potential sources of heterogeneity (Additional file 7).
-
Overall postural stability (15 studies [24, 26, 27, 57, 86,87,88,89,90, 92, 95, 98, 99, 101, 102]): Moderate level evidence indicated that there is no difference between people with and without PFP for overall balance (SMD 0.38, 95% CI − 0.05–0.82; Fig. 4 and Table 1). Publication bias was not detected (Egger’s test p = 0.813, Additional file 3). Subgroup analyses indicated that the sex is a potential source of heterogeneity (Additional file 7). Meta-regression indicated that age is not a source of heterogeneity (Additional file 7).
-
Posteromedial (PM) postural stability (4 studies [85, 95, 96, 98]): Moderate level evidence indicated a very large and non-significant effect for lower reach during SEBT PM in people with PFP (SMD 1.22, 95% CI − 0.59–3.02; Fig. 5 and Table 1).
-
Posterolateral (PL) postural stability (4 studies [85, 95, 96, 98]): Moderate level evidence indicated a large and non-significant effect for lower reach during SEBT PL in people with PFP (SMD 1.06, 95% CI − 0.54–2.66; Fig. 6 and Table 1).
-
AP CoP Velocity (6 studies [58, 87, 88, 91, 97, 100]): Moderate level evidence indicated that there is no difference between people with and without PFP for AP CoP velocity (SMD 0.28, 95% CI − 0.07–0.64; Fig. 7 and Table 1).
-
ML CoP Velocity (6 studies [58, 87, 88, 91, 95, 100]): Moderate level evidence indicated a moderate and non-significant effect for greater ML CoP velocity in people with PFP compared to controls (SMD 0.67, 95% CI − 0.20–1.55; Fig. 8 and Table 1).
-
Overall CoP Velocity (4 studies [24, 58, 87, 92]): Moderate level evidence indicated that people with PFP present greater overall CoP velocity with a very large effect compared to controls (SMD 1.24, 95% CI 0.33–2.15; Fig. 9 and Table 1).
-
Influence of vision: Felicio et al. [88] was the only study that exclusively assessed their participants with eyes closed (AP, ML and overall balance) and a sensitivity analysis was performed to verify the impact of pooling assessments performed with open and closed eyes for all meta-analyses. We concluded that the inclusion of the Felicio et al. [88] study did not impact the results.
-
Studies not included in meta-analysis: Naserpour et al. [103] was the only study that evaluated the time to CoP stabilisation and reported statistical difference between groups (PFP group took a longer time to stabilise); and Stensdotter et al. [104] did not report their data in detail (Additional file 8).
Question 2: Interventions Targeting Balance Improvements for People with PFP
-
Study characteristics: The studies of Maryan et al. [105] and Maryan et al. [106] reported identical data and were considered as one study for analyses. From 23 included studies, 755 people with PFP were included (Additional file 5). SEBT-related tasks, posturography (single- and double-legged stance) and CoP behaviour were applied to assess balance performance. CoP behaviour was assessed during single-legged squat, step tasks, seated position and single-legged stance. Interventions included neurofeedback, taping, exercises, braces, manual therapy, dry needling, and virtual reality (Additional file 5).
-
Risk of bias: The mean PEDro score was 2.9 (1.4), with 50% presenting high RoB (n = 11), 36% moderate RoB (n = 8), and 14% presenting low RoB (n = 3) (Additional file 6).
-
AP postural stability (11 studies [28, 30, 62, 94, 107,108,109,110,111,112,113]): low-level evidence indicated that interventions are moderately effective to improve AP balance compared to control interventions (SMD 0.59, 95% CI 0.04–1.14, Fig. 10 and Table 2). Publication bias was not detected (Egger’s test p = 0.681, Additional file 3). Subgroup analyses indicated that the type of comparator, type of experimental intervention, and design are potential sources of heterogeneity (Additional file 7). Meta-regression indicated that age and treatment duration are not sources of heterogeneity (Additional file 7).
-
ML postural stability (4 studies [28, 29, 109, 111]): very low level evidence indicated that interventions are not effective to improve ML balance compared to control interventions (SMD 0.01, 95% CI − 0.51–0.53, Fig. 11 and Table 2).
-
Overall postural stability (7 studies [28, 107, 109, 111, 114,115,116]): low-level evidence indicated a non-significant small effect in favour of interventions compared to control interventions (SMD 0.49, 95% CI − 0.14–1.11, Fig. 12 and Table 2).
-
PM postural stability (5 studies [62, 107, 108, 110, 113]): moderate level evidence indicated that interventions lead to small improvement for SEBT PM (SMD 0.37, 95% CI 0.08–0.65) compared to control interventions (Fig. 13 and Table 2).
-
PL postural stability (5 studies [62, 107, 108, 110, 113]): moderate level evidence indicated that interventions lead to small improvement for SEBT PL (SMD 0.31, 95% CI 0.02–0.59) compared to control interventions (Fig. 14 and Table 2).
-
Studies not included in meta-analysis: Loudon et al. [31] was the only study that evaluated intervention effects using the balance and reach test, and reported that exercise is able to improve balance in people with PFP; Miller et al. [117], and Maryam et al. [105, 106] did not report their data in detail; and the studies by Demirci et al. [118], Ojaghi et al. [119], Sinaei et al. [120] and Fang et al. [121] compared two types of experimental interventions without a control condition (Additional file 8).
Question 3: Balance Interventions Targeting Pain and Function for People with PFP
-
Study characteristics: Of 14 included studies, 907 people with PFP were included (Additional file 5). Visual analogue scale (VAS), numeric pain rating scale (NPRS), and Knee injury and Osteoarthritis Outcome Score (KOOS) subscale pain were used to assess pain level. Anterior Knee Pain Scale (AKPS), Knee Outcome Survey—Activities of Daily Living Scale (KOS-ADLS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), KOOS subscales and functional tests were used to assess functional status. A description of balance interventions is presented in Additional file 5.
-
Risk of bias: The mean PEDro score was 3.6 (1.8), with 50% presenting low RoB (n = 7), 14% moderate RoB (n = 2), and 36% presenting high RoB (n = 5) (Additional file 6).
-
Pain data (14 studies [28, 31, 32, 61, 107, 113, 114, 122,123,124,125,126,127,128]): low-level evidence indicated that balance interventions were largely effective to improve pain compared to non-balance interventions (SMD 0.82, 95% CI 0.30–1.33, Fig. 15 and Table 3). The Egger’s test was not significant for publication bias (p = 0.054); however, as we found a marginal p value, we performed the sensitivity analyses which showed different results. Trim-and-fill analysis indicated that balance interventions have no effect on pain (low-level evidence, SMD 0.38, 95% CI − 0.28–1.03; Table 3) and the analysis by removing the outliers [61, 125, 128] indicated that balance interventions have a small effect in improving pain in people with PFP compared to non-balance interventions (low-level evidence, SMD 0.40, 95% CI 0.04–0.76; Table 3) (Additional file 3). Subgroup and meta-regression analyses did not indicate possible sources of heterogeneity (Additional file 7).
-
Function data: considering patient-reported outcome measures (10 studies [28, 31, 32, 61, 113, 122,123,124, 126, 127]), moderate level evidence indicated that balance interventions have a small effect in improving function compared to non-balance interventions (SMD 0.45, 95% CI 0.13–0.78, Fig. 16 and Table 3). Subgroup and meta-regression analyses did not indicate possible sources of heterogeneity (Additional file 7). Considering functional tests (5 studies [28, 31, 113, 125, 126]), very low level evidence indicated that balance interventions have a moderate and non-significant effect in improving function compared to non-balance interventions (SMD 0.67, 95% CI − 0.04–1.38, Fig. 17 and Table 3).
Discussion
PFP Versus Control (Asymptomatic) on Balance (Q1)
The cross-sectional data revealed ambiguous results because most meta-analyses reported non-significant results, but the meta-analyses with more included studies reported impairments in the balance of people with PFP. Most meta-analyses (six out of eight) reported non-significant results based on moderate-level evidence, indicating that balance is not impaired in people with PFP. However, the non-significant results raise questions regarding the actual presence of balance impairments in the population with PFP. The non-significant results for SEBT PM and PL, and ML CoP velocity were obtained from meta-analyses with few included studies (n = 4–6), with magnitude effects varying from moderate to very large for worse balance in people with PFP, which does not the support a consistent conclusion.
Considering the results from the three meta-analyses with more studies included (n = 13–19 studies), there are some circumstances which require attention for the interpretation. The results for ML postural stability reported more coherent findings that people with PFP have impairments in ML balance based on moderate-level evidence. The results for AP postural stability are based on very low level evidence and seem to be highly influenced by the small-study effect, suggesting that publication bias interfered with the results. Although the trim-and-fill analysis reported non-significant results, in the analysis by removing the outliers, we could observe a significant result as the original analysis (moderate effect), and the level of evidence increased to moderate. The findings for overall postural stability seem to be highly affected by one study which likely influenced the meta-analysis towards a non-significant result. Although the publication bias analysis did not indicate outliers, only one study presented results suggesting that people with PFP have less overall balance sway [26] with the other 18 studies presenting that people with PFP have a worse overall balance or no difference compared to controls (Fig. 4). Therefore, based on the present results, we cannot affirm that people with PFP have impairments in balance compared to asymptomatic people. However, there are some findings suggesting that balance is a physical factor likely altered in people with PFP. Our results show the need for more high-quality with large sample size investigations in order to confirm whether balance impairments are present in the PFP population.
Different aspects affecting people with PFP may compromise postural control, such as impairments in central control [129], proprioception [130], muscle activity [131] or muscle capacity [65, 132]. Previous studies reported that postural control in people with PFP is correlated with knee muscle strength [23], hip muscle strength [87, 100] and knee proprioception [98]. In contrast, studies reported controversial results on the correlation between postural control and pain [23, 101, 102]. Nevertheless, little is known about the cause–consequence relationship between PFP and postural control. A recent study indicated that balance impairments could be a risk factor for PFP development [133]. Contrarily, some experimental research reported that induced knee pain impairs balance and quadriceps coordination [134, 135]. Therefore, a better understanding of how balance is affected as PFP develops could help clinicians in their decisions regarding possible interventions aiming at treating or preventing PFP [136].
Interventions Addressing Balance in People with PFP (Q2)
This is the first review pooling information on balance from people with PFP and interestingly, 23 randomised controlled trials used balance measures as an outcome to determine the efficacy of their interventions. Based on the meta-analyses for Q2, we cannot affirm that balance is a modifiable outcome in people with PFP. The results present a number of issues which makes the conclusion fragile. The positive results for improvements on AP, PM and PL balance include a confidence interval which does not warrant concluding a clinical significance of these interventions [137]. Additionally, the AP balance result was likely influenced by a study with a very large effect in favour of the experimental intervention (SMD = 3.26) [112]. The non-significant results for ML and overall balance and the low to very low level evidence provide further information to question the effectiveness of interventions to improve balance in people with PFP.
We can affirm that the inconclusive results are related to the diversity of interventions, including neurofeedback [111], taping [30, 94, 105, 106, 109, 112, 115, 117,118,119,120], exercises [28, 31, 62, 107, 114, 116, 121], braces [29], manual therapy [108, 117, 118], dry needling [110] and virtual reality [113]. Few studies included exercises addressing specifically balance deficits [28, 31, 107, 114]. The study by Steinberg et al. [107] included single-legged ballet-related exercises, and the study by Foroughi et al. [28] included an exercise in which the participants were required to maintain balance on an unstable seat apparatus; and the subgroup analysis including these two studies showed a large effect in favour of the interventions on AP balance (very low level evidence). The study by Mahmoud and Kamel [114] included a progressive balance exercise programme and presented a very large effect in favour of interventions in improving overall balance (study with moderate RoB). The study by Loudon et al. [31] included single-legged stance and reach tasks and reported improvements in the balance and reach test (study with high RoB). Therefore, we may infer that balance is a potential modifiable factor in people with PFP, but the lack of specific and high-quality studies does not allow a clear conclusion. Further investigation is needed to ascertain whether interventions are effective to improve balance in people with PFP.
Balance Interventions on Pain and Function in People with PFP (Q3)
The results for Q3 suggest that interventions which included balance exercises are not clearly effective for function improvement or pain reduction. The effect on function measured using patient-reported outcome measures was small and included a confidence interval which does not justify concluding clinical relevance [137]. Additionally, no significant effect was observed for function measured using functional tests. For pain reduction, the meta-analysis reported a large effect in favour of interventions including balance exercises, based on low-level evidence and including a confidence interval with the lower limit that most clinicians and researchers would be considered to be not clinically significant [137].
The literature reports strong evidence supporting the effectiveness of some interventions addressing pain and function in people with PFP, such as hip- and knee-targeted exercise therapy [7, 12]. Comparing the evidence level of previous results and our findings, we could suggest that balance exercises are less essential for PFP rehabilitation. Nonetheless, beyond the fact that subgroup analyses were performed for heterogeneity investigation, their results suggest the importance of multimodal exercise programmes, including balance exercises as one component to reduce pain in people with PFP. Multimodal programmes that included balance exercises moderately reduced pain compared to control interventions without balance exercises. Additionally, the subgroup analysis pooling studies that specifically aimed to verify the effects of balance reported a very large effect in favour of interventions. However, we should consider these results with caution; if the pain explains impairments in balance, targeting balance on its own might not be relevant. Therefore, the inconclusive results about the effects of balance intervention on pain and function do not justify clinical application, but the results encourage further investigations in the field.
Limitations
An important limitation of the findings is the heterogeneity which was present in all meta-analyses. It suggests that the diversity in participants’ characteristics, interventions or methodological aspects exceeds the diversity expected by chance and likely influences the results [45]. The subgroup analysis indicated some potential factors which may explain the heterogeneity, such as the assessment method and sex for Q1; and the type of comparator, the design and specificity of interventions for Q2. Even with these factors, we could not conclude which factors strongly influence the statistical heterogeneity. Therefore, other factors should be explored, such as pain [20, 21] and body mass index [138, 139] which may have an important role in moderating postural control. The study by Yelvar et al. [102] reported that pain and body mass index are moderately correlated with postural control in people with PFP. We intended to perform meta-regression analyses to verify whether pain and body mass index could explain the heterogeneity; however, the included studies poorly reported these variables (Additional file 5) which prevented such analysis. We may speculate that the heterogeneity reflects the multifactorial aspect of PFP, along with a possible high level of heterogeneity in many characteristics among this population that could affect results. Additionally, other systematic reviews investigating the influence of balance on different populations, such as older people [34, 140] and people with chronic ankle instability [141, 142], also reported heterogeneity in their results, which suggest that heterogeneity may be inherent in this topic. Nevertheless, the high heterogeneity of the present findings is important and should be considered for the interpretation of the results. Another limitation is that some results are based on low or very low level evidence which compromises the trustworthiness of the reported effects. Also, for some meta-analyses, we could not assess the presence of publication bias due to the number of included studies. Therefore, the present results should be interpreted with caution and additional studies with low RoB and homogeneous data may change our conclusions.
Conclusions
People with PFP likely present balance impairments compared to asymptomatic people. There was insufficient evidence to support the efficacy of interventions to improve or modify balance in people with PFP. Also, there was insufficient evidence to support the efficacy of balance exercises to improve pain and function in people with PFP.
Availability of Data and Materials
The data sets that support the findings of this study are presented in the supplementary materials and are also available upon request.
Abbreviations
- AP:
-
Anteroposterior
- CI:
-
Confidence interval
- CoP:
-
Centre of pressure
- EIA:
-
Epidemiological appraisal instrument
- GRADE:
-
Grading of recommendations assessment, development and evaluation
- ML:
-
Mediolateral
- PEDro:
-
Physiotherapy evidence database
- PFP:
-
Patellofemoral pain
- PL:
-
Posterolateral
- PM:
-
Posteromedial
- RoB:
-
Risk of bias
- SEBT:
-
Star excursion balance test
- SMD:
-
Standardised mean differences
References
Smith BE, Selfe J, Thacker D, Hendrick P, Bateman M, Moffatt F, et al. Incidence and prevalence of patellofemoral pain: a systematic review and meta-analysis. PLoS ONE. 2018;13: e0190892. https://doi.org/10.1371/journal.pone.0190892.
Glaviano NR, Kew M, Hart JM, Saliba S. Demographic and epidemiological trends in patellofemoral pain. Int J Sports Phys Ther. 2015;10:281–90.
Rathleff MS, Rathleff CR, Olesen JL, Rasmussen S, Roos EM. Is knee pain during adolescence a self-limiting condition? Prognosis of patellofemoral pain and other types of knee pain. Am J Sports Med. 2016;44:1165–71. https://doi.org/10.1177/0363546515622456.
van Middelkoop M, van der Heijden RA, Bierma-Zeinstra SMA. Characteristics and outcome of patellofemoral pain in adolescents: Do they differ from adults? J Orthop Sports Phys Ther. 2017;47:801–5. https://doi.org/10.2519/jospt.2017.7326.
van der Heijden RA, Lankhorst NE, van Linschoten R, Bierma-Zeinstra SM, van Middelkoop M. Exercise for treating patellofemoral pain syndrome. Cochrane Database Syst Rev. 2015;1:CD010387. https://doi.org/10.1002/14651858.CD010387.pub2.
Lack S, Barton C, Sohan O, Crossley K, Morrissey D. Proximal muscle rehabilitation is effective for patellofemoral pain: a systematic review with meta-analysis. Br J Sports Med. 2015;49:1365–76. https://doi.org/10.1136/bjsports-2015-094723.
Winters M, Holden S, Lura CB, Welton NJ, Caldwell DM, Vicenzino BT, et al. Comparative effectiveness of treatments for patellofemoral pain: a living systematic review with network meta-analysis. Br J Sports Med. 2021;55:369–77. https://doi.org/10.1136/bjsports-2020-102819.
Dischiavi SL, Wright AA, Tarara DT, Bleakley CM. Do exercises for patellofemoral pain reflect common injury mechanisms? A systematic review. J Sci Med Sport Elsevier. 2021;24:229–40. https://doi.org/10.1016/j.jsams.2020.09.001.
Collins NJ, Bierma-Zeinstra SMA, Crossley KM, van Linschoten RL, Vicenzino B, van Middelkoop M. Prognostic factors for patellofemoral pain: a multicentre observational analysis. Br J Sports Med. 2013;47:227–33. https://doi.org/10.1136/bjsports-2012-091696.
Lankhorst NE, van Middelkoop M, Crossley KM, Bierma-Zeinstra SMA, Oei EHG, Vicenzino B, et al. Factors that predict a poor outcome 5–8 years after the diagnosis of patellofemoral pain: a multicentre observational analysis. Br J Sports Med. 2016;50:881–6. https://doi.org/10.1136/bjsports-2015-094664.
Powers CM, Witvrouw E, Davis IS, Crossley KM. Evidence-based framework for a pathomechanical model of patellofemoral pain: 2017 patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester, UK: part 3. Br J Sports Med. 2017;51:1713–23. https://doi.org/10.1136/bjsports-2017-098717.
Collins NJ, Barton CJ, van Middelkoop M, Callaghan MJ, Rathleff MS, Vicenzino BT, et al. 2018 Consensus statement on exercise therapy and physical interventions (orthoses, taping and manual therapy) to treat patellofemoral pain: recommendations from the 5th International Patellofemoral Pain Research Retreat, Gold Coast, Australia, 2017. Br J Sports Med. 2018;52:1170–8. https://doi.org/10.1136/bjsports-2018-099397.
Crossley KM, Stefanik JJ, Selfe J, Collins NJ, Davis IS, Powers CM, et al. Patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester. Part 1: Terminology, definitions, clinical examination, natural history, patellofemoral osteoarthritis and patient-reported outcome measures. Br J Sports Med. 2016. https://doi.org/10.1136/bjsports-2016-096384.
Witvrouw E, Callaghan MJ, Stefanik JJ, Noehren B, Bazett-Jones DM, Willson JD, et al. Patellofemoral pain: consensus statement from the 3rd International Patellofemoral Pain Research Retreat held in Vancouver, September 2013. Br J Sports Med. 2014;48:411–4. https://doi.org/10.1136/bjsports-2014-093450.
Creath R, Kiemel T, Horak F, Jeka JJ. The role of vestibular and somatosensory systems in intersegmental control of upright stance. J Vestib Res Equilib Orientat. 2008;18:39–49.
Fukuoka Y, Nagata T, Ishida A, Minamitani H. Characteristics of somatosensory feedback in postural control during standing. IEEE Trans Neural Syst Rehabil Eng. 2001;9:145–53. https://doi.org/10.1109/7333.928574.
Winter D. Human balance and posture control during standing and walking. Gait Posture. 1995;3:193–214. https://doi.org/10.1016/0966-6362(96)82849-9.
de Oliveira SD, Magalhães FH, Faria NC, Pazzinatto MF, Ferrari D, Pappas E, et al. Lower amplitude of the Hoffmann reflex in women with patellofemoral pain: thinking beyond proximal, local, and distal factors. Arch Phys Med Rehabil. 2016;97:1115–20. https://doi.org/10.1016/j.apmr.2015.12.017.
Witvrouw E, Sneyers C, Lysens R, Victor J, Bellemans J. Reflex response times of vastus medialis oblique and vastus lateralis in normal subjects and in subjects with patellofemoral pain syndrome. J Orthop Sports Phys Ther. 1996;24:160–5. https://doi.org/10.2519/jospt.1996.24.3.160.
Solomonow M, Krogsgaard M. Sensorimotor control of knee stability: a review. Scand J Med Sci Sports. 2001;11:64–80. https://doi.org/10.1034/j.1600-0838.2001.011002064.x.
Kristjansson E, Treleaven J. Sensorimotor function and dizziness in neck pain: implications for assessment and management. J Orthop Sports Phys Ther. 2009;39:364–77. https://doi.org/10.2519/jospt.2009.2834.
Shumway-Cook A, Woollacott MH. Motor Control: Translating Research into Clinical Practice. 5th ed. Philadelphia: LWW; 2016.
Citaker S, Kaya D, Yuksel I, Yosmaoglu B, Nyland J, Atay OA, et al. Static balance in patients with patellofemoral pain syndrome. Sports Health. 2011;3:524–7. https://doi.org/10.1177/1941738111420803.
Zeinalzadeh A, Talebian S, Naghdi S, Salavati M, Nazary-Moghadam S, Zeynalzadeh GB. Effects of vision and cognitive load on static postural control in subjects with and without patellofemoral pain syndrome. Physiother Theory Pract. 2018;34:276–85. https://doi.org/10.1080/09593985.2017.1391360.
Goto S, Aminaka N, Gribble PA. Lower extremity muscle activity, kinematics, and dynamic postural control in individuals with patellofemoral pain. J Sport Rehabil. 2017. https://doi.org/10.1123/jsr.2016-0100.
Silva DO, Magalhães FH, Pazzinatto MF, Briani RV, Ferreira AS, Aragão FA, et al. Contribution of altered hip, knee and foot kinematics to dynamic postural impairments in females with patellofemoral pain during stair ascent. Knee. 2016;23:376–81. https://doi.org/10.1016/j.knee.2016.01.014.
Saad MC, Felício LR, de Masullo CL, Liporaci RF, Bevilaqua-Grossi D. Analysis of the center of pressure displacement, ground reaction force and muscular activity during step exercises. J Electromyogr Kinesiol. 2011;21:712–8. https://doi.org/10.1016/j.jelekin.2011.07.014.
Foroughi F, Sobhani S, Yoosefinejad AK, Motealleh A. Added value of isolated core postural control training on knee pain and function in women with patellofemoral pain syndrome: a randomized controlled trial. Arch Phys Med Rehabil. 2019;100:220–9. https://doi.org/10.1016/j.apmr.2018.08.180.
Lee S-P, Souza RB, Powers CM. The influence of hip abductor muscle performance on dynamic postural stability in females with patellofemoral pain. Gait Posture. 2012;36:425–9. https://doi.org/10.1016/j.gaitpost.2012.03.024.
Aminaka N, Gribble PA. Patellar taping, patellofemoral pain syndrome, lower extremity kinematics, and dynamic postural control. J Athl Train. 2008;43:21–8.
Loudon JK, Gajewski B, Goist-Foley HL, Loudon KL. The effectiveness of exercise in treating patellofemoral-pain syndrome. J Sport Rehabil. 2004;13:323–42. https://doi.org/10.1123/jsr.13.4.323.
Rabelo NDDA, Costa LOP, de Lima BM, Dos Reis AC, Bley AS, Fukuda TY, et al. Adding motor control training to muscle strengthening did not substantially improve the effects on clinical or kinematic outcomes in women with patellofemoral pain: a randomised controlled trial. Gait Posture. 2017;58:280–6. https://doi.org/10.1016/j.gaitpost.2017.08.018.
Howe TE, Rochester L, Neil F, Skelton DA, Ballinger C. Exercise for improving balance in older people. Cochrane Database Syst Rev. 2011;11:CD004963. https://doi.org/10.1002/14651858.CD004963.pub3.
Lesinski M, Hortobágyi T, Muehlbauer T, Gollhofer A, Granacher U. Effects of balance training on balance performance in healthy older adults: a systematic review and meta-analysis. Sports Med. 2015;45:1721–38. https://doi.org/10.1007/s40279-015-0375-y.
Powers CM. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33:639–46. https://doi.org/10.2519/jospt.2003.33.11.639.
Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40:42–51. https://doi.org/10.2519/jospt.2010.3337.
Tiberio D. The effect of excessive subtalar joint pronation on patellofemoral mechanics: a theoretical model. J Orthop Sports Phys Ther. 1987;9:160–5.
Boling MC, Padua DA, Marshall SW, Guskiewicz K, Pyne S, Beutler A. A prospective investigation of biomechanical risk factors for patellofemoral pain syndrome: the Joint Undertaking to Monitor and Prevent ACL Injury (JUMP-ACL) cohort. Am J Sports Med. 2009;37:2108–16. https://doi.org/10.1177/0363546509337934.
Neal BS, Lack SD, Lankhorst NE, Raye A, Morrissey D, van Middelkoop M. Risk factors for patellofemoral pain: a systematic review and meta-analysis. Br J Sports Med. 2019;53:270–81. https://doi.org/10.1136/bjsports-2017-098890.
Barton CJ, Levinger P, Menz HB, Webster KE. Kinematic gait characteristics associated with patellofemoral pain syndrome: a systematic review. Gait Posture. 2009;30:405–16. https://doi.org/10.1016/j.gaitpost.2009.07.109.
Neal BS, Barton CJ, Gallie R, O’Halloran P, Morrissey D. Runners with patellofemoral pain have altered biomechanics which targeted interventions can modify: a systematic review and meta-analysis. Gait Posture. 2016;45:69–82. https://doi.org/10.1016/j.gaitpost.2015.11.018.
Barton CJ, Munteanu SE, Menz HB, Crossley KM. The efficacy of foot orthoses in the treatment of individuals with patellofemoral pain syndrome: a systematic review. Sports Med. 2010;40:377–95. https://doi.org/10.2165/11530780-000000000-00000.
Horak FB. Clinical measurement of postural control in adults. Phys Ther. 1987;67:1881–5.
Pollock AS, Durward BR, Rowe PJ, Paul JP. What is balance? Clin Rehabil. 2000;14:402–6. https://doi.org/10.1191/0269215500cr342oa.
Higgins JPT, Thomas J, Chandler J, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg Lond Engl. 2010;8:336–41. https://doi.org/10.1016/j.ijsu.2010.02.007.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. https://doi.org/10.1136/bmj.39489.470347.AD.
Schünemann H, Brożek J, Guyatt G, et al (editors). GRADE handbook for grading quality of evidence and strength of recommendations (updated October 2013). The GRADE Working Group, 2013. Available from guidelinedevelopment.org/handbook.
van Tulder M, Furlan A, Bombardier C, Bouter L. Editorial Board of the Cochrane Collaboration Back Review Group. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine. 2003;28:1290–9. https://doi.org/10.1097/01.BRS.0000065484.95996.AF.
Duarte M, Freitas SMSF. Revision of posturography based on force plate for balance evaluation. Braz J Phys Ther. 2010;14:183–92. https://doi.org/10.1590/S1413-35552010000300003.
Lack S, Barton C, Vicenzino B, Morrissey D. Outcome predictors for conservative patellofemoral pain management: a systematic review and meta-analysis. Sports Med. 2014;44:1703–16. https://doi.org/10.1007/s40279-014-0231-5.
Farlie MK, Robins L, Haas R, Keating JL, Molloy E, Haines TP. Programme frequency, type, time and duration do not explain the effects of balance exercise in older adults: a systematic review with a meta-regression analysis. Br J Sports Med. 2019;53:996–1002. https://doi.org/10.1136/bjsports-2016-096874.
Holden S, Rathleff MS, Jensen MB, Barton CJ. How can we implement exercise therapy for patellofemoral pain if we don’t know what was prescribed? A systematic review. Br J Sports Med. 2018;52:385–385. https://doi.org/10.1136/bjsports-2017-097547.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan: a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. https://doi.org/10.1186/s13643-016-0384-4.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
Barton CJ, Silva DDO, Morton S, Collins NJ, Rathleff MS, Vicenzino B, et al. REPORT-PFP: a consensus from the International Patellofemoral Research Network to improve REPORTing of quantitative PatelloFemoral Pain studies. Br J Sports Med. 2021;55:1135–43. https://doi.org/10.1136/bjsports-2020-103700.
Carry PM, Gala R, Worster K, Kanai S, Miller NH, James D, et al. Postural stability and kinetic change in subjects with patellofemoral pain after a nine-week hip and core strengthening intervention. Int J Sports Phys Ther. 2017;12:314–23.
Gwynne CR. Alterations in center of pressure during single-limb loading in individuals with patellofemoral pain. J Am Podiatr Med Assoc. 2020;110:5. https://doi.org/10.7547/18-070.
Ankit Rohatgi. WebPlotDigitizer version 4.5. Available from: https://automeris.io/WebPlotDigitizer.
Burda BU, O’Connor EA, Webber EM, Redmond N, Perdue LA. Estimating data from figures with a Web-based program: considerations for a systematic review. Res Synth Methods. 2017;8:258–62. https://doi.org/10.1002/jrsm.1232.
da Boitrago MVS, de Mello NN, Barin FR, Júnior PL, de Souza Borges JH, Oliveira M. Effects of proprioceptive exercises and strengthening on pain and functionality for patellofemoral pain syndrome in women: a randomized controlled trial. J Clin Orthop Trauma. 2021;18:94–9. https://doi.org/10.1016/j.jcot.2021.04.017.
Motealleh A, Mohamadi M, Moghadam MB, Nejati N, Arjang N, Ebrahimi N. Effects of core neuromuscular training on pain, balance, and functional performance in women with patellofemoral pain syndrome: a clinical trial. J Chiropr Med. 2019;18:9–18. https://doi.org/10.1016/j.jcm.2018.07.006.
McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A. DEPRESsion Screening Data (DEPRESSD) Collaboration. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020. https://doi.org/10.1177/0962280219889080.
Genaidy AM, Lemasters GK, Lockey J, Succop P, Deddens J, Sobeih T, et al. An epidemiological appraisal instrument: a tool for evaluation of epidemiological studies. Ergonomics. 2007;50:920–60. https://doi.org/10.1080/00140130701237667.
Rathleff MS, Rathleff CR, Crossley KM, Barton CJ. Is hip strength a risk factor for patellofemoral pain? A systematic review and meta-analysis. Br J Sports Med. 2014;48:1088. https://doi.org/10.1136/bjsports-2013-093305.
Nunes GS, Scattone Silva R, dos Santos AF, Fernandes RAS, Serrão FV, de Noronha M. Methods to assess patellofemoral joint stress: a systematic review. Gait Posture. 2018;61:188–96. https://doi.org/10.1016/j.gaitpost.2017.12.018.
Collins NJ, Bisset LM, Crossley KM, Vicenzino PB. Efficacy of nonsurgical interventions for anterior knee pain. Sports Med. 2012;42:31–49. https://doi.org/10.2165/11594460-000000000-00000.
Reutimann S, Hill-Strathy M, Krewer C, Bergmann J, Müller F, Jahn K, et al. Influence of footwear on postural sway: a systematic review and meta-analysis on barefoot and shod bipedal static posturography in patients and healthy subjects. Gait Posture. 2022;92:302–14. https://doi.org/10.1016/j.gaitpost.2021.11.022.
Low DC, Walsh GS, Arkesteijn M. Effectiveness of exercise interventions to improve postural control in older adults: a systematic review and meta-analyses of centre of pressure measurements. Sports Med. 2017;47:101–12. https://doi.org/10.1007/s40279-016-0559-0.
Kenny RPW, Atkinson G, Eaves DL, Martin D, Burn N, Dixon J. The effects of textured materials on static balance in healthy young and older adults: a systematic review with meta-analysis. Gait Posture. 2019;71:79–86. https://doi.org/10.1016/j.gaitpost.2019.04.017.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Sullivan GM, Feinn R. Using effect size—or why the P value is not enough. J Grad Med Educ. 2012;4:279–82. https://doi.org/10.4300/JGME-D-12-00156.1.
Fletcher J. What is heterogeneity and is it important? BMJ. 2007;334:94–6. https://doi.org/10.1136/bmj.39057.406644.68.
Schroll JB, Moustgaard R, Gøtzsche PC. Dealing with substantial heterogeneity in Cochrane reviews Cross-sectional study. BMC Med Res Methodol. 2011;11:22. https://doi.org/10.1186/1471-2288-11-22.
Israel H, Richter RR. A guide to understanding meta-analysis. J Orthop Sports Phys Ther. 2011;41:496–504. https://doi.org/10.2519/jospt.2011.3333.
Higgins JPT, Thomas J, Chandler J, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions - Chapter 9 Analysing data and undertaking meta-analyses. Available from: https://handbook-5-1.cochrane.org/chapter_9/9_analysing_data_and_undertaking_meta_analyses.htm.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. https://doi.org/10.1136/bmj.315.7109.629.
Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. https://doi.org/10.1111/j.0006-341x.2000.00455.x.
Terrin N, Schmid CH, Lau J, Olkin I. Adjusting for publication bias in the presence of heterogeneity. Stat Med. 2003;22:2113–26. https://doi.org/10.1002/sim.1461.
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med. 2007;26:4544–62. https://doi.org/10.1002/sim.2889.
Murad MH, Chu H, Lin L, Wang Z. The effect of publication bias magnitude and direction on the certainty in evidence. BMJ Evid-Based Med. 2018;23:84–6. https://doi.org/10.1136/bmjebm-2018-110891.
Cristea IA, Kok RN, Cuijpers P. Efficacy of cognitive bias modification interventions in anxiety and depression: meta-analysis. Br J Psychiatry. 2015;206:7–16. https://doi.org/10.1192/bjp.bp.114.146761.
de Wit L, Luppino F, van Straten A, Penninx B, Zitman F, Cuijpers P. Depression and obesity: a meta-analysis of community-based studies. Psychiatry Res. 2010;178:230–5. https://doi.org/10.1016/j.psychres.2009.04.01.
Nunes GS, Feldkircher JM, Tessarin BM, Bender PU, da Luz CM, de Noronha M. Kinesio taping does not improve ankle functional or performance in people with or without ankle injuries: systematic review and meta-analysis. Clin Rehabil. 2021;35:182–99. https://doi.org/10.1177/0269215520963846.
Steinberg N, Tenenbaum S, Waddington G, Adams R, Zakin G, Zeev A, et al. Unilateral and bilateral patellofemoral pain in young female dancers: associated factors. J Sports Sci. 2020;38:719–30. https://doi.org/10.1080/02640414.2020.1727822.
Ibrahim Salem M, Alayat M, Shousha T. Evaluation of postural stability in patellofemoral pain syndrome patients. Indian J Physiother Occup Ther. 2014. https://doi.org/10.5958/j.0973-5674.8.2.068.
de Carvalho-E-Silva APMC, Almeida GPL, Magalhães MO, França FJR, Ramos LAV, Comachio J, et al. Dynamic postural stability and muscle strength in patellofemoral pain: Is there a correlation? Knee. 2016;23:616–21. https://doi.org/10.1016/j.knee.2016.04.013.
Felicio LR, de Masullo CL, Saad MC, Bevilaqua-Grossi D. The effect of a patellar bandage on the postural control of individuals with patellofemoral pain syndrome. J Phys Ther Sci. 2014;26:461–4. https://doi.org/10.1589/jpts.26.461.
Akhbari B, Salavati M, Mohammadi F, Safavi-farokhi Z. Intra- and inter-session reliability of static and dynamic postural control in participants with and without patellofemoral pain syndrome. Physiother Can. 2015;67:248–53. https://doi.org/10.3138/ptc.2014-51.
Negahban H, Etemadi M, Naghibi S, Emrani A, Shaterzadeh Yazdi MJ, Salehi R, et al. The effects of muscle fatigue on dynamic standing balance in people with and without patellofemoral pain syndrome. Gait Posture. 2013;37:336–9. https://doi.org/10.1016/j.gaitpost.2012.07.025.
Nasab MS, Mostamand J, Jamshidi N, Tahririan M. The comparison of hip abductors with hip external rotator muscles fatigue on static standing balance in subjects with and without patellofemoral pain syndrome. Phys Treat. 2014;4:47–54.
Motealleh A, Kordi Yoosefinejad A, Ghoddosi M, Azhdari N, Pirouzi S. Trunk postural control during unstable sitting differs between patients with patellofemoral pain syndrome and healthy people: a cross-sectional study. Knee. 2019;26:26–32. https://doi.org/10.1016/j.knee.2018.10.002.
Loudon JK, Wiesner D, Goist-Foley HL, Asjes C, Loudon KL. Intrarater reliability of functional performance tests for subjects with patellofemoral pain syndrome. J Athl Train. 2002;37:256–61.
Song C-Y, Lin J-J, Chang AH. Effects of femoral rotational taping on dynamic postural stability in female patients with patellofemoral pain. Clin J Sport Med. 2017;27:438–43. https://doi.org/10.1097/JSM.0000000000000392.
Zamboti CL, da Silva RA, Gobbi C, Shigaki L, de Macedo CSG. Analysis of pain, functional capacity, muscular strength and balance in young women with patellofemoral pain syndrome. Fisioter Em Mov. 2017;30:433–41. https://doi.org/10.1590/1980-5918.030.003.AO01.
Arun B, Vakkachan T, Abraham BA. Comparison of dynamic postural control with and without patellofemoral pain syndrome using star excursion balance test. Res Rev J Med Sci Technol. 2019;2:1–6.
Stensdotter AK, Grip H, Hodges PW, Häger-Ross C. Quadriceps activity and movement reactions in response to unpredictable sagittal support-surface translations in women with patellofemoral pain. J Electromyogr Kinesiol. 2008;18:298–307. https://doi.org/10.1016/j.jelekin.2006.10.004.
Coelho VK, Gomes BSQ, Lopes TJA, Corrêa LA, Telles GF, Nogueira LAC. Knee proprioceptive function and physical performance of patients with patellofemoral pain: a matched case-control study. Knee. 2021;33:49–57. https://doi.org/10.1016/j.knee.2021.08.031.
Kim C, Yeom S, Ahn S, Kang N, Park K, Jeon K. Effects of patellofemoral pain syndrome on changes in dynamic postural stability during landing in adult women. Appl Bionics Biomech. 2022;2022:7452229. https://doi.org/10.1155/2022/7452229.
Manojlović D, Zorko M, Spudić D, Šarabon N. Strength, flexibility and postural control of the trunk and lower body in participants with and without patellofemoral pain. Appl Sci. 2022;12:3238. https://doi.org/10.3390/app12073238.
Priore LB, Azevedo FM, Pazzinatto MF, Ferreira AS, Hart HF, Barton C, et al. Influence of kinesiophobia and pain catastrophism on objective function in women with patellofemoral pain. Phys Ther Sport. 2019;35:116–21. https://doi.org/10.1016/j.ptsp.2018.11.013.
Yelvar GDY, Çirak Y, Dalkilinç M, Demir YP, Baltaci G, Kömürcü M, et al. Impairments of postural stability, core endurance, fall index and functional mobility skills in patients with patello femoral pain syndrome. J Back Musculoskelet Rehabil. 2017;30:163–70. https://doi.org/10.3233/BMR-160729.
Naserpour M, Goharpey S, Saki A, Mohammadi Z. Dynamic postural control during step down task in patients with patellofemoral pain syndrome. J Phys Ther Sci. 2018;30:1289–92. https://doi.org/10.1589/jpts.30.1289.
Stensdotter A-K, Guerra JB, Häger-Ross C. Limb support in response to balance provocations in women with patellofemoral pain. Adv Physiother. 2009;11:97–103. https://doi.org/10.1080/14038190802425575.
Maryam E, Mahbobeh S, Farzaneh M, Fatemeh K, Zohreh N. The effects of two taping in patients with patellofemoral pain syndrome. Ann Trop Med Public Health. 2018;1:34–55. https://doi.org/10.1002/central/CN-01959246.
Maryam E, Mahbobeh S, Farzaneh M, Fatemeh K, Zohreh N. A comparison of the effect of knee muscle taping versus core muscle taping on balance, pain and functional performance in patients with patellofemoral pain syndrome. Physiother Q. 2022;30:38–45. https://doi.org/10.5114/pq.2021.108670.
Steinberg N, Tenenbaum S, Waddington G, Adams R, Zakin G, Zeev A, et al. Isometric exercises and somatosensory training as intervention programmes for patellofemoral pain in young dancers. Eur J Sport Sci. 2020;20:845–57. https://doi.org/10.1080/17461391.2019.1675766.
Motealleh A, Barzegar A, Abbasi L. The immediate effect of lumbopelvic manipulation on knee pain, knee position sense, and balance in patients with patellofemoral pain: a randomized controlled trial. J Bodyw Mov Ther. 2020;24:71–7. https://doi.org/10.1016/j.jbmt.2020.01.006.
Ferreira DC, da Silva Junior RA, Araújo CGA, Mantovani PR, de Macedo CSG. McConnell patellar taping on postural control of women with patellofemoral pain syndrome: randomized clinical trial. Fisioter Em Mov. 2020. https://doi.org/10.1590/1980-5918.033.AO57.
Zarei H, Bervis S, Piroozi S, Motealleh A. Added value of gluteus medius and quadratus lumborum dry needling in improving knee pain and function in female athletes with patellofemoral pain syndrome: a randomized clinical trial. Arch Phys Med Rehabil. 2020;101:265–74. https://doi.org/10.1016/j.apmr.2019.07.009.
Ahmadi M, Yalfani A, Gandomi F, Rashid K. The effect of twelve-week neurofeedback training on pain, proprioception, strength and postural balance in men with patellofemoral pain syndrome: a double-blind randomized control trial. J Rehabil Sci Res. 2020;7:66–74. https://doi.org/10.30476/jrsr.2020.84868.1067.
Goel N, Bhatia S. Effect of patellar taping on dynamic balance during star excursion balance test in patients with patellofemoral pain syndrome. Indian J Physiother Occup Ther- Int J. 2012;6:145–8.
Ebrahimi N, Rojhani-Shirazi Z, Yoosefinejad AK, Nami M. The effects of virtual reality training on clinical indices and brain mapping of women with patellofemoral pain: a randomized clinical trial. BMC Musculoskelet Disord. 2021;22:900. https://doi.org/10.1186/s12891-021-04785-6.
Mahmoud W, Kamel E. The effect of additional balance training program to gluteus medius strengthening exercises on patellofemoral pain syndrome. Int J Ther Rehabil Res. 2015;4:7. https://doi.org/10.5455/ijtrr.00000051.
Aytar A, Ozunlu N, Surenkok O, Baltacı G, Oztop P, Karatas M. Initial effects of kinesio taping in patients with patellofemoral pain syndrome: a randomized, double-blind study. Isokinet Exerc Sci. 2011;19:135–42. https://doi.org/10.3233/IES-2011-0413.
Chevidikunnan MF, Al Saif A, Gaowgzeh RA, Mamdouh KA. Effectiveness of core muscle strengthening for improving pain and dynamic balance among female patients with patellofemoral pain syndrome. J Phys Ther Sci. 2016;28:1518–23. https://doi.org/10.1589/jpts.28.1518.
Miller J, Westrick R, Diebal A, Marks C, Gerber JP. Immediate effects of lumbopelvic manipulation and lateral gluteal kinesio taping on unilateral patellofemoral pain syndrome: a pilot study. Sports Health. 2013;5:214–9. https://doi.org/10.1177/1941738112473561.
Demirci S, Kinikli GI, Callaghan MJ, Tunay VB. Comparison of short-term effects of mobilization with movement and Kinesiotaping on pain, function and balance in patellofemoral pain. Acta Orthop Traumatol Turc. 2017;51:442–7. https://doi.org/10.1016/j.aott.2017.09.005.
Ojaghi SM, Kamali F, Ghanbari A, Ebrahimi S, Nematollahi AR. Effects of taping and elastic bandage on postural control in athletes with patellofemoral pain: a randomized control trial. GMJ. 2015;4:82–9.
Sinaei E, Foroozantabar V, Yoosefinejad AK, Sobhani S, Motealleh A. Electromyographic comparison of vastus medialis obliquus facilitatory versus vastus lateralis inhibitory kinesio taping in athletes with patellofemoral pain: a randomized clinical trial. J Bodyw Mov Ther. 2021;28:157–63. https://doi.org/10.1016/j.jbmt.2021.07.017.
Fang B, Kim Y-H, Choi M-Y. Effects of high-intensity aquatic or bicycling training in athletes with unilateral patellofemoral pain syndrome. Int J Environ Res Public Health. 2022;19:4675. https://doi.org/10.3390/ijerph19084675.
Ferber R, Bolgla L, Earl-Boehm JE, Emery C, Hamstra-Wright K. Strengthening of the hip and core versus knee muscles for the treatment of patellofemoral pain: a multicenter randomized controlled trial. J Athl Train. 2015;50:366–77. https://doi.org/10.4085/1062-6050-49.3.70.
van Linschoten R, van Middelkoop M, Berger MY, Heintjes EM, Verhaar JAN, Willemsen SP, et al. Supervised exercise therapy versus usual care for patellofemoral pain syndrome: an open label randomised controlled trial. BMJ. 2009;339:4074. https://doi.org/10.1136/bmj.b4074.
Clark D, Downing N, Mitchell J, Coulson L, Syzpryt E, Doherty M. Physiotherapy for anterior knee pain: a randomised controlled trial. Ann Rheum Dis. 2000;59:700–4. https://doi.org/10.1136/ard.59.9.700.
Emamvirdi M, Letafatkar A, Khaleghi TM. The effect of valgus control instruction exercises on pain, strength, and functionality in active females with patellofemoral pain syndrome. Sports Health. 2019;11:223–37. https://doi.org/10.1177/1941738119837622.
Shadloo N, Kamali F, Salehi DN. A comparison between whole-body vibration and conventional training on pain and performance in athletes with patellofemoral pain. J Bodyw Mov Ther. 2021;27:661–6. https://doi.org/10.1016/j.jbmt.2021.03.003.
Mølgaard CM, Rathleff MS, Andreasen J, Christensen M, Lundbye-Christensen S, Simonsen O, et al. Foot exercises and foot orthoses are more effective than knee focused exercises in individuals with patellofemoral pain. J Sci Med Sport. 2018;21:10–5. https://doi.org/10.1016/j.jsams.2017.05.019.
Yalfani A, Ahmadi M, Gandomi F. The effects of 12-weeks of sensorimotor exercise on pain, strength, pelvic drop, and dynamic knee valgus in males with patellofemoral pain syndrome. Phys Treat. 2020;10:159–68. https://doi.org/10.32598/ptj.10.3.442.1.
Diekfuss JA, Grooms DR, Nissen KS, Coghill RC, Bonnette S, Barber Foss KD, et al. Does central nervous system dysfunction underlie patellofemoral pain in young females? Examining brain functional connectivity in association with patient-reported outcomes. J Orthop Res. 2021. https://doi.org/10.1002/jor.25152.
Baker V, Bennell K, Stillman B, Cowan S, Crossley K. Abnormal knee joint position sense in individuals with patellofemoral pain syndrome. J Orthop Res. 2002;20:208–14. https://doi.org/10.1016/S0736-0266(01)00106-1.
Barton CJ, Lack S, Malliaras P, Morrissey D. Gluteal muscle activity and patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47:207–14. https://doi.org/10.1136/bjsports-2012-090953.
Nunes GS, Barton CJ, Serrão FV. Hip rate of force development and strength are impaired in females with patellofemoral pain without signs of altered gluteus medius and maximus morphology. J Sci Med Sport. 2018;21:123–8. https://doi.org/10.1016/j.jsams.2017.05.014.
Nakagawa TH, Dos Santos AF, Lessi GC, Petersen RS, Scattone SR. Y-balance test asymmetry and frontal plane knee projection angle during single-leg squat as predictors of patellofemoral pain in male military recruits. Phys Ther Sport. 2020;44:121–7. https://doi.org/10.1016/j.ptsp.2020.05.011.
Hodges PW, Mellor R, Crossley K, Bennell K. Pain induced by injection of hypertonic saline into the infrapatellar fat pad and effect on coordination of the quadriceps muscles. Arthritis Care Res. 2009;61:70–7. https://doi.org/10.1002/art.24089.
Hirata RP, Arendt-Nielsen L, Shiozawa S, Graven-Nielsen T. Experimental knee pain impairs postural stability during quiet stance but not after perturbations. Eur J Appl Physiol. 2012;112:2511–21. https://doi.org/10.1007/s00421-011-2226-3.
Culvenor AG, van Middelkoop M, Macri EM, Crossley KM. Is patellofemoral pain preventable? A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2020. https://doi.org/10.1136/bjsports-2020-102973.
Ferreira M. The smallest worthwhile effect of a health intervention. J Physiother. 2018;64:272–4. https://doi.org/10.1016/j.jphys.2018.07.008.
Yin L, Qin J, Chen Y, Xie J, Hong C, Huang J, et al. Impact of body mass index on static postural control in adults with and without diabetes: a cross-sectional study. Front Endocrinol. 2021;12: 768185. https://doi.org/10.3389/fendo.2021.768185.
Tsiros MD, Brinsley J, Mackintosh S, Thewlis D. Relationships between adiposity and postural control in girls during balance tasks of varying difficulty. Obes Res Clin Pract. 2019;13:358–64. https://doi.org/10.1016/j.orcp.2019.06.003.
Kozinc Ž, Löfler S, Hofer C, Carraro U, Šarabon N. Diagnostic balance tests for assessing risk of falls and distinguishing older adult fallers and non-fallers: a systematic review with meta-Analysis. Diagnostics. 2020;10:667. https://doi.org/10.3390/diagnostics10090667.
Thompson C, Schabrun S, Romero R, Bialocerkowski A, van Dieen J, Marshall P. Factors contributing to chronic ankle instability: a systematic review and meta-analysis of systematic reviews. Sports Med. 2018;48:189–205. https://doi.org/10.1007/s40279-017-0781-4.
Song K, Jang J, Nolte T, Wikstrom EA. Dynamic reach deficits in those with chronic ankle instability: a systematic review and meta-analysis. Phys Ther Sport. 2022;53:40–50. https://doi.org/10.1016/j.ptsp.2021.11.004.
Acknowledgements
The authors would like to thank the Federal University of Santa Maria and the Rio Grande do Sul Research Foundation (FAPERGS) for the financial support.
Funding
This work was supported under public notices 013/2021 [PROBIC – Rio Grande do Sul Research Foundation/Federal University of Santa Maria (FAPERGS/UFSM); scholarship to Diênifer Zilmer Rodrigues] and 010/2020 (ARD FAPERGS process number 21/2551-0000708-7).
Author information
Authors and Affiliations
Contributions
GSN was responsible for the study conceptualization; GSN, FVS and MN designed the study; GSN and MN formulated the search strategy and carried out the searches; GSN, DZR, LH, BMT and IP performed the selection process and data extraction; GSN, BMT and IP assessed the risk of bias; GSN and MN performed all the statistical analyses; GSN, BMT and FVS performed the GRADE assessment; GSN, DZR and LH drafted the manuscript; GSN and MN are the study guarantor; all authors reviewed and edited the draft; all authors read and consented to the content of the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing interests
Guilherme Nunes, Diênifer Rodrigues, Luiza Hörbe, Bruna Tessarin, Fábio Serrão, Izabela Prates and Marcos de Noronha declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Strategy searches.
Additional file 2.
Excluded studies.
Additional file 3.
Funnel plots.
Additional file 4.
GRADE description.
Additional file 5.
Study characteristics.
Additional file 6.
Risk of bias assessment.
Additional file 7.
Subgroup and meta-regression analysis.
Additional file 8.
Data of the included studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nunes, G.S., Rodrigues, D.Z., Hörbe, L. et al. Is Postural Control Affected in People with Patellofemoral Pain and Should it be Part of Rehabilitation? A Systematic Review with Meta-analysis. Sports Med - Open 8, 144 (2022). https://doi.org/10.1186/s40798-022-00538-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40798-022-00538-4